Abstract
The aim of the study is to compare selected carbohydrates that differed in the glycaemic index: maltodextrin, three native starches (wheat, rice, maize), and two disaccharides (trehalose and lactose) used to encapsulation of model oil (in this case cold-pressed pumpkin oil). Encapsulation efficiency of pumpkin oil by spray drying, size of obtained capsules, oxidative stability of encapsulated oil, and retention of tocopherols, squalene, and sterols in surface and core material of capsules were determined. It was found that encapsulation efficiency varied from 35% for rice starch to 68–71% for wheat starch, maltodextrin, and lactose. The bulk density of capsules was independent of the used carbohydrate type (189–198 kg/m3), while their size was significantly lower for samples of pumpkin oil encapsulated in native starches (over 2 times compared to capsules with trehalose). Of the best lipid capturing agents (native wheat starch, maltodextrin, and lactose), wheat starch mainly bound tocopherols, squalene, and sterols to the capsule surface, while lactose to the core material of the capsules (35.5–81.2%). Among tested carbohydrates, native wheat starch acted as the best antioxidant agent (oxidative stability was 15.1 h vs. 9.4 h for pure pumpkin oil).
1. Introduction
Many encapsulation techniques have been developed to avoid oxidative degradation of various bioactive lipophilic food ingredients (such as polyunsaturated fatty acids, tocopherols, phytosterols, carotenoids, etc.) and to increase their use in hydrophilic food recipes (such as drinks, juices, etc.). Among them, the complex coacervates, Pickering emulsions, core-shell structure microcapsules, cross-linked polymer gels, and self-assembled structures are the most widely used [1].
In the case of spray-dried prepared microcapsules, the choice of appropriate carrier material is an essential and decisive stage. According to Desai and Park [2] and Ray et al. [3] the ideal wall material should be characterized by the ability to disperse or emulsify the active material and stabilize the emulsion produced, by the non-reactivity with the active core materials and the ability for providing maximum protection to the active material against environmental conditions (e.g., oxygen, heat, light, humidity). In addition, an ideal carrier material should be neutral in flavour and inexpensive.
Maltodextrins with a mild flavour and reasonable price meet these requirements and are widely used [4,5,6]. Chemically, they are a group of compounds derived from acid or enzymatic hydrolysis of starch containing oligomers or/and polymers of α-(1,4) D-glucose, and consist of partly hydrolysed amylose and amylopectin fractions, glucose and maltose [7]. Maltodextrins are used as neutral fillers, gel forming ingredients, emulsion and foam stabilizers, and coating materials. The ability of maltodextrins to inhibit the formation of ice crystals at low temperatures, prevent sugar crystallization, and increase the viscosity and density of liquids is especially appreciated. In addition, maltodextrin gives the products the desired sensory properties, in particular replacing fat and binders with prebiotic properties [8,9]. The additional functions of maltodextrins in food are extending shelf life, improving powdery appearance, mitigating sweetness, preventing melting, averting or retard granulation, and reducing nutrient losses [10]. Maltodextrins may be used in the production of food for children, act as an ingredient of all kinds of pomades and spacers in confectionery, are carriers in the process of drying dyes, flavours, and juices, are utilized to produce food concentrates, soups and sauces, spices, etc., and are ingredients of nourishing and strengthening drinks [9], information from the website of the company selling maltodextrin [https://pepees.pl/en/index.php?wiad=58 (accessed on 16 December 2021)]. Their properties depend on the dextrose equivalent, which measures reducing sugar content and usually takes values between 3 and 20 percent [9,11].
Unfortunately, maltodextrins can affect the pH of dental plaque in vivo [12]. A study of the acidogenic potential of various maltodextrins concluded that though maltodextrins appeared to be significantly less acidogenic than sucrose, they can lead to a substantial drop in plaque pH and may therefore have the potential to cause demineralization of enamel [13]. Although maltodextrin has an acidogenic potential lower than sucrose, studies reporting the cariogenic potential of maltodextrin and its association with other carbohydrates, are still scarce [14]. According to Rytz et al. [15] glycaemic index calculated according to the modified FAO-model is 110 for maltodextrin, while only 47 for lactose, 70 for trehalose, and 20 for fructose and galactose. It points, that maltodextrins should be used sparingly by diabetic and/or hypoglycaemic individuals, and under the advice of a healthcare practitioner [10,16,17].
In this context, the aim of the current study is to compare selected carbohydrates differed in the glycaemic index: maltodextrin, three native starches (wheat, rice, maize), and two disaccharides (trehalose and lactose) used to encapsulation of model oil (in this case cold-pressed pumpkin oil). Encapsulation efficiency, size of obtained capsules, oxidative stability of model oil in capsules, and core/surface deposition of tocopherols, squalene, and sterols in obtained capsules were determined. This study should explain if commercial maltodextrin can be replaced by other non-processed carbohydrate materials.
2. Materials and Methods
2.1. Materials
Pumpkin oil, carbohydrates (commercial maltodextrin, wheat, rice and maize starches, lactose, trehalose), and other wall materials (guar gum, whey protein concentrate) were used in the study. Cold pressed pumpkin oil was purchased from Szarłat S.C. Company (Łomża, Poland). Maltodextrin (DE = 17.5, moisture 7.3%, pH 4.8) was purchased from Pepees S.A. Company (Łomża, Poland). Wheat starch (Maritena 200–moisture 11.9%, ash ≤ 0.3, protein ≤ 0.3, pH 5–7) was purchased from Brenntag Poland Sp. z o. o. Company (Kędzierzyn-Koźle, Poland). Rice starch (Remy FG P–moisture 11.2%, pH 6, protein 0.6% d.m., ash 0.4% d.m.) was purchased from Beneo (Leuven-Wijgmaal, Belgium). Maize starch (C Gel 03401–CAS No 9005-25-8, moisture 11.3%, pH 4–6, protein 0.4%, ash 0.1%) and trehalose (C Ascend 16400–CAS No 6138-23-4, trehalose content in dry mass min 98%, moisture 9.1%) were purchased from Cargill Poland Sp. z o.o. (Warsaw, Poland). Lactose (moisture 4.5%) and guar gum were purchased from Edpol Food and Innovation Company (Łomża, Poland). Whey protein concentrate (WPC 80) was purchased from Ostrowia Company (Ostrów Mazowiecka, Poland).
2.2. Determination of Geometrical and Thermal Properties of Carbohydrate Materials
The particle size of carbohydrates was determined using laser diffraction analysis with Mastersizer 2000 (Malvern Instruments Ltd., Worcestershire, UK). The size was described by D (3,2)—Sauter mean diameter (surface weighted mean diameter) and D (4,3)—De Brouckere mean diameter (volume weighted mean diameter).
Morphology of the carbohydrates was imaged with scanning electron microscopy (SEM Quanta 200, FEI Company, Hillsboro, OR, USA) according to Damerau et al. [18]. The samples were analysed using the SEM operating an accelerating voltage of 30 kV with ×800 or ×100 (for trehalose) magnifications.
Pasting properties were determined only for three used starches in a Brabender amylograph (800145 type, C.W. Brabender Instruments Inc., South Hackensack, NJ, USA) according to Wang et al. [19]. Each starch was suspended in distilled water to form a 6% starch suspension and heated from 30 °C to 95 °C at a heating rate of 1.5 °C/min. The viscosity of the starch paste was expressed in Brabender Units (BU).
2.3. Formulation of Oil-in-Water Emulsions
The composition of emulsion of the core material (pumpkin oil) in the wall material solution was established based on preliminary studies [20]. The emulsion was expected to be stable after 24 h storage at room temperature. Bearing in mind this limit, finally pumpkin oil (10.2%, w/w) was blended with each carbohydrate material (15.4%, w/w): maltodextrin (M), wheat starch (WS), rice starch (RS), maize starch (MS), lactose (L) or trehalose (T), whey protein concentrate (3.9%, w/w), guar gum (0.5%, w/w) and water (70%, w/w) using Thermomix equipment (Vorwerk, Wuppertal, Germany) operated at 9000 rpm for 120 s at 40 °C. Then each emulsion was homogenized at 24 MPa (I step) and 4 MPa (II step) using a high-pressure laboratory valve homogenizer (Panda 2K, GEA Niro Soavi, Parma, Italy).
2.4. Droplet Size and Microstructural Characteristic of Oil-in-Water Emulsions
The size of a dispersed phase in emulsions was determined by laser diffraction analysis with Mastersizer 2000. The median size of the droplet distribution was measured.
Microstructural analysis of the emulsions was conducted using a Nikon Eclipse reversed confocal microscope (Nikon, Tokyo, Japan) with an oil immersion objective at a magnification of ×60 [18].
2.5. Spray Drying of Oil-in-Water Emulsions
The spray dried encapsulated pumpkin oil was prepared using a pilot-plant spray-dryer (A/S Niro Atomizer, Copenhagen, Denmark; spraying mechanism—disc with a 110 mm diameter, 6400 rpm number of revolution). A constant feeding speed of emulsion was in a spray dryer chamber. The drying parameters were controlled to keep 130 °C inlet temperature, and 90 °C outlet temperature, and feed flow rate was 77 mL/min.
The encapsulation process for each emulsion was done in triplicate and powders from three repetitions were mixed before physical and chemical analyses.
2.6. Determination of Oil Content in Capsules and Encapsulation Efficiency
The total oil content (TO) in the powders was determined according to the method described by Takeungwongtrakul et al. [21]. The oil was extracted three times as follow: 0.5 g of powder was mixed with 20 mL of chloroform/methanol mixture (2:1, v/v) (both solvents were purchased from Sigma-Aldrich, Poznań, Poland), ultrasonicated for 10 min and shaken for 20 min at 30 rpm/min using Multi-Rotator Multi RS-60 (Biosan, Riga, Latvia), and then was centrifuged (10,000 rpm for 10 min) in a 5417R-type Eppendorf centrifuge (Eppendorf AG, Hamburg, Germany). The supernatants were combined in a separatory funnel and mixed with 15 mL of 0.58% sodium chloride (POCH, Gliwice, Poland). After phase separation, the chloroform phase was dried with anhydrous sodium sulphate (POCH) and evaporated to dryness in the rotary evaporator (Büchi Labortechnik AG, Flawil, Switzerland) under an N2 stream. The total oil content was determined expressed as a percentage of powder.
The surface oil content (SO) in the powders was extracted three times as follow [21]: 2 g of powder was mixed with 15 mL of n-hexane (Sigma-Aldrich) and shaken for 2 min at 30 rpm/min using Multi-Rotator Multi RS-60 (Biosan) at room temperature. Then solvent was filtered, evaporated using a rotary evaporator (Büchi Labortechnik), and weighted. The surface oil content (SO) was expressed as a percentage of the powder.
The difference between total oil content and surface oil content was calculated to determine the core oil content (CO) in the powders.
The encapsulation efficiency (EE) of emulsions by spray drying was calculated based on the fallowing equation:
2.7. Determination of Properties of Encapsulated Pumpkin Oil Capsules
The moisture content of the powders was measured according to the AOAC Method [22]. The bulk density of the powders was measured by the standard method using a 61–400 apparent density tester (Coesfeld Materialtest, Dortmund, Germany).
The particle size and morphology of the powders were determined similarly as in the case of carbohydrates.
The colour of the powders was determined using equipment for digital image analysis (DIA) [23]. The equipment was consisted of CCD (charge-coupled device) Nikon DXM-1200 colour camera, Kaiser RB 5004 HF–High Frequency Daylight Copy Light set with 4 × 36 W fluorescent light tubes (colour temperature about 5400 K) (Kaiser Fototechnik GmbH and Co., KG, Buchen, Germany) and LUCIA (Laboratory Universal Computer Image Analysis) G v. 4.8 software. The results were expressed in the CIEL*a*b* colour model [24]. The total colour difference (ΔE) was calculated based on the fallowing equation:
The whiteness index (WI) was calculated based on the following equation:
Oxidative stability (OSI) of the powders was evaluated by a 743 Rancimat (Metrom, Switzerland) eight-channel oxidative stability instrument. 2.5 g of each sample was weighed into a reaction vessel. The capped vessel was placed in a thermostated electric heating block. The temperature was set at 110 °C and an air flow rate of 20 L/h was applied. The induction time (expressed as hours) was measured for each sample.
2.8. Determination of Content of Bioactive Unsaponifiable Compounds in Native and Encapsulated Pumpkin Oil
The content of tocopherols was determined according to the method described by Czaplicki et al. [25]. The powder was diluted in n-hexane and centrifuged (11,500× g for 10 min) in a 5417R-type Eppendorf centrifuge (Eppendorf AG). The tocopherols were analysed using an Agilent Technologies 1200 RP-HPLC apparatus with a fluorescence detector (Santa Clara, CA, USA). Separation was performed on a LiChrospher Si60 column (Merck, Darmstadt, Germany). The mobile phase was 0.7% iso-propanol in a n-hexane solution and flow rate was at 1 mL/min. The fluorescence detector was set at excitation and emission wavelengths of 296 nm and 330 nm, respectively. The content of tocopherols was calculated based on the external calibration curves.
The content of sterols and squalene was determined according to the method described by Roszkowska et al. [26]. The sample was dissolved in n-hexane with 5α-cholestane solution as an internal standard. The mixture was saponified in 2 M KOH at temperature 70 °C for 30 min. Sterols and squalene were extracted with diethyl ether. The dry extract was re-dissolved in pyridine and N,O-bis (trimethylsilyl) trifluoroacetamide (BSTFA) with 1% trimethylchlorosilane (TMCS), and derivatization was conducted at 60 °C for 60 min. The mixture dissolved in heptane was analysed using GC-MS QP2010 PLUS system (Shimadzu, Kyoto, Japan). Separation was performed on a ZB-5ms capillary column (Phenomenex, Torrance, CA, USA). Helium was used as a carrier gas. The temperatures were: injector 230 °C, the column in range from 70 °C to 310 °C, the interface of GC-MS 240 °C, the ion source 220 °C, and the electron energy was 70 eV. The content of sterols and squalene was calculated based on the internal standard method.
2.9. Statistical Analysis
The analyses were performed in triplicate and obtained results were analysed statistically using Statistica 12.5 PL software (StatSoft, Kraków, Poland). The analysis of variance (ANOVA) with Duncan’s test for α = 0.05 was used for testing of significant differences between the samples.
3. Results and Discussion
3.1. Characterization of Carbohydrate Materials and Prepared Emulsions with Pumpkin Oil
3.1.1. Carbohydrate Material Properties
The native starches selected for this study had different granule size, with rice as the smallest one followed by maize, and wheat, and these granules were characterized by different shapes observed in SEM images in Figure 1. Other carbohydrates used in the study differed significantly from used starches regarding the granule size and microstructure. It was observed that native starches have spherical shapes, similar to previously presented by Li et al. [27,28]. Bertoft [29] reported that size and shape of starch granules differ among plant species and can be angular, oval, round, spherical or irregular. As shown in Figure 1, rice starch particles, as well as wheat and maize, are angular in shape, and are clustered as compound granules. This type of rice starch granules aggregation was also described by Amagliani et al. [30]. SEM images of lactose and trehalose showed that they have a similar shape, distinct from the shape of native starches and maltodextrin particles. The particle structure of these samples resembles crystals with various sizes, which is typical for lactose [31] and trehalose [32].
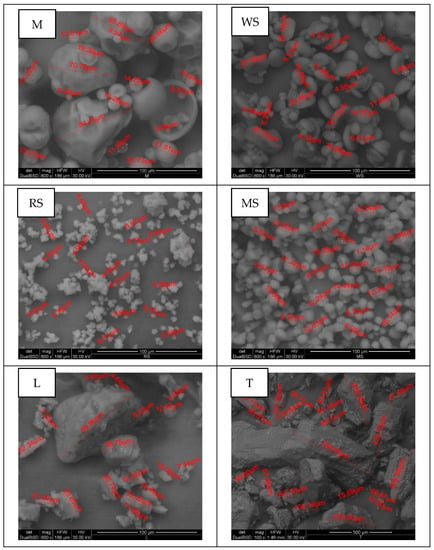
Figure 1.
SEM images of used carbohydrate materials: maltodextrin (M), wheat starch (WS), rice starch (RS), maize starch (MS), lactose (L) and trehalose (T).
Table 1 shows the average particle sizes and amylograph properties of used carbohydrates. The smallest D (4,3) and D (3,2) diameters were observed for rice starch (6.4 and 5.2 µm), respectively. Wheat and maize starches were characterized by about three times higher mean diameters compared to rice starch. The largest mean diameters, both D (4,3) and D (3,2), were determined for trehalose. Li et al. [27] and Amagliani et al. [30] also reported that rice starch granules are the smallest among starches of cereal grains.

Table 1.
Mean diameters and amylograph properties of used carbohydrate materials.
One of the most important functional characteristics of starch is its gelatinization properties. There are specific temperature ranges for gelatinization corresponding to each type of starch. In our study, the highest initial gelatinization temperature was determined for wheat starch (84 °C), while the lowest for rice starch (66 °C). Additionally, rice starch had a lower final gelatinization temperature (88 °C) compared to wheat and maize starches (93 °C). Rice starch had the highest amylograph viscosity (1210 BU) compared to maize (520 BU) and wheat starch (150 BU). Observed phenomenon is due to natural differences in molecular structure of starch from different sources [33], but low viscosity of gelatinized wheat starch indicates the high depolymerization of amylose and amylopectin, probably processed by native α-amylase [34] during wheat plant growing, grain storage or starch isolation. Such hydrolysed starch subunits can more effectively absorb hydrophobic moieties, as in this study, pumpkin oil [35,36]. This shows that wheat starch should have the greatest pumpkin oil lipid-binding capacity.
3.1.2. Emulsion Properties
Figure 2 shows the droplet size distributions of prepared emulsions. The emulsion with wheat starch showed the widest distribution of droplets, in which the size varied from 5 to 45 µm, with a peak at 20 µm. The emulsions with maize and rice starches contained droplets with a diameter ranging from 5 to about 30 µm, with a peak at 15 µm. In turn, the emulsions with maltodextrin, lactose and trehalose had similar size distributions, and particle diameter was in the range of 0.2–10 µm. Gómez-Luría et al. [37] used maize, rice and wheat starches to stabilize oil-in water emulsion and also observed the highest mean droplet size for wheat starch. Similarly, the low particle size of emulsions with maltodextrin and trehalose was confirmed by Teo et al. [32]. The authors emphasized that the small droplet size provides good stability of emulsion during spray drying.
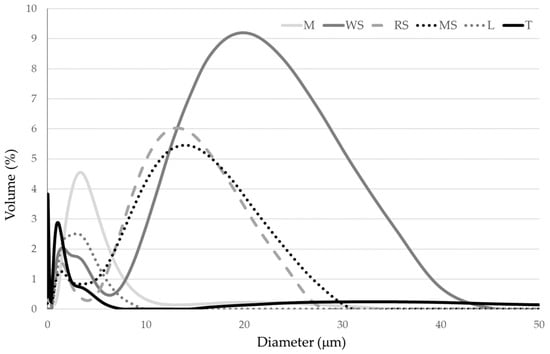
Figure 2.
Particle size distribution of emulsions prepared with pumpkin oil and carbohydrate materials: maltodextrin (M), wheat starch (WS), rice starch (RS), maize starch (MS), lactose (L) and trehalose (T).
To verify droplet size distribution data and to assess droplet flocculation, emulsion microstructure was visualised using a confocal microscope (Figure 3). It was observed the presence of starch granules in the emulsions with native cereal starches, which is probably a result of relative low temperature of emulsion preparation (40 °C). This is consistent with the study of Błaszczak and Lewandowicz [38], who found that at room temperature potato starch granules remained unmodified and light microscopy micrographs showed them as black spots. As reported Noranizan et al. [39] all native starches show increasing solubility as the temperature and time of heating are increased. Furthermore, the solubility of wheat starch is lower than the other starches, which is result of the presence amylose-lipid complex, which inhibits swelling, cracking and dispersion of the granules.
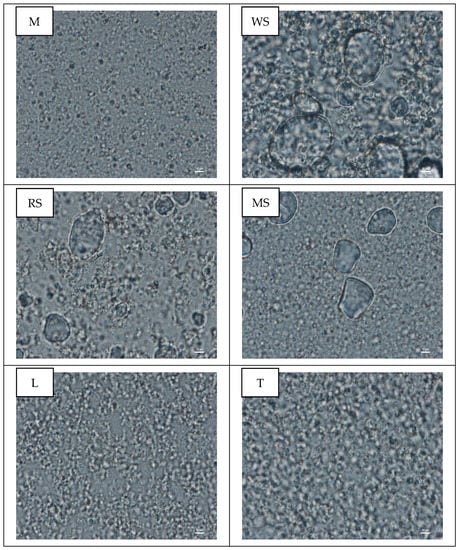
Figure 3.
Confocal microscope images of emulsions prepared with pumpkin oil and carbohydrate materials: maltodextrin (M), wheat starch (WS), rice starch (RS), maize starch (MS), lactose (L) and trehalose (T).
Li et al. [27] reported that the surface roughness of native rice starch had a negative impact on emulsifying power possibility because the reduced surface contact considerably lessens the interfacial potential well holding smooth particles at the interface.
3.2. Encapsulation Efficiency of Pumpkin Oil in Carbohydrate Shells by Spray Drying
Encapsulation efficiency is the most important parameter for determining the high efficiency of encapsulation process and is an indicator of coated and surface oil content. This parameter is significantly influenced by the composition of wall material [40]. In current study the highest EE (71%) was determined in powder utilising lactose (Table 2). The best properties of lactose can be attributed to the highest possibility of Maillard reaction products formation. It was confirmed by Aghbashlo et al. [41], who found that the skim milk powder and lactose coated microcapsules had significantly higher EE than treatment with skim milk powder and maltodextrin (84.96% vs. 76.22%). The authors explained this phenomenon by faster crust formation in droplets containing lactose, and therefore the oil could not diffuse out easily to the surface. In the study of Yang et al. [42], sodium caseinate blends with lactose had higher EE than gum Arabic and soy protein. They stated that lactose can form conjugates through Maillard reaction with whey protein, which stabilize encapsulated oil [42,43].

Table 2.
Oil content in capsules and encapsulation efficiency of spray drying process of pumpkin oil with carbohydrate materials.
It was found that the powder containing wheat starch was characterized by the lowest content of surface oil. This phenomenon can be explained by the thermal properties of used wheat starch, especially low amylograph viscosity (Table 1). Esposito et al. [44] reported that a too high viscosity could obstruct the atomizer nozzle, causing also incomplete solvent evaporation, and low process yields also negatively affecting the particle morphology. Hee et al. [45] confirmed that encapsulation efficiency is affected by the emulsion viscosity, which is a result of wall material composition. They found that addition of gum Arabic increased the emulsion viscosity and decreased encapsulation efficiency. It is highlighted that usefulness of maltodextrin as a coating material is related to its good solubility in water and low viscosity even at high concentrations. The combining other coating materials (i.e., gums, proteins) with maltodextrin improve its emulsifying properties and allow it to form stable capsules [40,46].
3.3. Characterization of Encapsulated Pumpkin Oil in Carbohydrate Shells
3.3.1. Basic Parameters of Capsules
In the current study, Table 3 shows that the moisture content was the highest in the powder containing wheat starch (3.11%). In turn, the lowest values were detected for lactose (1.40%) and trehalose (1.85%).

Table 3.
Moisture content, physical properties and oxidative stability of encapsulated pumpkin oil in carbohydrate shells.
Bulk density is a measure of the mass of powder which occupies a fixed volume. In this study bulk density was in the range from 189 kg/m3 (powder with maize starch) to 198 kg/m3 (powder with lactose) (Table 3). Teo et al. [32] obtained similar results of bulk density for encapsulated corn oil with trehalose and maltodextrin (for 10% addition it was 180–280 kg/m3).
3.3.2. Size, Colour and Microstructure of Prepared Capsules
The powders obtained by spray drying were characterized by similar particle sizes of D (4,3) and D (3,2) (Table 3). The highest mean diameters were detected for particles of encapsulated pumpkin oil with trehalose. The colour of the bulk capsules was comparable for most of variants. Additionally, whiteness index (WI) was only slightly differed between samples. The greater colour difference compared to capsules with maltodextrin (ΔE) was found for the capsules obtained with the use of wheat starch, which were found to be lighter and less yellow than other ones.
Figure 4 presents micrographs of encapsulated pumpkin oil in used carbohydrate shells. These powders were composed of particles with a similar external topography, characterized generally by rounded shapes and a continuous wall with no apparent cracks or fissures on the outer surface. This globular morphology results from the fast water evaporation during the spray drying process [47]. It was observed that capsules prepared with maltodextrin and native starches showed larger depressions on the outer surface than those prepared with lactose and trehalose. Additionally, the capsules of powders with rice and maize starches had visible small particles (starch granules) taped to the surface, which probably resulted from the high content of surface oil in these samples.

Figure 4.
SEM images of encapsulated pumpkin oil in carbohydrate shells: maltodextrin (M), wheat starch (WS), rice starch (RS), maize starch (MS), lactose (L) and trehalose (T).
3.3.3. Oxidative Stability of Encapsulated Pumpkin Oil
Pumpkin oil powders were characterized by high oxidation stability (from 9.5 h for wall material with trehalose to 15.1 h for wall material with wheat starch). It is worth pointing out that the oxidative stability of cold pressed pumpkin oil is in the range of 6.8–9.4 h [48,49,50]. High OSI of powders can be explained by the possibility of carbohydrate wall materials to form a glassy shell that blocks the surface pores and creates a barrier, which can limit oxygen diffusion toward the core material [44]. The lowest OSI powder with trehalose can be explained by the hygroscopic nature of trehalose, which increases the tendency to absorb water from the environment, resulting in possibility of crystallization. Drusch et al. [51] reported, that in the glassy state trehalose decreases lipid oxidation. Additionally, powder with lactose compared to powder with wheat starch had low OSI (12.4 h). According to Chandrapala et al. [52] lactose is very hygroscopic, and under varying storage conditions, the amorphous lactose undergoes an irreversible transition to stable crystalline forms. This crystallization leads to a coating of crystals on the surface of the spray dried powder particles which decreases the shelf life of the powders.
3.4. Distribution of Unsaponifiable Lipophilic Compounds in Surface and Core Oils of Encapsulated Pumpkin Oil
Used in current study pumpkin oil contained 320, 260 and 50.8 mg/100 g of sterols, squalene and tocopherols, respectively (Table 4). Among tocopherols two compounds were determined: α-tocopherol (16.7%) and γ-tocopherol (83.3% of total). This content and composition of pumpkin oil is similar to those found previously by Ogrodowska et al. [49] and Konopka et al. [50]. In the case of sterols, shares of spinasterol + β-sitosterol, Δ7,22,25-stigmastatrienol, Δ7-stigmasterol and Δ7,25-stigmastadienol were 52.8, 21.3, 4.4 and 21.6%, respectively. Presented sterol composition is similar to that reported by Konopka et al. [50] and Hrabovski et al. [53], who showed that Δ7-sterols are predominating in pumpkin oil. Such unique composition of pumpkin sterols was recently confirmed by the study conducted in Italy, which showed the ca. 95% of total sterols of Berrettina pumpkin seed oil are composed of Δ7-sterols [54]. Usually, these sterols groups account for at least 91% of pumpkin oil, so their lower level can indicate pumpkin oil adulteration [49,55]. In general, sterols, tocopherols, and squalene content and composition are relatively constant between pumpkin seed oils produced from plants cultivated in various world regions [56].

Table 4.
Bioactive unsaponifiable compounds in native pumpkin oil.
Encapsulation efficiency of each identified compound in sterols and tocopherols groups and of squalene was determined only for three carbohydrates with the highest encapsulation efficiency of pumpkin oil, which were maltodextrin, wheat starch and lactose (Table 2). Among used carbohydrates the best properties to entrap all lipophilic unsaponifiable compounds were found for maltodextrin (encapsulation efficiency in range 67.5–75.3%) (Table 5). In the case of maltodextrin, surface and core oils were mostly similar in the composition of entrapped lipophilic unsaponifiable compounds, which pointed on an aligned affinity to internal and external parts of capsules. In the case of wheat starch, majority of components were deposited in surface oil, with only two exceptions for comparable contents of α-tocopherol and sum of spinasterol + β-sitosterol in surface and core oil (Table 4). Opposite to wheat starch phenomenon was found for lactose. For this disaccharide, the majority of bioactive lipophilic compounds were entrapped by core oil. Summarizing all studied lipophilic compounds, it is worth noting, that only α-tocopherol was better entrapped in core oil by lactose (81.2%) than by maltodextrin (70.3%). All other components were better core encapsulated by maltodextrin.

Table 5.
Comparison of bioactive unsaponifiable compounds contents in surface and total oil of capsules with the greatest encapsulation efficiency.
The highest ability of maltodextrin to bind low in mass and volume lipophilic pumpkin oil compounds resulted from the highest availability of partly hydrolysed amylose and amylopectin helices to absorb hydrophobic compounds after emulsion and spry-drying stages of capsules production [57]. It was previously found that a variety of physicochemical mechanisms may either favour or oppose binding, including electrostatic interactions, hydrophobic interactions, hydrogen bonding, and configurational entropy [58]. It is also well known that linear chains within maltodextrin molecules form helical structures upon interacting with hydrophobic tails of amphiphilic lipid molecules (more details in Wangsakan et al. [58]). In turn, in the case of native wheat starch, starch granules are not fully degraded and only part of its polymers can absorb lipophilic compounds from prepared emulsion. In this regard, ability of native starch to form inclusion complexes is significantly lower than for chemically or enzymatically hydrolysed maltodextrins. On the other hand, the highest lactose ability to entrap pumpkin oil tocopherols, sterols and squalene in core of capsules can be explained by the properties of created emulsion (Figure 3). In the case of components of more comparable molecular volumes of sterols, tocopherols, squalene and disaccharides, obtained emulsion seems to be more uniform and hydrophilic and hydrophobic parts better segregated. In general, disaccharides are active compounds in creation of protein-lipid bilayers [59]. Nonreducing disaccharides, such as trehalose, strongly destabilize the phase separation leading to uniformly mixed membranes as opposed to reducing disaccharides (like lactose) [59]. However, in emulsion solution, reducing sugars can react with the amino groups of amino acids leading to Maillard products. Such products can enhance the stability of protein-polysaccharides emulsions during oil encapsulation by spray-drying [60].
4. Conclusions
Results of the study showed that maltodextrin can be replaced by other carbohydrates during encapsulation of pumpkin oil. The use of native rice and maize starches as a wall component is useless, since their application for preparing emulsion causes significant decrease of its encapsulation efficiency (up to 42%), oil leakage, and consequently lower oxidative stability of the encapsulated pumpkin oil. In turn, the use of native wheat starch and lactose proved to be the best replacements of maltodextrin, since the encapsulation efficiency and oxidative stability were the highest. It was confirmed that maltodextrin had the highest ability to entrap unsaponifiable compounds of pumpkin oil, but significant part of these compounds was found as the surface lipids, so can be easily oxidized in food matrices. The amount of pumpkin oil entrapped by the natural starches was inversely proportional to the viscosity of the gelatinized paste. Lactose exhibited the lowest efficiency of oil entrapping, but majority of unsaponifiable lipophilic compounds of pumpkin oil were deposited in the core of capsules.
Author Contributions
Conceptualization, D.O., I.Z.K. and M.T.; methodology, D.O., I.Z.K., W.B.; validation, D.O., I.Z.K. and M.T.; formal analysis, D.O., I.Z.K. and M.T.; investigation, D.O., W.B. and B.P.; resources, D.O.; writing—original draft preparation, D.O., I.Z.K. and M.T.; writing—review and editing, D.O., I.Z.K. and M.T.; visualization, D.O., I.Z.K., M.T. and W.B.; supervision, I.Z.K. and M.T.; project administration, D.O.; funding acquisition, D.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bao, C.; Jiang, P.; Chai, J.; Jiang, Y.; Li, D.; Bao, W.; Liu, B.; Liu, B.; Norde, W.; Li, Y. The delivery of sensitive food bioactive ingredients: Absorption mechanisms, influencing factors, encapsulation techniques and evaluation models. Food Res. Int. 2019, 120, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Raychaudhuri, U.; Chakraborty, R. An overview of encapsulation of active compounds used in food products by drying technology. Food Biosci. 2016, 13, 76–83. [Google Scholar] [CrossRef]
- Apintanapong, M.; Noomhorm, A. The use of spray drying to microencapsulate 2-acetyl-1-pyrroline, a major flavour component of aromatic rice. Int. J. Food Sci. Technol. 2003, 38, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, J.; Borges, S.; Amorim, M.; Pereira, M.J.; Oliveira, A.; Pintado, M.E.; Teixeira, P. Comparison of spray drying, freeze drying and convective hot air drying for the production of a probiotic orange powder. J. Funct. Foods 2015, 17, 340–351. [Google Scholar] [CrossRef]
- Zhang, L.; Zeng, X.; Fu, N.; Tang, X.; Sun, Y.; Lin, L. Maltodextrin: A consummate carrier for spray-drying of xylooligosaccharides. Food Res. Int. 2018, 106, 383–393. [Google Scholar] [CrossRef]
- Desai, K.G.H.; Jin Park, H. Recent developments in microencapsulation of food ingredients. Dry. Technol. 2005, 23, 1361–1394. [Google Scholar] [CrossRef]
- Masum, A.K.M.; Chandrapala, J.; Huppertz, T.; Adhikari, B.; Zisu, B. Production and characterization of infant milk formula powders: A review. Dry. Technol. 2020, 39, 1492–1512. [Google Scholar] [CrossRef]
- Valenzuela, C.; Aguilera, J.M. Effects of maltodextrin on hygroscopicity and crispness of apple leathers. J. Food Eng. 2015, 144, 1–9. [Google Scholar] [CrossRef]
- Hofman, D.L.; Van Buul, V.J.; Brouns, F.J. Nutrition, health, and regulatory aspects of digestible maltodextrins. Crit. Rev. Food Sci. Nutr. 2016, 56, 2091–2100. [Google Scholar] [CrossRef]
- Marcus, J.B. Flavor Enhancement Ingredients. Aging, Nutrition and Taste; Academic Press: Cambridge, MA, USA, 2019; pp. 173–206. [Google Scholar]
- Siemons, I.; Politiek, R.G.A.; Boom, R.M.; Van der Sman, R.G.M.; Schutyser, M.A.I. Dextrose equivalence of maltodextrins determines particle morphology development during single sessile droplet drying. Food Res. Int. 2020, 131, 108988. [Google Scholar] [CrossRef]
- Tahmassebi, J.; Duggal, M.S.; Malik-Kotru, G.; Curzon, M.E.J. Soft drinks and dental health: A review of the current literature. J. Dent. 2006, 34, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Al-Khatib, G.R.; Duggal, M.S.; Toumba, K.J. An evaluation of the acidogenic potential of maltodextrins in vivo. J. Dent. 2001, 29, 409–414. [Google Scholar] [CrossRef]
- Rezende, G.; Hashizume, L.N. Maltodextrin and dental caries: A literature review. RGO-Rev. Gaúch. Odontol. 2018, 66, 257–262. [Google Scholar] [CrossRef] [Green Version]
- Rytz, A.; Adeline, D.; Lê, K.A.; Tan, D.; Lamothe, L.; Roger, O.; Macé, K. Predicting glycemic index and glycemic load from macronutrients to accelerate development of foods and beverages with lower glucose responses. Nutrients 2019, 11, 1172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkins, D.J.; Kendall, C.W.; Augustin, L.S.; Franceschi, S.; Hamidi, M.; Marchie, A.; Jenkins, A.L.; Axelsen, M. Glycemic index: Overview of implications in health and disease. Am. J. Clin. Nutr. 2002, 76, 266S–273S. [Google Scholar] [CrossRef] [PubMed]
- Yoshizane, C.; Mizote, A.; Yamada, M.; Arai, N.; Arai, S.; Maruta, K.; Mitsuzumi, H.; Ariyasu, T.; Ushio, S.; Fukuda, S. Glycemic, insulinemic and incretin responses after oral trehalose ingestion in healthy subjects. Nutr. J. 2017, 16, 9. [Google Scholar] [CrossRef] [Green Version]
- Damerau, A.; Ogrodowska, D.; Banaszczyk, P.; Dajnowiec, F.; Tańska, M.; Linderborg, K.M. Baltic herring (Clupea harengus membras) oil encapsulation by spray drying using a rice and whey protein blend as a coating material. J. Food Eng. 2022, 314, 110769. [Google Scholar] [CrossRef]
- Wang, W.; Guan, L.; Seib, P.A.; Shi, Y.C. Settling volume and morphology changes in cross-linked and unmodified starches from wheat, waxy wheat, and waxy maize in relation to their pasting properties. Carbohydr. Polym. 2018, 196, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Ogrodowska, D.; Tańska, M.; Brandt, W. The influence of drying process conditions on the physical properties, bioactive compounds and stability of encapsulated pumpkin seed oil. Food Bioproccess Technol. 2017, 10, 1265–1280. [Google Scholar] [CrossRef] [Green Version]
- Takeungwongtrakul, S.; Benjakul, S.; H-kittikun, A. Wall materials and the presence of antioxidants influence encapsulation efficiency and oxidative stability of micro-encapsulated shrimp oil. Eur. J. Lipid Sci. Technol. 2015, 117, 450–459. [Google Scholar] [CrossRef]
- AOAC. Association of Official Analytical Chemists, 16th ed.; Official Methods of Analysis: Gaithersburg, MD, USA, 1995. [Google Scholar]
- Tańska, M.; Rotkiewicz, D.; Kozirok, W.; Konopka, I. Measurement of the geometrical features and surface color of rapeseeds using digital image analysis. Food Res. Int. 2005, 38, 741–750. [Google Scholar] [CrossRef]
- Smarzyński, K.; Sarbak, P.; Kowalczewski, P.Ł.; Różańska, M.B.; Rybicka, I.; Polanowska, K.; Fedko, M.; Kmiecik, D.; Masewicz, Ł.; Nowicki, M.; et al. Low-field NMR study of shortcake biscuits with cricket powder, and their nutritional and physical characteristics. Molecules 2021, 26, 5417. [Google Scholar] [CrossRef] [PubMed]
- Czaplicki, S.; Ogrodowska, D.; Derewiaka, D.; Tańska, M.; Zadernowski, R. Bioactive compounds in unsaponifiable fraction of oils from unconventional sources. Eur. J. Lipid Sci. Technol. 2011, 113, 1456–1464. [Google Scholar] [CrossRef]
- Roszkowska, B.; Tańska, M.; Czaplicki, S.; Konopka, I. Variation in the composition and oxidative stability of commercial rapeseed oils during their shelf life. Eur. J. Lipid Sci. Technol. 2015, 117, 673–683. [Google Scholar] [CrossRef]
- Li, C.; Li, Y.; Sun, P.; Yang, C. Pickering emulsions stabilized by native starch granules. Colloids Surf. A Physicochem. Eng. Asp. 2013, 431, 142–149. [Google Scholar] [CrossRef]
- Li, H.; Turner, M.S.; Dhital, S. Encapsulation of Lactobacillus plantarum in porous maize starch. LWT 2016, 74, 542–549. [Google Scholar] [CrossRef] [Green Version]
- Bertoft, E. Understanding starch structure: Recent progress. Agronomy 2017, 7, 56. [Google Scholar] [CrossRef]
- Amagliani, L.; O’Regan, J.; Kelly, A.L.; O’Mahony, J.A. Chemistry, structure, functionality and applications of rice starch. J. Cereal Sci. 2016, 70, 291–300. [Google Scholar] [CrossRef]
- Lara-Mota, E.E.; Nicolás-Vázquez, M.I.; López-Martínez, L.A.; Espinosa-Solis, V.; Cruz-Alcantar, P.; Toxqui-Teran, A.; Saavedra-Leos, M.Z. Phenomenological study of the synthesis of pure anhydrous β-lactose in alcoholic solution. Food Chem. 2021, 340, 128054. [Google Scholar] [CrossRef]
- Teo, A.; Lam, Y.; Lee, S.J.; Goh, K.K. Spray drying of whey protein stabilized nanoemulsions containing different wall materials–maltodextrin or trehalose. LWT 2021, 136, 110344. [Google Scholar] [CrossRef]
- Pojić, M.; Hadnađev, M.; Hadnađev, T.D. Gelatinization properties of wheat flour as determined by empirical and fundamental rheometric method. Eur. Food Res. Technol. 2013, 237, 299–307. [Google Scholar] [CrossRef]
- Miłek, J. Determination of activation energies and the optimum temperatures of hydrolysis of starch by α-amylase from porcine pancreas. Molecules 2021, 26, 4117. [Google Scholar] [CrossRef] [PubMed]
- Alcázar-Alay, S.C.; Meireles, M.A.A. Physicochemical properties, modifications and applications of starches from different botanical sources. Food Sci. Technol. 2015, 35, 215–236. [Google Scholar] [CrossRef] [Green Version]
- Kawai, K.; Takato, S.; Sasaki, T.; Kajiwara, K. Complex formation, thermal properties, and in-vitro digestibility of gelatinized potato starch-fatty acid mixtures. Food Hydrocoll. 2012, 27, 228–234. [Google Scholar] [CrossRef]
- Gómez-Luría, D.; Vernon-Carter, E.J.; Alvarez-Ramirez, J.; Cruz-Sosa, F. Insights of the ability of gelatinized fractions from non-chemical modified corn, rice, wheat, and waxy corn starches to stabilize O/W emulsions. Food Hydrocoll. 2019, 89, 726–734. [Google Scholar] [CrossRef]
- Błaszczak, W.; Lewandowicz, G. Light microscopy as a tool to evaluate the functionality of starch in food. Foods 2020, 9, 670. [Google Scholar] [CrossRef] [PubMed]
- Noranizan, M.A.; Dzulkifly, M.H.; Russly, A.R. Effect of heat treatment on the physico-chemical properties of starch from different botanical sources. Int. Food Res. J. 2010, 17, 127–135. [Google Scholar]
- Koç, M.; Güngör, Ö.; Zungur, A.; Yalçın, B.; Selek, İ.; Ertekin, F.K.; Ötles, S. Microencapsulation of extra virgin olive oil by spray drying: Effect of wall materials composition, process conditions, and emulsification method. Food Bioprocess Technol. 2015, 8, 301–318. [Google Scholar] [CrossRef]
- Aghbashlo, M.; Mobli, H.; Madadlou, A.; Rafiee, S. The correlation of wall material composition with flow characteristics and encapsulation behavior of fish oil emulsion. Food Res. Int. 2012, 49, 379–388. [Google Scholar] [CrossRef]
- Yang, X.; Li, S.; Yan, J.; Xia, J.; Huang, L.; Li, M.; Ding, H.; Xu, L. Effect of different combinations of emulsifier and wall materials on physical properties of spray-dried microencapsulated swida wilsoniana oil. J. Bioresour. Bioprod. 2020, 5, 44–50. [Google Scholar] [CrossRef]
- Goyal, A.; Sharma, V.; Sihag, M.K.; Tomar, S.K.; Arora, S.; Sabikhi, L.; Singh, A.K. Development and physico-chemical characterization of microencapsulated flaxseed oil powder: A functional ingredient for omega-3 fortification. Powder Technol. 2015, 286, 527–537. [Google Scholar] [CrossRef]
- Esposito, T.; Mencherini, T.; Del Gaudio, P.; Auriemma, G.; Franceschelli, S.; Picerno, P.; Aquino, R.; Sansone, F. Design and development of spray-dried microsystems to improve technological and functional properties of bioactive compounds from hazelnut shells. Molecules 2020, 25, 1273. [Google Scholar] [CrossRef] [Green Version]
- Hee, Y.Y.; Tan, C.P.; Rahman, R.A.; Adzahan, N.M.; Lai, W.T.; Chong, G.H. Influence of different wall materials on the microencapsulation of virgin coconut oil by spray drying. Int. J. Food Eng. 2015, 11, 61–69. [Google Scholar] [CrossRef]
- Lim, H.K.; Tan, C.P.; Bakar, J.; Ng, S.P. Effects of different wall materials on the physicochemical properties and oxidative stability of spray-dried microencapsulated red-fleshed pitaya (Hylocereus polyrhizus) seed oil. Food Bioproccess Technol. 2012, 5, 1220–1227. [Google Scholar] [CrossRef]
- Silva, V.M.; Vieira, G.S.; Hubinger, M.D. Influence of different combinations of wall materials and homogenisation pressure on the microencapsulation of green coffee oil by spray drying. Food Res. Int. 2014, 61, 132–143. [Google Scholar] [CrossRef]
- Murkovic, M.; Pfannhauser, W. Stability of pumpkin seed oil. Eur. J. Lipid Sci. Technol. 2000, 102, 607–611. [Google Scholar] [CrossRef]
- Ogrodowska, D.; Laaksonen, O.; Tańska, M.; Konopka, I.; Linderborg, K.M. Pumpkin oil addition and encapsulation process as methods to improve oxidative stability of fish oil. LWT 2020, 124, 109142. [Google Scholar] [CrossRef]
- Konopka, I.; Roszkowska, B.; Czaplicki, S.; Tańska, M. Optimization of pumpkin oil recovery by using aqueous enzymatic extraction and comparison of the quality of the obtained oil with the quality of cold-pressed oil. Food Technol. Biotechnol. 2016, 54, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Drusch, S.; Serfert, Y.; Heuvel, A.V.D.; Schwarz, K. Physicochemical characterization and oxidative stability of fish oil encapsulated in an amorphous matrix containing trehalose. Food Res. Int. 2006, 39, 807–815. [Google Scholar] [CrossRef]
- Chandrapala, J.; Vasiljevic, T. Properties of spray dried lactose powders influenced by presence of lactic acid and calcium. J. Food Eng. 2017, 198, 63–71. [Google Scholar] [CrossRef]
- Hrabovski, N.; Sinadinović-Fišer, S.; Nikolovski, B.; Sovilj, M.; Borota, O. Phytosterols in pumpkin seed oil extracted by organic solvents and supercritical CO2. Eur. J. Lipid Sci. Technol. 2012, 114, 1204–1211. [Google Scholar] [CrossRef]
- Montesano, D.; Blasi, F.; Simonetti, M.S.; Santini, A.; Cossignani, L. Chemical and nutritional characterization of seed oil from Cucurbita maxima L. (var. Berrettina) pumpkin. Foods 2018, 7, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandl, A.; Reich, G.; Lindner, W. Detection of adulteration of pumpkin seed oil by analysis of content and composition of specific Δ7-phytosterols. Eur. Food Res. Technol. 1999, 209, 400–406. [Google Scholar] [CrossRef]
- Tańska, M.; Ogrodowska, D.; Bartoszewski, G.; Korzeniewska, A.; Konopka, I. Seed lipid composition of new hybrids of Styrian oil pumpkin grown in Poland. Agronomy 2020, 10, 1104. [Google Scholar] [CrossRef]
- Kanyuck, K.M.; Mills, T.B.; Norton, I.T.; Norton-Welch, A.B. Temperature influences on network formation of low DE maltodextrin gels. Carbohydr. Polym. 2019, 218, 170–178. [Google Scholar] [CrossRef]
- Wangsakan, A.; Chinachoti, P.; McClements, D.J. Maltodextrin—anionic surfactant interactions: Isothermal titration calorimetry and surface tension study. J. Agric. Food Chem. 2001, 49, 5039–5045. [Google Scholar] [CrossRef]
- Moiset, G.; López, C.A.; Bartelds, R.; Syga, L.; Rijpkema, E.; Cukkemane, A.; Baldus, M.; Poolman, B.; Marrink, S.J. Disaccharides impact the lateral organization of lipid membranes. J. Am. Chem. Soc. 2014, 136, 16167–16175. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Woo, M.W.; Patel, H.; Selomulya, C. Enhancing the stability of protein-polysaccharides emulsions via Maillard reaction for better oil encapsulation in spray-dried powders by pH adjustment. Food Hydrocoll. 2017, 69, 121–131. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).