Unveiling the Influence of Carbon Nanotube Diameter and Surface Modification on the Anchorage of L-Asparaginase
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials, Enzyme and Chemicals
2.2. Characterization Techniques
2.3. Determination of ASNase Isoelectric Point
2.4. MWCNTs Functionalization
2.5. ASNase Immobilization over MWCNT
2.6. ASNase Activity Measurement
2.7. Operational Stability of Immobilized ASNase
2.8. Enzymatic Kinetic Parameters
3. Results and Discussion
3.1. Characterization of MWCNTs
3.2. Determination of ASNase Isoelectric Point
3.3. Effect of pH on ASNase Immobilization onto MWCNTs
3.4. Effect of Contact Time on ASNase Immobilization onto MWCNTs
3.5. Effect of Enzyme Concentration on ASNase Immobilization onto MWCNTs
3.6. Operational Stability
3.7. Kinetic Parameters Determination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jozala, A.F.; Geraldes, D.C.; Tundisi, L.L.; Feitosa, V.A.; Breyer, C.A.; Cardoso, S.L.; Mazzola, P.G.; Oliveira-Nascimento, L.; Rangel-Yagui, C.O.; Magalhães, P.O.; et al. Biopharmaceuticals from microorganisms: From production to purification. Braz. J. Microbiol. Publ. Braz. Soc. Microbiol. 2016, 47 (Suppl. 1), 51–63. [Google Scholar] [CrossRef] [PubMed]
- Panke, S.; Wubbolts, M.G. Enzyme technology and bioprocess engineering. Curr. Opin. Biotechnol. 2002, 13, 111–116. [Google Scholar] [CrossRef]
- Miranda-Filho, A.; Piñeros, M.; Ferlay, J.; Soerjomataram, I.; Monnereau, A.; Bray, F. Epidemiological patterns of leukaemia in 184 countries: A population-based study. Lancet. Haematol. 2018, 5, e14–e24. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-Q.; Tao, M.-L.; Shen, W.-D.; Zhou, Y.-Z.; Ding, Y.; Ma, Y.; Zhou, W.-L. Immobilization of l-asparaginase on the microparticles of the natural silk sericin protein and its characters. Biomaterials 2004, 25, 3751–3759. [Google Scholar] [CrossRef] [PubMed]
- Ulu, A.; Ates, B. Immobilization of l-Asparaginase on Carrier Materials: A Comprehensive Review. Bioconjug. Chem. 2017, 28, 1598–1610. [Google Scholar] [CrossRef]
- Aiswarya, R.; Baskar, G. Enzymatic mitigation of acrylamide in fried potato chips using asparaginase from Aspergillus terreus. Int. J. Food Sci. Technol. 2018, 53, 491–498. [Google Scholar]
- Nunes, J.C.F.; Cristóvão, R.O.; Freire, M.G.; Santos-Ebinuma, V.C.; Faria, J.L.; Silva, C.G.; Tavares, A.P.M. Recent Strategies and Applications for l-Asparaginase Confinement. Molecules 2020, 25, 5827. [Google Scholar] [CrossRef]
- Haroun, A.; Mostafa, H.; Ahmed, E. Functionalized Multi-walled Carbon Nanotubes as Emerging Carrier for Biological Applications. In Proceedings of the 5th World Congress on New Technologies (NewTech’19), Lisbon, Portugal, 18–20 August 2019. [Google Scholar]
- Cristóvão, R.O.; Almeida, M.R.; Barros, M.A.; Nunes, J.C.F.; Boaventura, R.A.R.; Loureiro, J.M.; Faria, J.L.; Neves, M.C.; Freire, M.G.; Ebinuma-Santos, V.C.; et al. Development and characterization of a novel l-asparaginase/MWCNT nanobioconjugate. RSC Adv. 2020, 10, 31205–31213. [Google Scholar] [CrossRef]
- Verma, S.K.; Modi, A.; Bellare, J. Polyethersulfone-carbon nanotubes composite hollow fiber membranes with improved biocompatibility for bioartificial liver. Colloids Surf. B Biointerfaces 2019, 181, 890–895. [Google Scholar]
- Zhao, X.; Tian, K.; Zhou, T.; Jia, X.; Li, J.; Liu, P. PEGylated multi-walled carbon nanotubes as versatile vector for tumor-specific intracellular triggered release with enhanced anti-cancer efficiency: Optimization of length and PEGylation degree. Colloids Surfaces B Biointerfaces 2018, 168, 43–49. [Google Scholar] [CrossRef]
- Costa, J.B.; Lima, M.J.; Sampaio, M.J.; Neves, M.C.; Faria, J.L.; Morales-Torres, S.; Tavares, A.P.M.; Silva, C.G. Enhanced biocatalytic sustainability of laccase by immobilization on functionalized carbon nanotubes/polysulfone membranes. Chem. Eng. J. 2019, 355, 974–985. [Google Scholar] [CrossRef]
- Mohamad, N.R.; Marzuki, N.H.C.; Buang, N.A.; Huyop, F.; Wahab, R.A. An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnol. Biotechnol. Equip. 2015, 29, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, G.; Lin, Y. Amperometric choline biosensor fabricated through electrostatic assembly of bienzyme/polyelectrolyte hybrid layers on carbon nanotubes. Analyst 2006, 131, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.G.; Tavares, A.P.M.; Dražić, G.; Silva, A.M.T.; Loureiro, J.M.; Faria, J.L. Controlling the Surface Chemistry of Multiwalled Carbon Nanotubes for the Production of Highly Efficient and Stable Laccase-Based Biocatalysts. ChemPlusChem 2014, 79, 1116–1122. [Google Scholar] [CrossRef]
- Elena, V.E. Graphical Approach to Compare Concentration Constants of Hill and Michaelis-Menten Equations. J. Biotechnol. Biomed. Sci. 2018, 1, 94–99. [Google Scholar]
- Zhou, Y.; Fang, Y.; Ramasamy, R.P. Non-Covalent Functionalization of Carbon Nanotubes for Electrochemical Biosensor Development. Sensors 2019, 19, 392. [Google Scholar] [CrossRef]
- Hu, C.-Y.; Xu, Y.-J.; Duo, S.-W.; Zhang, R.-F.; Li, M.-S. Non-Covalent Functionalization of Carbon Nanotubes with Surfactants and Polymers. J. Chin. Chem. Soc. 2009, 56, 234–239. [Google Scholar] [CrossRef]
- Ali Mohammadi, Z.; Aghamiri, S.F.; Zarrabi, A.; Talaie, M.R. A comparative study on non-covalent functionalization of carbon nanotubes by chitosan and its derivatives for delivery of doxorubicin. Chem. Phys. Lett. 2015, 642, 22–28. [Google Scholar] [CrossRef]
- Ulu, A.; Karaman, M.; Yapıcı, F.; Naz, M.; Sayın, S.; Saygılı, E.İ.; Ateş, B. The Carboxylated Multi-walled Carbon Nanotubes/l-Asparaginase Doped Calcium-Alginate Beads: Structural and Biocatalytic Characterization. Catal. Lett. 2020, 150, 1679–1691. [Google Scholar] [CrossRef]
- Haroun, A.A.A.; Ahmed, H.M.; Mossa, A.; Mohafrash, S.M.M.; Ahmed, E.F. Production, characterization and immobilization of Aspergillus versicolor L-asparaginase onto multi-walled carbon nanotubes. Biointerface Res. Appl. Chem. 2020, 10, 733740. [Google Scholar]
- Almeida, M.R.; Cristóvão, R.O.; Barros, M.A.; Nunes, J.C.F.; Boaventura, R.A.R.; Loureiro, J.M.; Faria, J.L.; Neves, M.C.; Freire, M.G.; Santos-Ebinuma, V.C.; et al. Superior operational stability of immobilized l-asparaginase over surface-modified carbon nanotubes. Sci. Rep. 2021, 11, 21529. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Lu, D.; Hao, F.; Liu, R. Exploring the diameter and surface dependent conformational changes in carbon nanotube-protein corona and the related cytotoxicity. J. Hazard. Mater. 2015, 292, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Mu, Q.; Liu, W.; Xing, Y.; Zhou, H.; Li, Z.; Zhang, Y.; Ji, L.; Wang, F.; Si, Z.; Zhang, B.; et al. Protein Binding by Functionalized Multiwalled Carbon Nanotubes Is Governed by the Surface Chemistry of Both Parties and the Nanotube Diameter. J. Phys. Chem. C 2008, 112, 3300–3307. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Bautista-Toledo, I.; Ferro-García, M.A.; Moreno-Castilla, C. Activated carbon surface modifications by adsorption of bacteria and their effect on aqueous lead adsorption. J. Chem. Technol. Biotechnol. 2001, 76, 1209–1215. [Google Scholar] [CrossRef] [Green Version]
- Marques, R.; Machado, B.; Faria, J.; Silva, A. Controlled generation of oxygen functionalities on the surface of Single-Walled Carbon Nanotubes by HNO3 hydrothermal oxidation. Carbon 2010, 48, 1515–1523. [Google Scholar] [CrossRef]
- Barros, R.A.M.; Cristóvão, R.O.; Carabineiro, S.A.C.; Neves, M.C.; Freire, M.G.; Faria, J.L.; Santos-Ebinuma, V.C.; Tavares, A.P.M.; Silva, C.G. Immobilization and Characterization of L-Asparaginase over Carbon Xerogels. BioTech 2022, 11, 10. [Google Scholar] [CrossRef]
- Walters, D.E. Enzymes A: Practical Introduction to Structure, Mechanism, and Data Analysis, 2nd ed.; Copeland, R.A., Ed.; Wiley-VCH: New York, NY, USA, 2000; pp. xvi, 397. ISBN 0-471-35929-7. [Google Scholar]
- Feng, W.; Ji, P. Enzymes immobilized on carbon nanotubes. Biotechnol. Adv. 2011, 29, 889–895. [Google Scholar] [CrossRef]
- Shim, M.; Shi Kam, N.W.; Chen, R.J.; Li, Y.; Dai, H. Functionalization of Carbon Nanotubes for Biocompatibility and Biomolecular Recognition. Nano Lett. 2002, 2, 285–288. [Google Scholar] [CrossRef]
- Wang, L.; Wei, L.; Chen, Y.; Jiang, R. Specific and reversible immobilization of NADH oxidase on functionalized carbon nanotubes. J. Biotechnol. 2010, 150, 57–63. [Google Scholar] [CrossRef]
- Hou, J.; Xu, L.; Han, Y.; Tang, Y.; Wan, H.; Xu, Z.; Zheng, S. Deactivation and regeneration of carbon nanotubes and nitrogen-doped carbon nanotubes in catalytic peroxymonosulfate activation for phenol degradation: Variation of surface functionalities. RSC Adv. 2019, 9, 974–983. [Google Scholar] [CrossRef]
- Peigney, A.; Laurent, C.; Flahaut, E.; Bacsa, R.R.; Rousset, A. Specific surface area of carbon nanotubes and bundles of carbon nanotubes. Carbon 2001, 39, 507–514. [Google Scholar] [CrossRef]
- Chen, M.; Yu, H.-W.; Chen, J.-H.; Koo, H.-S. Effect of purification treatment on adsorption characteristics of carbon nanotubes. Diam. Relat. Mater. 2007, 16, 1110–1115. [Google Scholar] [CrossRef]
- Lemes, A.P.; Montanheiro, T.L.d.A.; da Silva, A.P.; Durán, N. PHBV/MWCNT Films: Hydrophobicity, Thermal and Mechanical Properties as a Function of MWCNT Concentration. J. Compos. Sci. 2019, 3, 12. [Google Scholar] [CrossRef]
- Amaral Montanheiro, T.L.; Montagna, L.S.; Machado, J.P.B.; Lemes, A.P. Covalent functionalization of MWCNT with PHBV chains: Evaluation of the functionalization and production of nanocomposites. Polym. Compos. 2019, 40, 288–295. [Google Scholar] [CrossRef] [Green Version]
- Salgin, S.; Salgin, U.; Bahadir, S. Zeta Potentials and Isoelectric Points of Biomolecules: The Effects of Ion Types and Ionic Strengths. Int. J. Electrochem. Sci. 2012, 7, 12404–12414. [Google Scholar]
- Bae, N.; Pollak, A.; Lubec, G. Proteins from Erwinia asparaginase Erwinase® and E. coli asparaginase 2 MEDAC® for treatment of human leukemia, show a multitude of modifications for which the consequences are completely unclear. Electrophoresis 2011, 32, 1824–1828. [Google Scholar] [CrossRef]
- Liu, Y.; Pietzsch, M.; Ulrich, J. Purification of L-asparaginase II by crystallization. Front. Chem. Sci. Eng. 2013, 7, 37–42. [Google Scholar] [CrossRef]
- Zhu, J.H.; Yan, X.L.; Chen, H.J.; Wang, Z.H. In situ extraction of intracellular L-asparaginase using thermoseparating aqueous two-phase systems. J. Chromatogr. A 2007, 1147, 127–134. [Google Scholar] [CrossRef]
- Noma, S.A.A.; Ulu, A.; Acet, Ö.; Sanz, R.; Sanz-Pérez, E.S.; Odabaşı, M.; Ateş, B. Comparative study of ASNase immobilization on tannic acid-modified magnetic Fe3O4/SBA-15 nanoparticles to enhance stability and reusability. New J. Chem. 2020, 44, 4440–4451. [Google Scholar] [CrossRef]
- Verma, S.; Mehta, R.K.; Maiti, P.; Röhm, K.H.; Sonawane, A. Improvement of stability and enzymatic activity by site-directed mutagenesis of E. coli asparaginase II. Biochim. Et Biophys. Acta 2014, 1844, 1219–1230. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Fan, H.; Ai, S.; Han, R.; Qiu, Y. Hemoglobin (Hb) immobilized on amino-modified magnetic nanoparticles for the catalytic removal of bisphenol A. Chemosphere 2011, 83, 255–264. [Google Scholar] [CrossRef]
- Li, P.; Ma, X.-H.; Dong, Y.-M.; Jin, L.; Chen, J. α-Glucosidase immobilization on polydopamine-coated cellulose filter paper and enzyme inhibitor screening. Anal. Biochem. 2020, 605, 113832. [Google Scholar] [CrossRef] [PubMed]
- Bahreini, E.; Aghaiypour, K.; Abbasalipourkabir, R.; Mokarram, A.R.; Goodarzi, M.T.; Saidijam, M. Preparation and nanoencapsulation of l-asparaginase II in chitosan-tripolyphosphate nanoparticles and in vitro release study. Nanoscale Res. Lett. 2014, 9, 340. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Sharma, I.; Prajapati, B.P.; Suryawanshi, R.K.; Kango, N. Catalytic characteristics and application of l-asparaginase immobilized on aluminum oxide pellets. Int. J. Biol. Macromol. 2018, 114, 504–511. [Google Scholar] [CrossRef]
- Golestaneh, D.; Varshosaz, J. Enhancement in Biological Activity of L-Asparginase by its Conjugation on Silica Nanoparticles. Recent Pat. Nanotechnol. 2018, 12, 70–82. [Google Scholar] [CrossRef]
- Ulu, A. Metal–organic frameworks (MOFs): A novel support platform for ASNase immobilization. J. Mater. Sci. 2020, 55, 6130–6144. [Google Scholar] [CrossRef]
- Ali Noma, S.A.; Acet, Ö.; Ulu, A.; Önal, B.; Odabaşı, M.; Ateş, B. l-asparaginase immobilized p(HEMA-GMA) cryogels: A recent study for biochemical, thermodynamic and kinetic parameters. Polym. Test. 2021, 93, 106980. [Google Scholar] [CrossRef]
- Silverstein, T.P.; Goodney, D.E. Enzyme-Linked Biosensors: Michaelis−Menten Kinetics Need Not Apply. J. Chem. Educ. 2010, 87, 905–907. [Google Scholar] [CrossRef]
- Valencia, P.; Ibañez, F. Estimation of the Effectiveness Factor for Immobilized Enzyme Catalysts through a Simple Conversion Assay. Catalysts 2019, 9, 930. [Google Scholar] [CrossRef]
- Hosseinipour, S.L.; Khiabani, M.S.; Hamishehkar, H.; Salehi, R. Enhanced stability and catalytic activity of immobilized α-amylase on modified Fe3O4 nanoparticles for potential application in food industries. J. Nanopart. Res. 2015, 17, 382. [Google Scholar] [CrossRef]
- Magri, A.; Soler, M.F.; Lopes, A.M.; Cilli, E.M.; Barber, P.S.; Pessoa, A., Jr.; Pereira, J.F.B. A critical analysis of L-asparaginase activity quantification methods-colorimetric methods versus high-performance liquid chromatography. Anal. Bioanal. Chem. 2018, 410, 6985–6990. [Google Scholar] [CrossRef] [PubMed]
- Nunes, J.C.F.; Cristóvão, R.O.; Santos-Ebinuma, V.C.; Faria, J.L.; Silva, C.G.; Neves, M.C.; Freire, M.G.; Tavares, A.P.M. L-Asparaginase-Based Biosensors. Encyclopedia 2021, 1, 848–858. [Google Scholar] [CrossRef]
- Tundisi, L.L.; Coêlho, D.F.; Zanchetta, B.; Moriel, P.; Pessoa, A.; Tambourgi, E.B.; Silveira, E.; Mazzola, P.G. L-Asparaginase Purification. Sep. Purif. Rev. 2017, 46, 35–43. [Google Scholar] [CrossRef]
- Gashtasbi, F.; Ahmadian, G.; Noghabi, K.A. New insights into the effectiveness of alpha-amylase enzyme presentation on the Bacillus subtilis spore surface by adsorption and covalent immobilization. Enzym. Microb. Technol. 2014, 64–65, 17–23. [Google Scholar] [CrossRef]
- Orhan, H.; Aktaş Uygun, D. Immobilization of L-Asparaginase on Magnetic Nanoparticles for Cancer Treatment. Appl. Biochem. Biotechnol. 2020, 191, 1432–1443. [Google Scholar] [CrossRef]


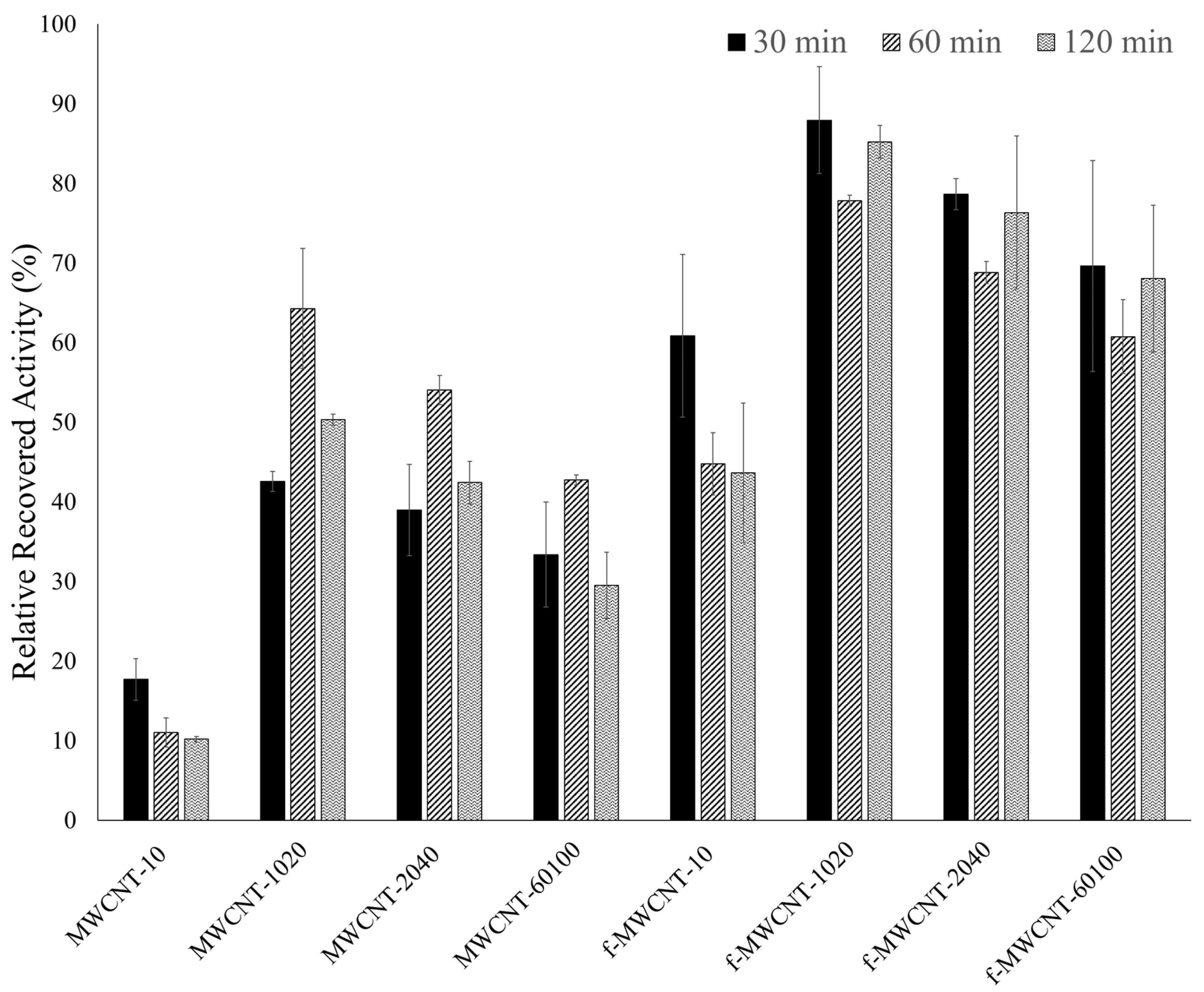
 ). ASNase immobilization onto 2 mg of pristine multi-walled carbon nanotubes (MWCNTs) at pH 8.0 for 60 min. Error bars correspond to the standard deviation between replicates.
). ASNase immobilization onto 2 mg of pristine multi-walled carbon nanotubes (MWCNTs) at pH 8.0 for 60 min. Error bars correspond to the standard deviation between replicates.
 ). ASNase immobilization onto 2 mg of pristine multi-walled carbon nanotubes (MWCNTs) at pH 8.0 for 60 min. Error bars correspond to the standard deviation between replicates.
). ASNase immobilization onto 2 mg of pristine multi-walled carbon nanotubes (MWCNTs) at pH 8.0 for 60 min. Error bars correspond to the standard deviation between replicates.
 ). ASNase immobilization onto 2 mg of functionalized multi-walled carbon nanotubes (f-MWCNTs) at pH 8.0 for 30 min. Error bars correspond to the standard deviation between replicates.
). ASNase immobilization onto 2 mg of functionalized multi-walled carbon nanotubes (f-MWCNTs) at pH 8.0 for 30 min. Error bars correspond to the standard deviation between replicates.
 ). ASNase immobilization onto 2 mg of functionalized multi-walled carbon nanotubes (f-MWCNTs) at pH 8.0 for 30 min. Error bars correspond to the standard deviation between replicates.
). ASNase immobilization onto 2 mg of functionalized multi-walled carbon nanotubes (f-MWCNTs) at pH 8.0 for 30 min. Error bars correspond to the standard deviation between replicates.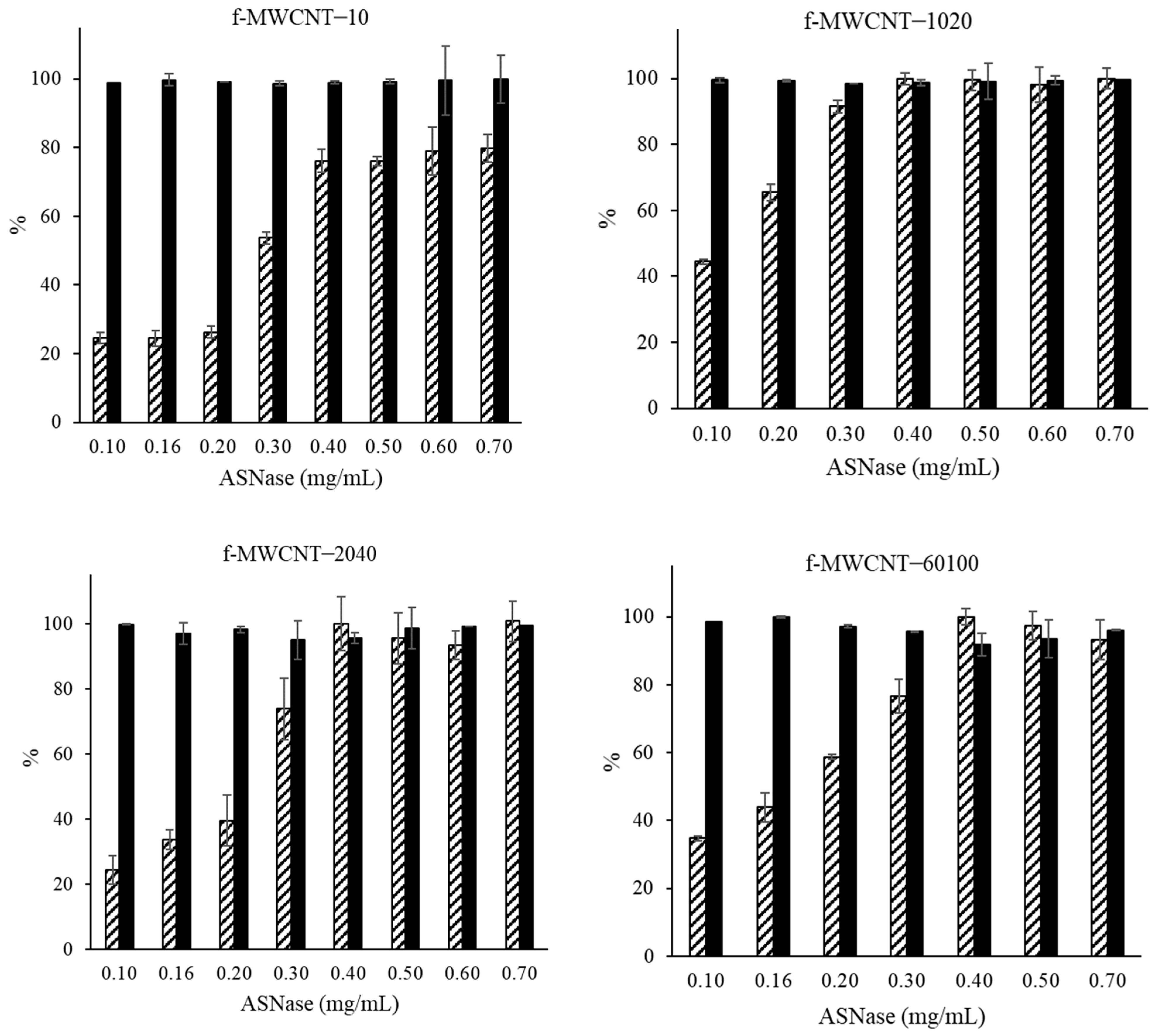


| Base Material | Diameter Range (nm) | Length (µm) | Purity (%) | Ash (wt %) | Specific Surface Area Range (m2/g) |
|---|---|---|---|---|---|
| MWCNT-10 | <10 | >5 | >97 | <3 | 250–500 |
| MWCNT-1020 | 10–20 | >5 | >97 | <3 | 100–160 |
| MWCNT-2040 | 20–40 | >5 | >97 | <3 | 80–140 |
| MWCNT-60100 | 60–100 | >5 | >97 | <3 | 40–70 |
| SBET (±5 m2/g) | ||
|---|---|---|
| MWCNT | F-MWCNT | |
| MWCNT-10 | 350 | 408 |
| MWCNT-1020 | 104 | 123 |
| MWCNT-2040 | 67 | 85 |
| MWCNT-60100 | 30 | 33 |
| Organism Source | Immobilization Support | Immobilization Method | IY | RRA | Ref |
|---|---|---|---|---|---|
| Aspergillus versicolor | f-MWCNTs | Physical adsorption | 54% | 100% | [46] |
| E. coli | chitosan-tripolyphosphate nanoparticles | Entrapment | - | 72% | [47] |
| E. coli | aluminum oxide pellets | Covalent linkage | 85% | - | [48] |
| E. coli | silica nanoparticles | Cross-linkage | 95% | - | [49] |
| E. coli | f-MWCNTs | Physical adsorption | 99% | 100% | This work |
| MWCNT | f-MWCNT | |||
|---|---|---|---|---|
| Free ASNase (0.3 mg/mL) | Immobilized ASNase | Free ASNase (0.4 mg/mL) | Immobilized ASNase | |
| vmax (mM min−1) | 0.044 | 0.017 | 0.043 | 0.010 |
| S50 (mM) | 258.0 | 113.6 | 288.3 | 73.8 |
| h | 1.7 | 1.6 | 1.6 | 2.1 |
| kcat/S50 (mM−1 min−1) | 4.65 | 1.83 | 3.40 | 0.81 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cristóvão, R.O.; Barros, R.A.M.; Pinho, J.G.; Teixeira, L.S.; Neves, M.C.; Freire, M.G.; Faria, J.L.; Santos-Ebinuma, V.C.; Tavares, A.P.M.; Silva, C.G. Unveiling the Influence of Carbon Nanotube Diameter and Surface Modification on the Anchorage of L-Asparaginase. Appl. Sci. 2022, 12, 8924. https://doi.org/10.3390/app12178924
Cristóvão RO, Barros RAM, Pinho JG, Teixeira LS, Neves MC, Freire MG, Faria JL, Santos-Ebinuma VC, Tavares APM, Silva CG. Unveiling the Influence of Carbon Nanotube Diameter and Surface Modification on the Anchorage of L-Asparaginase. Applied Sciences. 2022; 12(17):8924. https://doi.org/10.3390/app12178924
Chicago/Turabian StyleCristóvão, Raquel O., Rita A. M. Barros, João G. Pinho, Lília S. Teixeira, Márcia C. Neves, Mara G. Freire, Joaquim L. Faria, Valéria C. Santos-Ebinuma, Ana P. M. Tavares, and Cláudia G. Silva. 2022. "Unveiling the Influence of Carbon Nanotube Diameter and Surface Modification on the Anchorage of L-Asparaginase" Applied Sciences 12, no. 17: 8924. https://doi.org/10.3390/app12178924
APA StyleCristóvão, R. O., Barros, R. A. M., Pinho, J. G., Teixeira, L. S., Neves, M. C., Freire, M. G., Faria, J. L., Santos-Ebinuma, V. C., Tavares, A. P. M., & Silva, C. G. (2022). Unveiling the Influence of Carbon Nanotube Diameter and Surface Modification on the Anchorage of L-Asparaginase. Applied Sciences, 12(17), 8924. https://doi.org/10.3390/app12178924











