In Vitro Assessment of Antiproliferative Activity and Cytotoxicity Modulation of Capsicum chinense By-Product Extracts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Obtaining Extracts
2.1.1. Plant Material
2.1.2. Drying of Capsicum chinense By-Products
2.1.3. Maceration Extraction (ME)
2.1.4. Soxhlet Extraction (SOX)
2.1.5. Supercritical Fluid Extraction (SFE)
2.2. Cell Lines
2.3. Cytotoxic Activity
2.4. Modulation of Cytotoxicity
2.5. Statistical Analysis
3. Results
3.1. Antiproliferative Activity
3.2. Modulation of Cytotoxicity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 4 November 2021).
- National Cancer Institute. Side Effects of Cancer Treatment. Available online: https://www.cancer.gov/about-cancer/treatment/side-effects (accessed on 4 November 2021).
- Vasan, N.; Baselga, J.; Hyman, D.M. A view on drug resistance in cancer. Nature 2019, 575, 299–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catalano, A.; Iacopetta, D.; Ceramella, J.; Scumaci, D.; Giuzio, F.; Saturnino, C.; Aquaro, S.; Rosano, C.; Sinicropi, M.S. Multidrug Resistance (MDR): A Widespread Phenomenon in Pharmacological Therapies. Molecules 2022, 27, 616. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Lin, S.R.; Chang, C.H.; Hsu, C.F.; Tsai, M.J.; Cheng, H.; Leong, M.K.; Sung, P.J.; Chen, J.C.; Weng, C.F. Natural compounds as potential adjuvants to cancer therapy: Preclinical evidence. Br. J. Pharmacol. 2020, 177, 1409–1423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Xu, J.; Liu, Y.; Zeng, Y.; Wu, G. A Review on anti-tumor mechanisms of coumarins. Front. Oncol. 2020, 10, 2720. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, P.B.; Ha, S.E.; Vetrivel, P.; Kim, H.H.; Kim, S.M.; Kim, G.S. Functions of polyphenols and its anticancer properties in biomedical research: A narrative review. Transl. Cancer Res. 2020, 9, 7619–7631. [Google Scholar] [CrossRef] [PubMed]
- Niedzwiecki, A.; Roomi, M.W.; Kalinovsky, T.; Rath, M. Anticancer efficacy of polyphenols and their combinations. Nutrients 2016, 8, 552. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, M.; Costa, D.; Sousa, Â. Flavonoids-Based delivery systems towards cancer therapies. Bioengineering 2022, 9, 197. [Google Scholar] [CrossRef]
- Louisa, M.; Mirawati-Soediro, T.; Dhyanagiri-Suyatna, F. In vitro modulation of P-glycoprotein, MRP-1 and BCRP expression by mangiferin in doxorubicin-treated MCF-7 cells. Asian Pac. J. Cancer Prev. 2014, 15, 1639–1642. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.P.; Li, S.; Chen, Y.M.; Li, H.B. Natural polyphenols for prevention and treatment of cancer. Nutrients 2016, 8, 515. [Google Scholar] [CrossRef]
- Msomi, N.Z.; Simelane, M.B. Herbal Medicine. In Herbal Medicine, 1st ed.; Builders, P., Ed.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- Isah, T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salehi, B.; Hernández-Álvarez, A.J.; Contreras, M.M.; Martorell, M.; Ramírez-Alarcón, K.; Melgar-Lalanne, G.; Matthews, K.R.; Sharifi-Rad, M.; Setzer, W.N.; Nadeem, M.; et al. Potential phytopharmacy and food applications of Capsicum spp.: A comprehensive review. Nat. Prot. Commun. 2018, 13, 1543–1556. [Google Scholar] [CrossRef] [Green Version]
- Sarwa, K.K.; Kiran, J.; Sahu, J.; Rudrapal, M.; Debnath, M. A short review on Capsicum chinense Jacq. J. Herb. Med. Toxicol. 2012, 6, 7–10. [Google Scholar]
- Chel-Guerrero, L.D.; Castañeda-Corral, G.; López-Castillo, M.; Scampicchio, M.; Morozova, K.; Oney-Montalvo, J.E.; Ferrentino, G.; Acevedo-Fernández, J.J.; Rodríguez-Buenfil, I.M. In vivo Anti-Inflammatory Effect, Antioxidant Activity, and Polyphenolic Content of Extracts from Capsicum chinense By-Products. Molecules 2022, 27, 1323. [Google Scholar] [CrossRef] [PubMed]
- Oney-Montalvo, J.; Uc-Varguez, A.; Ramírez-Rivera, E.; Ramírez-Sucre, M.; Rodríguez-Buenfil, I. Influence of soil composition on the profile and content of polyphenols in habanero peppers (Capsicum chinense Jacq.). Agronomy 2020, 10, 1234. [Google Scholar] [CrossRef]
- Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación. Chile habanero. In Agenda Técnica Agrícola Yucatán, 2nd ed.; SAGARPA, Ed.; SAGARPA: Ciudad de México, Mexico, 2015; pp. 21–32. [Google Scholar]
- SIAP. Anuario Estadístico de la Producción Agrícola. Available online: https://nube.siap.gob.mx/cierreagricola/ (accessed on 5 November 2021).
- Maksimova, V.; Gudeva, L.K.; Gulaboski, R.; Nieberc, K. Co-extracted bioactive compounds in Capsicum fruit extracts prevent the cytotoxic effects of capsaicin on B104 neuroblastoma cells. Rev. Bras. Farmacogn. 2016, 26, 744–750. [Google Scholar] [CrossRef] [Green Version]
- Chel-Guerrero, L.D.; Ruíz-Gutiérrez, M.C.; Rodríguez-Buenfil, I.M. Evaluación química y uso potencial de subproductos de Capsicum chinense Jacq., cultivado en dos tipos de suelo de Yucatán. In Metabolómica y Cultivo del Chile Habanero (Capsicum chinense Jacq) de la Península de Yucatán, 1st ed.; Rodríguez-Buenfil, I.M., Ramírez-Sucre, M.O., Ramírez-Rivera, E., Eds.; CIATEJ: Jalisco, Mexico, 2020; pp. 185–216. ISBN 978-607-8734-09-2. [Google Scholar]
- Chel-Guerrero, L.D.; Oney-Montalvo, J.E.; Rodríguez-Buenfil, I.M. Phytochemical characterization of by-products of habanero pepper grown in two different types of soils from Yucatán, Mexico. Plants 2021, 10, 779. [Google Scholar] [CrossRef]
- Ferrentino, G.; Morozova, K.; Kongi-Mosibo, O.; Ramezani, M.; Scampicchio, M. Biorecovery of antioxidants from apple pomace by supercritical fluid extraction. J. Clean. Prod. 2018, 186, 253–261. [Google Scholar] [CrossRef]
- Chel-Guerrero, L.D.; Sauri-Duch, E.; Fragoso-Serrano, M.C.; Pérez-Flores, L.J.; Gómez-Olivares, J.L.; Salinas-Arreortua, N.; Sierra-Palacios, E.C.; Mendoza-Espinoza, J.A. Phytochemical profile, toxicity, and pharmacological potential of peels from four species of tropical fruits. J. Med. Food 2018, 21, 734–743. [Google Scholar] [CrossRef]
- Ameer, K.; Shahbaz, H.M.; Kwon, J.H. Green extraction methods for polyphenols from plant matrices and their byproducts: A review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315. [Google Scholar] [CrossRef] [Green Version]
- Paes, J.; Dotta, R.; Barbero, G.F.; Martínez, J. Extraction of phenolic compounds and anthocyanins from blueberry (Vaccinium myrtillus L.) residues using supercritical CO2 and pressurized liquids. J. Supercrit. Fluids 2014, 95, 8–16. [Google Scholar] [CrossRef]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-González, G.; Jacobo-Herrera, N.; Zentella-Dehesa, A.; Pereda-Miranda, R. Reversal of multidrug resistance by Morning glory resin glycosides in human breast cancer cells. J. Nat. Prod. 2012, 75, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.A.; Wichern, D.W. Principal components. In Applied Multivariate Statistical Analysis, 6th ed.; Hoag, C., Ryan, D., Behrens, L.M., Wendelken, J., Eds.; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2007; pp. 430–480. ISBN 13 978-0-13-187715-3. [Google Scholar]
- Geran, R.I.; Greenberg, N.H.; MacDonald, M.M.; Schumacher, A.M.; Abbott, B.J. Protocols for screening chemical agents and natural products against animal tumours and other biological systems. Cancer Chemother. Rep. 1972, 3, 17–19. [Google Scholar]
- Mali, P.Y. Cytotoxicity activities of chloroform extract of Cichorium intybus seed against HCT-15 and Vero cell line. Int. J. Health Allied Sci. 2015, 4, 267–270. [Google Scholar] [CrossRef]
- Srisawat, T.; Chumkaew, P.; Heed-Chim, W.; Sukpondma, Y.; Kanokwiroon, K. Phytochemical screening and cytotoxicity of crude extracts of Vatica diospyroides Symington type LS. Trop. J. Pharm. Res. 2013, 12, 71–76. [Google Scholar] [CrossRef] [Green Version]
- Jeon, G.U.; Han, J.Y.; Choi, Y.M.; Lee, S.M.; Kim, H.T.; Lee, J.S. Antioxidant and Antiproliferative Activity of Pepper (Capsicum annuum L.) Leaves. J. Korean Soc. Food Sci. Nutr. 2008, 37, 1079–1083. [Google Scholar] [CrossRef]
- Guaouguaou, F.E.E.; Bebaha, M.A.A.; Taghzouti, K.; Bouyahya, A.; Bakri, Y.; Dakka, N.; Es-Safi, N.E. Cytotoxicological investigation of the essential oil and the extracts of Cotula cinerea and Salvia verbenaca from Morocco. BioMed Res. Int. 2018, 2018, 7163961. [Google Scholar] [CrossRef] [Green Version]
- Bagattoli, P.C.D.; Cipriani, D.C.; Mariano, L.N.B.; Correa, M.; Wagner, T.M.; Noldin, V.F.; Filho, V.C.; Niero, R. Phytochemical, antioxidant and anticancer activities of extracts of seven fruits found in the southern brazilian flora. Indian J. Pharm. Sci. 2016, 78, 34–40. [Google Scholar] [CrossRef] [Green Version]
- Dhawan, D.; Gupta, J. Comparison of different solvents for phytochemical extraction potential from Datura metel plant leaves. Int. J. Biol. Chem. 2017, 11, 17–22. [Google Scholar] [CrossRef] [Green Version]
- Rezayian, M.; Ebrahimzadeh, H.; Niknam, V. Nitric oxide stimulates antioxidant system and osmotic adjustment in soybean under drought stress. J. Soil Sci. Plant Nutr. 2020, 20, 1122–1132. [Google Scholar] [CrossRef]
- Sak, K. Cytotoxicity of dietary flavonoids on different human cancer types. Pharmacogn. Rev. 2014, 8, 122–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koosha, S.; Mohamed, Z.; Sinniah, A.; Alshawsh, M.A. Investigation into the molecular mechanisms underlying the anti-proliferative and antitumorigenesis activities of diosmetin against HCT-116 human colorectal cancer. Nature 2019, 9, 5148. [Google Scholar] [CrossRef] [Green Version]
- Prakash, V. Terpenoids as cytotoxic compounds: A perspective. Phcog. Rev. 2018, 12, 166–176. [Google Scholar] [CrossRef]
- Jaafari, A.; Tilaoui, M.; Mouse, H.A.; M’bark, L.A.; Aboufatima, R.; Chait, A.; Lepoivre, M.; Zyad, A. Comparative study of the antitumor effect of natural monoterpenes: Relationship to cell cycle analysis. Rev. Bras. Farmacogn. 2012, 22, 534–540. [Google Scholar] [CrossRef] [Green Version]
- Abubakar, A.R.; Haque, M. Preparation of medicinal plants: Basic extraction and fractionation procedures for experimental purposes. J. Pharm. Bioallied Sci. 2020, 12, 1–10. [Google Scholar] [CrossRef]
- Eun-Yi, K.; Weon-Jong, Y.; Hae-Won, L.; Soo-Jin, H.; Young-Hwan, K.; Shanura, I.P.F.; Kichul, C.; Chi-Heon, L.; Sung-Pyo, H.; Su-Hyeon, C.; et al. Anti-inflammatory effect of supercritical extract and its constituents from Ishige okamurae. EXCLI J. 2016, 15, 434–445. [Google Scholar] [CrossRef]
- Iqbal, J.; Abbasi, B.A.; Mahmood, T.; Kanwal, S.; Ali, B.; Shah, S.A.; Khalil, A.T. Plant-derived anticancer agents: A green anticancer approach. Asian Pac. J. Trop. Biomed. 2017, 7, 1129–1150. [Google Scholar] [CrossRef]
- Rosas-Ramírez, D.G.; Fragoso-Serrano, M.; Escandón-Rivera, S.; Vargas-Ramírez, A.L.; Reyes-Grajeda, J.P.; Soriano-García, M. Resistance-modifying activity in vinblastine resistant human breast cancer cells by oligosaccharides obtained from mucilage of chia seeds (Salvia hispanica). Phytother. Res. 2017, 31, 906–914. [Google Scholar] [CrossRef]
- Corona-Castañeda, B.; Rosas-Ramírez, D.; Castañeda-Gómez, J.; Aparicio-Cuevas, M.A.; Fragoso-Serrano, M.; Figueroa-González, G.; Pereda-Miranda, R. Resin glycosides from Ipomoea wolcottiana as modulators of the multidrug resistance phenotype in vitro. Phytochemistry 2016, 123, 48–57. [Google Scholar] [CrossRef]
- Cruz-Morales, S.; Castañeda-Gómez, J.; Figueroa-González, G.; Mendoza-García, A.D.; Lorence, A.; Pereda-Miranda, R. Mammalian multidrug resistance lipopentasaccharide inhibitors from Ipomoea alba seeds. J. Nat. Prod. 2012, 75, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Tinoush, B.; Shirdel, I.; Wink, M. Phytochemicals: Potential lead molecules for MDR reversal. Front. Pharmacol. 2020, 11, 832. [Google Scholar] [CrossRef] [PubMed]
- Ncube, N.S.; Afolayan, A.J.; Okoh, A.I. Assessment techniques of antimicrobial properties of natural compounds of plant origin: Current methods and future trends. Afr. J. Biotechnol. 2008, 7, 1797–1806. [Google Scholar] [CrossRef] [Green Version]


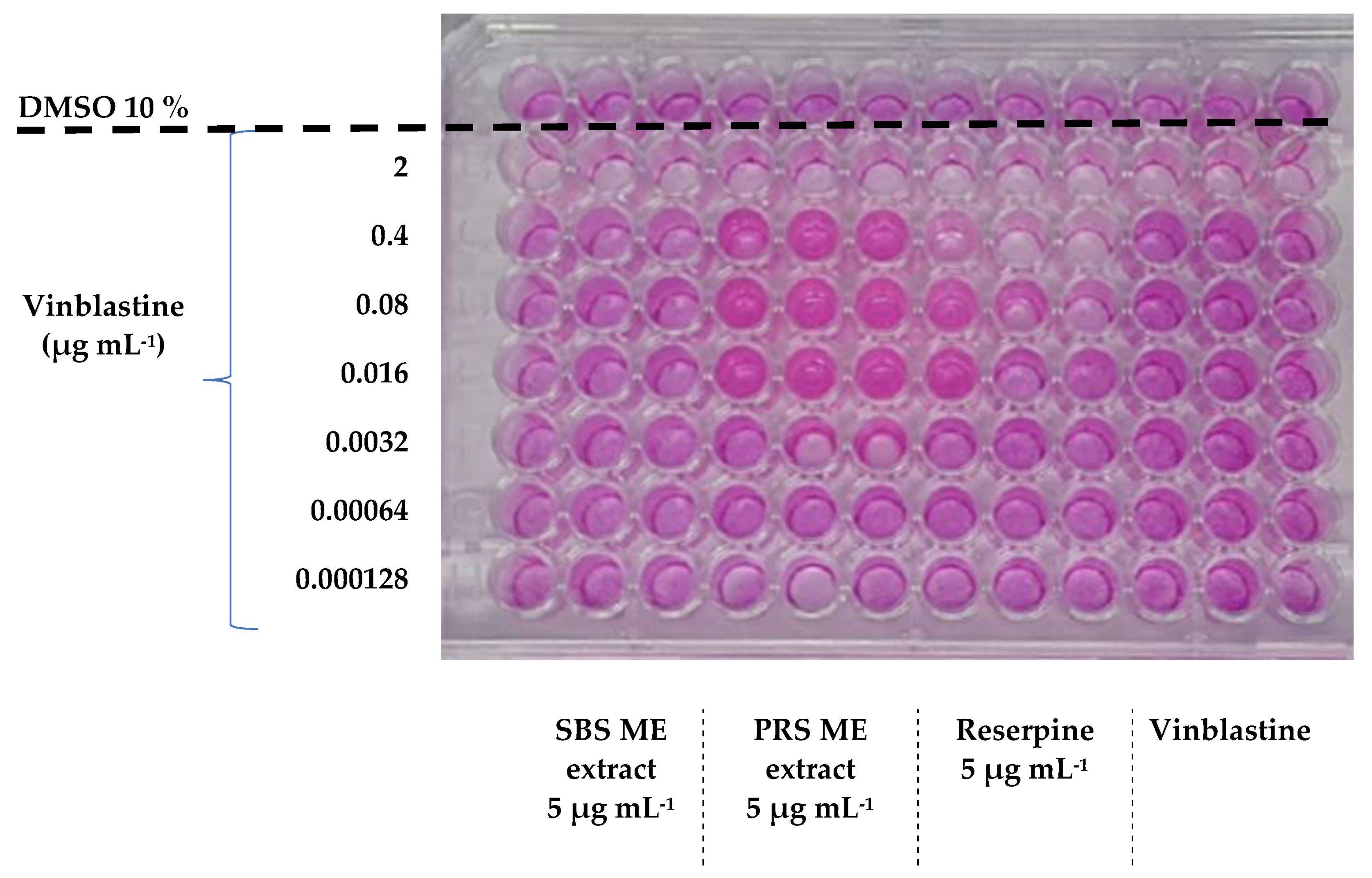
| Type of Habanero By-Product or Drug A | MCF–7/Sens B IC50 (µg mL−1) | RF E | MCF–7/Vin− C IC50 (µg mL−1) | RF | MCF–7/Vin+ D IC50 (µg mL−1) | RF |
|---|---|---|---|---|---|---|
| PBS ME | 0.0022 ± 0.0006 | 32.95 bcd | 0.46 ± 0.02 | 3.80 bc | 0.32 ± 0.07 g | 5.77 b |
| LBS ME | 0.0470 ± 0.0023 | 4.93 d | 0.38 ± 0.01 | 4.49 b | 0.36 ± 0.02 fg | 5.06 b |
| SBS ME | 0.0014 ± 0.0003 | 51.79 bc | 2.01 ± 0.33 | 0.87 gh | 0.23 ± 0.04 g | 7.78 a |
| PRS ME | 0.0010 ± 0.00006 | 72.50 b | 0.56 ± 0.04 | 3.15 bcd | 0.95 ± 0.07 | 1.94 cd |
| LRS ME | 0.0002 ± 0.00002 | 362.50 a | 0.61 ± 0.02 | 2.85 d | 0.55 ± 0.05 | 3.33 c |
| SRS ME | 0.0002 ± 0.00004 | 362.50 a | 0.59 ± 0.02 | 2.93 cd | 0.89 ± 0.02 | 2.06 cd |
| LBS SFE | 0.0045 ± 0.0012 | 16.11 cd | 3.74 ± 0.29 | 0.47 h | 1.73 ± 0.21 | 1.07 d |
| SBS SFE | 0.2213 ± 0.0065 | 0.33 d | 0.43 ± 0.14 | 4.05 b | 0.28 ± 0.09 | 6.59 ab |
| SRS SFE | 0.0141 ± 0.0031 | 5.14 d | 0.72 ± 0.16 | 2.40 dc | 1.84 ± 0.23 | 1.00 d |
| LBS SOX | 0.0084 ± 0.0017 | 8.63 cd | 0.94 ± 0.05 | 1.85 ef | 1.48 ± 0.06 | 1.24 d |
| SBS SOX | 0.0069 ± 0.0010 | 10.51 cd | 1.12 ± 0.14 | 1.56 fg | 1.19 ± 0.35 | 1.54 d |
| SRS SOX | 0.0036 ± 0.0006 | 20.35 cd | 0.46 ± 0.10 | 3.75 bcd | 1.96 ± 0.07 | 0.94 d |
| Reserpine F | 0.0022 ± 0.0001 | 32.95 bcd | 0.078 ± 0.01 | 22.42 a | 0.58 ± 0.05 | 3.14 c |
| Vinblastine | 0.0725 ± 0.0028 | 1.75 ± 0.03 | 1.84 ± 0.02 |
| Number of Component | Eigenvalue a | Variance Percentage | Accumulated Percentage |
|---|---|---|---|
| 1 | 1.5 | 50.6 | 50.6 |
| 2 | 0.8 | 27.1 | 77.7 |
| 3 | 0.7 | 22.3 | 100.0 |
| Variable | Component 1 | Component 2 | Component 3 |
|---|---|---|---|
| MCF–7/Sens | −0.575434 | 0.588499 | 0.567930 |
| MCF–7/Vin− | 0.531068 | 0.796973 | −0.287752 |
| MCF–7/Vin+ | 0.621967 | −0.136027 | 0.771138 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chel-Guerrero, L.D.; Scampicchio, M.; Ferrentino, G.; Rodríguez-Buenfil, I.M.; Fragoso-Serrano, M. In Vitro Assessment of Antiproliferative Activity and Cytotoxicity Modulation of Capsicum chinense By-Product Extracts. Appl. Sci. 2022, 12, 5818. https://doi.org/10.3390/app12125818
Chel-Guerrero LD, Scampicchio M, Ferrentino G, Rodríguez-Buenfil IM, Fragoso-Serrano M. In Vitro Assessment of Antiproliferative Activity and Cytotoxicity Modulation of Capsicum chinense By-Product Extracts. Applied Sciences. 2022; 12(12):5818. https://doi.org/10.3390/app12125818
Chicago/Turabian StyleChel-Guerrero, Lilian Dolores, Matteo Scampicchio, Giovanna Ferrentino, Ingrid Mayanín Rodríguez-Buenfil, and Mabel Fragoso-Serrano. 2022. "In Vitro Assessment of Antiproliferative Activity and Cytotoxicity Modulation of Capsicum chinense By-Product Extracts" Applied Sciences 12, no. 12: 5818. https://doi.org/10.3390/app12125818
APA StyleChel-Guerrero, L. D., Scampicchio, M., Ferrentino, G., Rodríguez-Buenfil, I. M., & Fragoso-Serrano, M. (2022). In Vitro Assessment of Antiproliferative Activity and Cytotoxicity Modulation of Capsicum chinense By-Product Extracts. Applied Sciences, 12(12), 5818. https://doi.org/10.3390/app12125818











