Assessment of Systemic and Maxillary Bone Loss in Cancer Patients with Endo-Periodontal Lesions Using Dkk-1 Biomarker and Dental Radiological Examinations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Inclusion and Exclusion Criteria
2.3. Clinical Examinations
2.4. Serum Sampling
2.5. ELISA
2.6. Statistical Analysis
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hristova, V.A.; Chan, D.W. Cancer biomarker discovery and translation: Proteomics and beyond. Expert Rev. Proteom. 2019, 16, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Lu, T.; Kuang, W. Prognostic role of dickkopf-1 in patients with cancer. Medicine 2020, 99, e20388. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gao, Y.; Yue, W. The Clinical Diagnostic and Prognostic Value of Dickkopf-1 in Cancer. Cancer Manag. Res. 2020, 12, 4253–4260. [Google Scholar] [CrossRef] [PubMed]
- Mazon, M.; Larouche, V.; St-Louis, M.; Schindler, D.; Carreau, M. Elevated blood levels of Dickkopf-1 are associated with acute infections. Immun. Inflamm. Dis. 2018, 6, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Song, J.; Chen, W.; Yuan, D.; Wang, W.; Chen, X.; Liu, H.; Su, H.; Zhu, J. Expression and Role of Dickkopf-1 (Dkk1) in Tumors: From the Cells to the Patients. Cancer Manag. Res. 2021, 13, 659–675. [Google Scholar] [CrossRef]
- Tung, E.K.-K.; Mak, C.K.-M.; Fatima, S.; Lo, R.C.-L.; Zhao, H.; Zhang, C.; Dai, H.; Poon, R.T.-P.; Yuen, M.-F.; Lai, C.-L.; et al. Clinicopathological and prognostic significance of serum and tissue Dickkopf-1 levels in human hepatocellular carcinoma. Liver Int. 2011, 31, 1494–1504. [Google Scholar] [CrossRef]
- Jaschke, N.; Hofbauer, L.C.; Göbel, A.; Rachner, T.D. Evolving functions of Dickkopf-1 in cancer and immunity. Cancer Lett. 2020, 482, 1–7. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, Y.; Wei, X.; Zhang, Q. Correlations of DKK1 with pathogenesis and prognosis of human multiple myeloma. Cancer Biomark. 2019, 24, 195–201. [Google Scholar] [CrossRef]
- Tan, X.; Huang, D.; Zhou, W.; Yan, L.; Yue, J.; Lu, W.; Song, D.; Zhou, X.; Ye, L.; Zhang, L. Dickkopf-1 may regulate bone coupling by attenuating wnt/β-catenin signaling in chronic apical periodontitis. Arch. Oral Biol. 2018, 86, 94–100. [Google Scholar] [CrossRef]
- Pinzone, J.J.; Hall, B.M.; Thudi, N.K.; Vonau, M.; Qiang, Y.W.; Rosol, T.J.; Shaughnessy, J.D., Jr. The role of Dickkopf-1 in bone development, homeostasis, and disease. Blood J. Am. Soc. Hematol. 2009, 113, 517–525. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Liu, L.; Liu, A. Dickkopf-1: Current knowledge and related diseases. Life Sci. 2018, 209, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Kong, Q.; Liu, L.; Huang, Y.; Zhang, F.; Wei, X.; Ling, J. The effect of octamer-binding transcription factor 4B1 on microRNA signals in human dental pulp cells with inflammatory response. J. Endod. 2014, 40, 101–108. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Wang, Z.; Zhou, Z.; Zhang, Y.; Zhu, Q.; Wei, K.; Lin, Y.; Cooper, P.; Smith, A.J.; Yu, Q. Lipopolysaccharide enhances Wnt5a expression through toll-like receptor 4, myeloid differentiating factor 88, phosphatidylinositol 3-OH kinase/AKT and nuclear factor kappa B pathways in human dental pulp stem cells. J. Endod. 2014, 40, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Müller, H.P.; Ulbrich, M.; Heinecke, A. Alveolar bone loss in adults as assessed on panoramic radiographs.(II) Multilevel models. Clin. Oral Investig. 2005, 9, 105–110. [Google Scholar] [CrossRef]
- Wang, S.Y.; Liu, Y.Y.; Ye, H.; Guo, J.P.; Li, R.; Liu, X.; Li, Z.G. Circulating Dickkopf-1 is correlated with bone erosion and inflammation in rheumatoid arthritis. J. Rheumatol. 2011, 38, 821–827. [Google Scholar] [CrossRef]
- Hartung, N.; Benary, U.; Wolf, J.; Kofahl, B. Paracrine and autocrine regulation of gene expression by Wnt-inhibitor Dickkopf in wild-type and mutant hepatocytes. BMC Syst. Biol. 2017, 11, 98. [Google Scholar] [CrossRef] [Green Version]
- Fujita, K.; Janz, S. Attenuation of WNT signaling by DKK-1 and -2 regulates BMP2-induced osteoblast differentiation and expression of OPG, RANKL and M-CSF. Mol. Cancer 2007, 6, 71. [Google Scholar] [CrossRef] [Green Version]
- Martu, A.; Rezus, E.; Sufaru, I.; Banu, C.; Martu, S.; Foia, L. Study on the clinical changes in general and oral status in patients with rheumatoid arthritis. Rom. J. Oral Rehabil. 2018, 10, 188–198. [Google Scholar]
- Martu, M.A.; Solomon, S.M.; Toma, V.; Maftei, G.A.; Iovan, A.; Gamen, A.; Hurjui, L.; Rezus, E.; Foia, L.; Forna, N.C. The importance of cytokines in periodontal disease and rheumatoid arthritis. Review. Rom. J. Oral Rehabil. 2019, 11, 220–240. [Google Scholar]
- Crotti, T.; Smith, M.D.; Hirsch, R.; Soukoulis, S.; Weedon, H.; Capone, M.; Ahern, M.J.; Haynes, D. Receptor activator NF kappaB ligand (RANKL) and osteoprotegerin (OPG) protein expression in periodontitis. J. Periodontal Res. 2003, 38, 380–387. [Google Scholar] [CrossRef]
- Niehrs, C. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene 2006, 25, 7469–7481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maithri, M.; Ballal, D.G.; Kumar, S.; Raghavendra, U.; Gudigar, A.; Chan, W.Y.; Macherla, S.; Vineetha, R.; Gopalkrishna, P.; Ciaccio, E.J.; et al. Development of a Computational Tool for the Estimation of Alveolar Bone Loss in Oral Radiographic Images. Computation 2022, 10, 8. [Google Scholar] [CrossRef]
- Mocanu, R.C.; Martu, M.-A.; Luchian, I.; Sufaru, I.G.; Maftei, G.A.; Ioanid, N.; Martu, S.; Tatarciuc, M. Microbiologic Profiles of Patients with Dental Prosthetic Treatment and Periodontitis before and after Photoactivation Therapy—Randomized Clinical Trial. Microorganisms 2021, 9, 713. [Google Scholar] [CrossRef] [PubMed]
- Pick, E.; Schäfer, T.; Al-Haj Husain, A.; Rupp, N.J.; Hingsammer, L.; Valdec, S. Clinical, Radiological, and Pathological Diagnosis of Fibro-Osseous Lesions of the Oral and Maxillofacial Region: A Retrospective Study. Diagnostics 2022, 12, 238. [Google Scholar] [CrossRef] [PubMed]
- Zaharescu, A.; Solomon, S.M.; Luca, M.G.; Toma, V.; Luchian, I.; Sufaru, I.G.; Martu, M.A.; Foia, L.; Martu, S. Quantification of proinflammatory molecules (IL1-α, IL1-β, IL2, IL12, IFN-γ, TNF-α) in crevicular fluid and serum in patients with endo-periodontal lesions. Rev. Chim. 2019, 70, 2252–2255. [Google Scholar] [CrossRef]
- Cohen, R.S.; Goldberger, T.; Merzlak, I.; Tsesis, I.; Chaushu, G.; Avishai, G.; Rosen, E. The Development of Large Radicular Cysts in Endodontically Versus Non-Endodontically Treated Maxillary Teeth. Medicina 2021, 57, 991. [Google Scholar] [CrossRef]
- Pinto, D.; Marques, A.; Pereira, J.F.; Palma, P.J.; Santos, J.M. Long-Term Prognosis of Endodontic Microsurgery—A Systematic Review and Meta-Analysis. Medicina 2020, 56, 447. [Google Scholar] [CrossRef]
- Galler, K.M.; Weber, M.; Korkmaz, Y.; Widbiller, M.; Feuerer, M. Inflammatory Response Mechanisms of the Dentine–Pulp Complex and the Periapical Tissues. Int. J. Mol. Sci. 2021, 22, 1480. [Google Scholar] [CrossRef]
- Tang, Z.; Liu, X.; Chen, K. Comparison of digital panoramic radiography versus cone beam computerized tomography for measuring alveolar bone. Head Face Med. 2017, 13, 2. [Google Scholar] [CrossRef] [Green Version]
- Tchorz, J.P.; Wrbas, K.T.; Von See, C.; Vach, K.; Patzelt, S.B.M. Accuracy of software-based three-dimensional root canal length measurements using cone-beam computed tomography. Eur. Endod. J. 2019, 4, 28–32. [Google Scholar] [CrossRef]
- Kruse, C.; Spin-Neto, R.; Wenzel, A.; Kirkevang, L.L. Cone beam computed tomography and periapical lesions: A systematic review analysing studies on diagnostic efficacy by a hierarchical model. Int. Endod. J. 2015, 48, 815–828. [Google Scholar] [CrossRef] [PubMed]
- Nesse, W.; Linde, A.; Abbas, F.; Spijkervet, F.K.; Dijkstra, P.U.; de Brabander, E.C.; Gerstenbluth, I.; Vissink, A. Dose-response relationship between periodontal inflamed surface area and HbA1c in type 2 diabetics. J. Clin. Periodontol. 2009, 36, 295–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maftei, G.-A.; Martu, M.-A.; Martu, M.-C.; Popescu, D.; Surlin, P.; Tatarciuc, D.; Popa, C.; Foia, L.-G. Correlations between Salivary Immuno-Biochemical Markers and HbA1c in Type 2 Diabetes Subjects before and after Dental Extraction. Antioxidants 2021, 10, 1741. [Google Scholar] [CrossRef] [PubMed]
- Marconcini, S.; Giammarinaro, E.; Cosola, S.; Oldoini, G.; Genovesi, A.; Covani, U. Effects of Non-Surgical Periodontal Treatment on Reactive Oxygen Metabolites and Glycemic Control in Diabetic Patients with Chronic Periodontitis. Antioxidants 2021, 10, 1056. [Google Scholar] [CrossRef]
- Kappenberg-Niţescu, D.C.; Luchian, I.; Mârțu, I.; Solomon, S.M.; Mârţu, S.; Păsărin, L.; Mârțu, A.; Sioustis, I.A.; Goriuc, A.; Tatarciuc, M. Periodontal effects of two innovative oral rinsing substances in oncologic patients. Exp. Ther. Med. 2021, 21, 98. [Google Scholar] [CrossRef]
- Nwizu, N.; Wactawski-Wende, J.; Genco, R.J. Periodontal disease and cancer: Epidemiologic studies and possible mechanisms. Periodontology 2020, 83, 213–233. [Google Scholar] [CrossRef]
- De Felice, F.; Tombolini, V.; Musio, D.; Polimeni, A. Radiation therapy and mandibular osteoradionecrosis: State of the art. Curr. Oncol. Rep. 2020, 22, 89. [Google Scholar] [CrossRef]
- Kaya, S.; Yavuz, İ.; Uysal, İ.; Akkuş, Z. Measuring bone density in healing periapical lesions by using cone beam computed tomography: A clinical investigation. J. Endod. 2012, 38, 28–31. [Google Scholar] [CrossRef]
- Nardi, C.; Calistri, L.; Pradella, S.; Desideri, I.; Lorini, C.; Colagrande, S. Accuracy of orthopantomography for apical periodontitis without endodontic treatment. J. Endod. 2017, 43, 1640–1646. [Google Scholar] [CrossRef]
- Antony, D.P.; Thomas, T.; Nivedhitha, M.S. Two-dimensional periapical, panoramic radiography versus three-dimensional cone-beam computed tomography in the detection of periapical lesion after endodontic treatment: A systematic review. Cureus 2020, 12, e7736. [Google Scholar] [CrossRef] [Green Version]
- Martu, M.A.; Maftei, G.A.; Luchian, I.; Popa, C.; Filioreanu, A.M.; Tatarciuc, D.; Nichitean, G.; Hurjui, L.-L.; Foia, L.-G. Wound healing of periodontal and oral tissues: Part II—Patho-phisiological conditions and metabolic diseases. Rom. J. Oral Rehabil. 2020, 12, 30–40. [Google Scholar]
- Martu, M.A.; Maftei, G.A.; Sufaru, I.G.; Jelihovschi, I.; Luchian, I.; Hurjui, L.; Martu, I.; Pasarin, L. COVID-19 and periodontal disease: Etiopathogenic and clinical immplications. Rom. J. Oral Rehabil. 2020, 12, 116–124. [Google Scholar]
- Popa, C.; Filioreanu, A.M.; Stelea, C.; Alexandru Maftei, G.A.; Popescu, E. Prevalence of oral lesions modulated by patients age: The young versus the elderly. Rom. J. Oral Rehabil. 2018, 10, 50–56. [Google Scholar]
- Menezes, M.E.; Devine, D.J.; Shevde, L.A.; Samant, R.S. Dickkopf1: A tumor suppressor or metastasis promoter? Int. J. Cancer 2012, 130, 1477–1483. [Google Scholar] [CrossRef] [Green Version]
- Giralt, I.; Gallo-Oller, G.; Navarro, N.; Zarzosa, P.; Pons, G.; Magdaleno, A.; Segura, M.F.; de Toledo, J.S.; Moreno, L.; Gallego, S.; et al. Dickkopf Proteins and Their Role in Cancer: A Family of Wnt Antagonists with a Dual Role. Pharmaceuticals 2021, 14, 810. [Google Scholar] [CrossRef]
- Ko, J.Y.; Wang, F.S.; Wang, C.J.; Wong, T.; Chou, W.Y.; Tseng, S.L. Increased Dickkopf-1 expression accelerates bone cell apoptosis in femoral head osteonecrosis. Bone 2010, 46, 584–591. [Google Scholar] [CrossRef]
- Yi, N.; Liao, Q.P.; Li, T.; Xiong, Y. Novel expression profiles and invasiveness-related biology function of DKK1 in endometrial carcinoma. Oncol. Rep. 2009, 21, 1421–1427. [Google Scholar]
- Hall, C.L.; Daignault, S.D.; Shah, R.B.; Pienta, K.J.; Keller, E.T. Dickkopf-1 expression increases early in prostate cancer development and decreases during progression from primary tumor to metastasis. Prostate 2008, 68, 1396–1404. [Google Scholar] [CrossRef] [Green Version]
- Mazon, M.; Masi, D.; Carreau, M. Modulating Dickkopf-1: A Strategy to Monitor or Treat Cancer? Cancers 2016, 8, 62. [Google Scholar] [CrossRef] [Green Version]
- Tian, E.; Zhan, F.; Walker, R.; Rasmussen, E.; Ma, Y.; Barlogie, B.; Shaughnessy, J.D., Jr. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N. Engl. J. Med. 2003, 349, 2483–2494. [Google Scholar] [CrossRef]
- Ramirez, J.G.; Smit, D.J.; Viol, F.; Schrader, J.; Ghadban, T.; Pantel, K.; Izbicki, J.R.; Reeh, M. High Serum Levels of Wnt Signaling Antagonist Dickkopf-Related Protein 1 Are Associated with Impaired Overall Survival and Recurrence in Esophageal Cancer Patients. Cancers 2021, 13, 4980. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-H.; Chung, S.W.; Auh, Q.-S.; Hong, S.-J.; Lee, Y.-A.; Jung, J.; Lee, G.-J.; Park, H.J.; Shin, S.-I.; Hong, J.-Y. Progress in Oral Microbiome Related to Oral and Systemic Diseases: An Update. Diagnostics 2021, 11, 1283. [Google Scholar] [CrossRef] [PubMed]
- Maftei, G.A.; Martu, C.M.; Popa, C.; Geletu, G.; Danila, V.; Jelihovschi, I.; Foia, L. The biomechanical properties of suture materials and their relationship to bacterial adherence. Mater. Plast. 2019, 56, 980–985. [Google Scholar] [CrossRef]


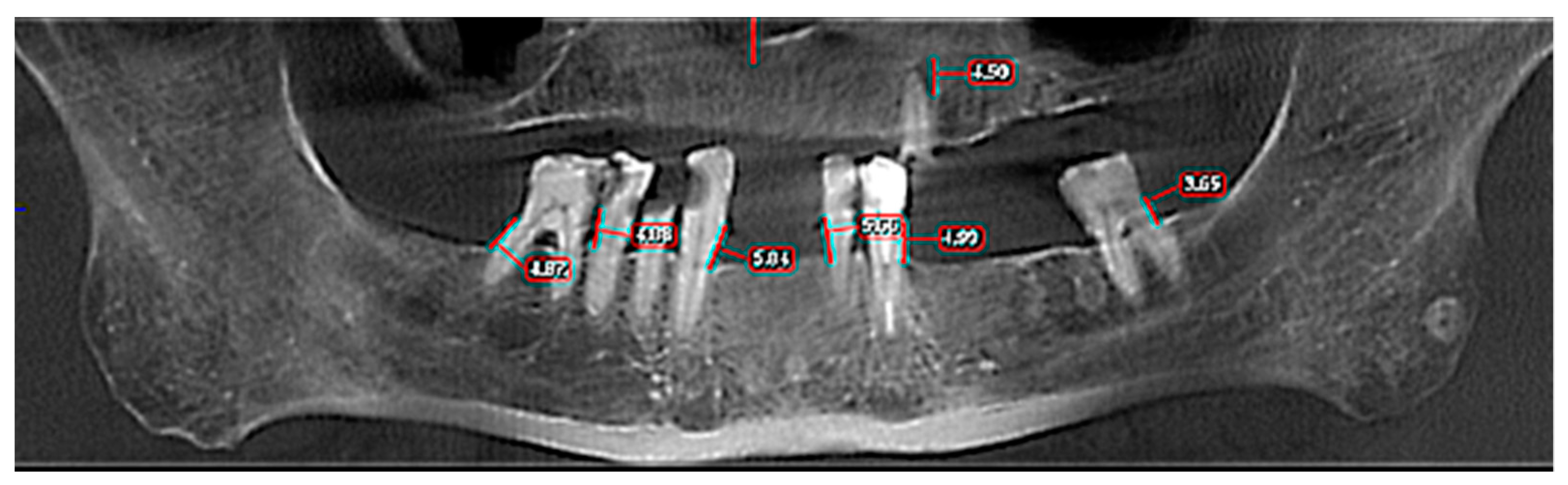
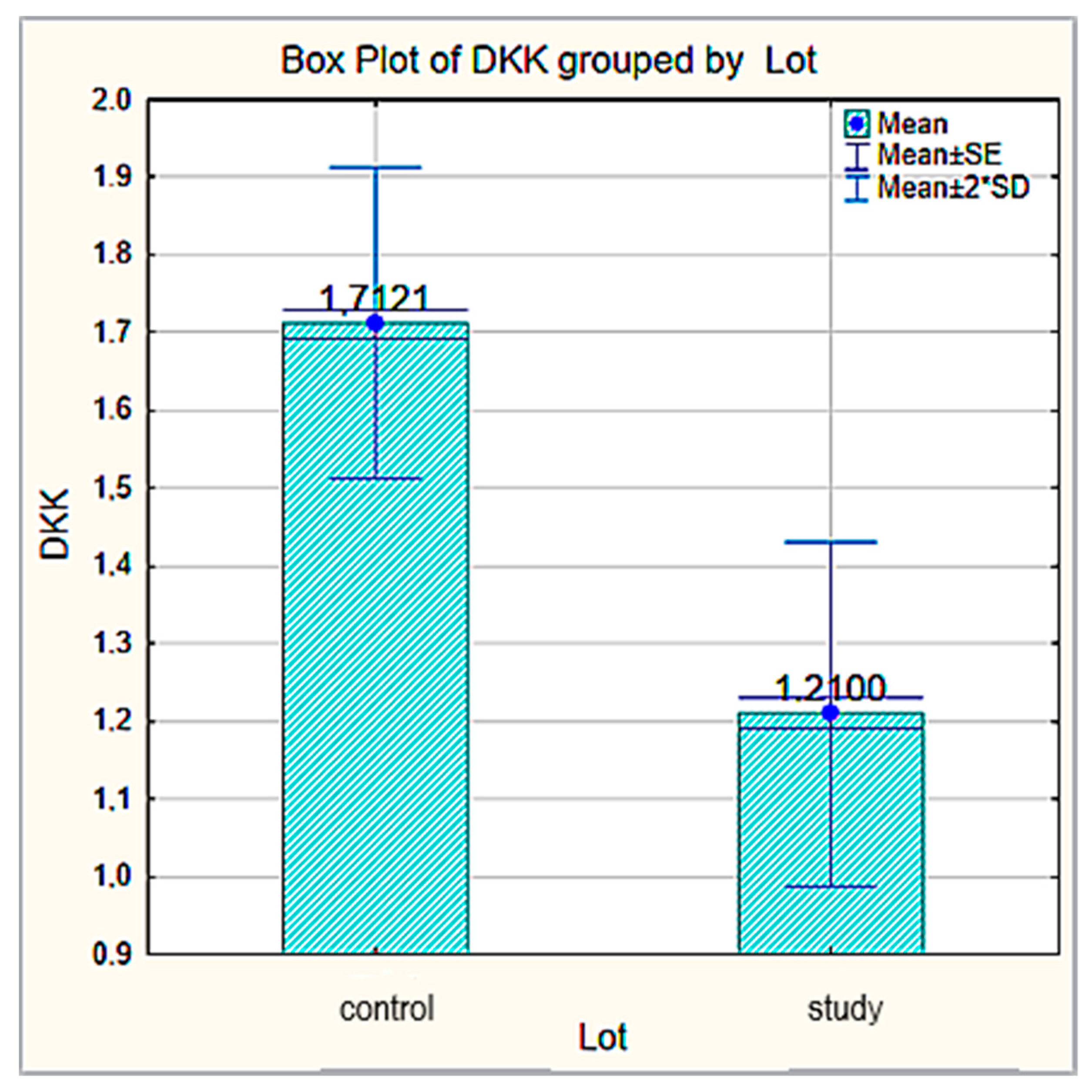
| Total (n = 63) | Control (n = 30) | ENT Cancer (n = 33) | p Value | |
|---|---|---|---|---|
Gender: n (%)
| 38 (60.3) 25 (39.7) | 15 (50.0) 15 (50.0) | 23 (69.7) 10 (30.3) | 0.110 |
Age: m ± SD
| 52.67 ± 12.241 52.84 ± 12.985 52.40 ± 11.269 | 53.87 ± 10.760 54.93 ± 12.567 52.80 ± 8.914 | 51.58 ± 13.521 51.48 ± 13.348 51.80 ± 14.642 | 0.463 |
Cancer type: n (%)
| 24 (72.7) 4 (12.1) 5 (15.2) | |||
Oral mucositis degree: n (%)
| 20 (60.6) 13 (39.4) | |||
Irradiation degree: n (%)
| 13 (39.4) 20 (60.6) |
| Horizontal Bone Loss—Max Size | Group | Number | Mean | Standard Average Error | SD | Min | Max | Median | Student’s t Test |
|---|---|---|---|---|---|---|---|---|---|
| OPT | Control | 30 | 4.930 | 0.594 | 3.258 | 0.0 | 13.2 | 4.60 | t = −0.373 |
| ENT Cancer | 33 | 5.191 | 0.367 | 2.109 | 0.0 | 11.3 | 5.00 | p = 0.711 | |
| Total | 63 | 5.067 | 0.340 | 2.698 | 0.0 | 13.2 | 4.80 | ||
| CBCT 3D | Control | 30 | 5.736 | 0.633 | 3.471 | 0.0 | 14.10 | 5.75 | t = −0.548 |
| ENT Cancer | 33 | 6.152 | 0.438 | 2.519 | 0.0 | 12.40 | 6.17 | p = 0.586 | |
| Total | 63 | 5.954 | 0.377 | 2.992 | 0.0 | 14.10 | 6.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antohi, C.; Salceanu, M.; Aminov, L.; Martu, M.-A.; Dascalu, C.G.; Dodi, G.; Stoica, G.; Bandol, G.; Iancu, D.; Dobrovat, B.; et al. Assessment of Systemic and Maxillary Bone Loss in Cancer Patients with Endo-Periodontal Lesions Using Dkk-1 Biomarker and Dental Radiological Examinations. Appl. Sci. 2022, 12, 5235. https://doi.org/10.3390/app12105235
Antohi C, Salceanu M, Aminov L, Martu M-A, Dascalu CG, Dodi G, Stoica G, Bandol G, Iancu D, Dobrovat B, et al. Assessment of Systemic and Maxillary Bone Loss in Cancer Patients with Endo-Periodontal Lesions Using Dkk-1 Biomarker and Dental Radiological Examinations. Applied Sciences. 2022; 12(10):5235. https://doi.org/10.3390/app12105235
Chicago/Turabian StyleAntohi, Cristina, Mihaela Salceanu, Liana Aminov, Maria-Alexandra Martu, Cristina Gena Dascalu, Gianina Dodi, George Stoica, Geanina Bandol, Dragos Iancu, Bogdan Dobrovat, and et al. 2022. "Assessment of Systemic and Maxillary Bone Loss in Cancer Patients with Endo-Periodontal Lesions Using Dkk-1 Biomarker and Dental Radiological Examinations" Applied Sciences 12, no. 10: 5235. https://doi.org/10.3390/app12105235
APA StyleAntohi, C., Salceanu, M., Aminov, L., Martu, M.-A., Dascalu, C. G., Dodi, G., Stoica, G., Bandol, G., Iancu, D., Dobrovat, B., & Haba, D. (2022). Assessment of Systemic and Maxillary Bone Loss in Cancer Patients with Endo-Periodontal Lesions Using Dkk-1 Biomarker and Dental Radiological Examinations. Applied Sciences, 12(10), 5235. https://doi.org/10.3390/app12105235








