Featured Application
The goal of this paper is to direct the discussion about individual risks and immunity breakthrough of infectious diseases, such as COVID-19, on quantitative effects concerning background and non-specific immunity, stimulation of antibody production and antibody decay, illustrated by a simple kinetic model.
Abstract
The personal risks of infection, as well as the conditions for achieving herd immunity, are strongly dependent on an individual’s response to the infective agents on the one hand, and the individual’s reactions to vaccination on the other hand. The main goal of this work is to illustrate the importance of quantitative individual effects for disease risk in a simple way. The applied model was able to illustrate the quantitative effects, in the cases of different individual reactions, after exposition to viruses or bacteria and vaccines. The model was based on simple kinetic equations for stimulation of antibody production using different concentrations of the infective agent, vaccine and antibodies. It gave a qualitative explanation for the individual differences in breakthrough risks and different requirements concerning a second, third or further vaccinations, reconsidering different efficiencies of the stimulation of an immune reaction.
1. Introduction
To date, COVID-19 virus has killed millions of people worldwide. Due to the COVID-19 development of different, efficient vaccines, many people are now protected from the virus, and the danger of further high numbers of victims appears to be curbed in many countries. However, individual immunity after recovering from the disease or after vaccination decays over weeks and month following infection. The mixture of decreasing immunity over time, a considerable percentage of the global population being unvaccinated and the appearance of new virus mutants, endangers herd immunity and demands an optimized strategy for re-vaccination and other measures such as social distancing.
Today, many details about the complex biomolecular and cellular mechanisms of immune responses are known and help us to understand the differences in individual responses to infection or vaccination. Despite this knowledge, predictions for herd immunity use simplified models based on yes/no states concerning the susceptibility for infective agents and achieving an immune state [1,2,3]. Moreover, as well as this “black and white” point of view, quantitative effects have to be taken into account in order to understand the dynamics of the evolution of a pandemic and for suitable management of a sustainable state of herd immunity [4]. Epidemic modeling should help to predict the efficiency of vaccination [5], pharmaceutical treatment and social distancing [6,7].
Temporal changes in personal susceptibility, different increases in rates of antibody production after stimulation, different decreases in rates of antibody concentration after infection or vaccination and low-level impact events [8] have to be included for consideration. The loss of antibodies over time [9], individual differences in antibody decay [10] and genetic factors of the immune system play an important role in the risk of infection and re-infection. Simulations confirm the important role of the frequency of physical contact in the spread of infection on the one hand and for non-pharmaceutical interventions for infection prevention on the other hand [11]. Such models support the decisions for a strict reduction in such contacts [12]. Moreover, persons with heavy illness and infected asymptomatic persons contribute significantly to the transfer of viruses [13].
Recent studies emphasize that vaccination lowers not only personal risk by reducing a person’s susceptibility to the virus, but it also reduces the density of infected persons in a given population [14]. The completeness of protection given by vaccination is also an important factor for achieving herd immunity. For reliable protection of a whole population, the number of people who must be vaccinated increases with decreasing efficacy of vaccines, which can be clearly demonstrated by mathematical modeling [15]. Simulations show that a vaccine efficacy of more than 70% is required for achieving herd immunity, but vaccination of 66% of a population is sufficient if the vaccine efficacy is above 90% [16]. Digital technologies offer powerful possibilities for data collection, data processing and simulation of the evolution of epidemics and on the efficacy of vaccines. Despite the advantages of the use of large databases and detailed physical modeling, simulations have to consider that individual behavior plays a deciding role in the dynamics of the spreading of infection [17,18]. Success in the fight against pandemics can be measured by the progress in vaccination and lowering incidence numbers in highly developed countries. The global character of pandemics has to be respected, which demands global control by vaccination all over the world [19].
Simulations of the spread of infection and the estimation of the importance of vaccine efficacy supply quantitative data for the evolution and possible containments of epidemics, as well as on the number of fatal cases [16]. In addition to quantitative relations in the evolution of epidemics in a population, the quantitative character of individual responses to virus exposition and vaccination has to be reconsidered. This concerns individual’s general state of health and non-specific sensitivity against any infection, concentration of viruses and the duration of virus exposition, the highly specific status of antibody availability as well as a reduction in infection susceptibility by less-specific immunity factors. In addition, for vaccination, an individual’s situation is important for achieving protection. This concerns the strength of stimulation for the production of specific antibodies and memory cells and their individual spontaneous decay in the weeks after vaccination or infection. In previous communication, a simple kinetic model was proposed for illustrating quantitative effects in individuals’ responses in the case of single and multiple expositions to different concentrations of infective agents [20]. In the following, a modified version of this model was applied in order to discuss the effects of simple and repeated vaccination. Thereby, individual differences in background immunity and responses to stimulation of the immune system are considered.
2. Concept of Combined Background Immunity and Specific Immunization
The following approach was based on the idea that immunity cannot be regarded as a strict non-susceptible state, in general, but as a state of lowered risk of infection only, in many cases. Herd immunity is composed of individuals with different degrees of moderate immunization and individuals with a “safe immunization state”. However, this “safe state” can also be quickly reduced to a less safe state by decreasing antibody concentration and by the emergence of infective mutants.
In addition, it has to be considered that several mechanisms contribute to the overall immunity of each single individual, among them is a non-specific general immunity background; a low-specific immunization, which is caused by occasional responses to related proteins or viruses; and the high specific immunization caused by the infective agent or related vaccines.
Finally, it is assumed that vaccination strategies have an impact on an individual’s immunity state and its degradation, which is caused by new mutants, reduction in antibody production and decay of antibody concentration in the blood and in the concerned tissues. Thus, the infective agent itself provokes a molecular immune response against, more or less, all relevant bio macromolecules, which are foreign to the target organism. In the following, the very complex behavior of the immune system and the immune response are reduced to a set of very simple equations. This set is neither suited for reflecting the true kinetic network of biomolecular reactions nor to supplying a real quantitative description of immunization and infection processes. However, it illustrates the quantitative effects in these processes and can give an idea about the importance of individual responsivities and occasional exposition events in the risk of primary infection or re-infection in the case of previous immunization.
The following parameters, for iterative simulation in time steps (1..100), are defined in the example of a viral infection:
- kvir = replication rate of a virus,
- kr = reaction rate between virus particles and antibodies,
- kst = rate of stimulation of antibody production by a virus,
- stoo = maximum for stimulation,
- voo = maximum concentration of a virus,
- cABv0 = initial concentration of antibodies,
- cBGim = non-specific and low-specific background immunity (counted as antibody equivalent),
- z = random noise of cBGim,
- DAB = decay rate of antibodies (specific protection),
- Dvacc = decay rate of primary applied vaccine.
The following variables are used in the simulation:
- cvir = concentration of virus particles,
- cABvir = concentration of antibodies formed due to the stimulation of virus particles,
- cABvacc = concentration of antibodies formed due to stimulation by a vaccine,
- cvacc = concentration of primary applied vaccine,
- cABtotal = total concentration of antibodies, including an antibody equivalent for cBGim.
The following operations were applied in iterative steps:
cABtotal = cABvacc + cABvir + cBGim
cvacc (t) = cvacc (t − 1) ∗ (1 − Dvacc)
cABvacc (t) = cABvacc (t − 1) ∗ (1 − DAB) + kst ∗ cvac
cvir (t) = roundoff3[1 + (kvir/cABtotal) ∗ (1 − cvir(t − 1)/voo)] ∗ kr ∗ cvir(t − 1) ∗ cABtotal
cABvir = cAvir(t − 1) ∗ sqrt[kst ∗ cABvir(t − 1) ∗ cvir(t − 1) ∗ (1 − kst ∗ cvir(t − 1)/stoo)] ∗ (1 − DAB) ∗ cABvir(t − 1) ∗ cvir(t − 1)
For simulation, a simple iterative method was sufficient. It could be run by an Excel table, for example. This allowed an easy change of parameters and start conditions. An example is given in the Table S1 (supplementary material).
3. Results: Illustration of Immunity Response after Vaccination and Infection
3.1. Immunity Decay after Vaccination
The immune reaction of the human body protects against a new infection after a survived infection or after vaccination, for a certain time. The reliability of protection is dependent on the following main factors:
- General immune status,
- Intensity of activation of the specific immune defense by the survived disease or vaccination,
- Intensity of a new virus exposition,
- Decay of specific immunity over time.
Therefore, the seriousness of a second infection is dependent on the time span between the first and second immunization. This simple relation is illustrated in Figure 1. Only a small effect can be expected in the case of an early small virus impact (Figure 1a). In this case, the immune response is slightly enforced, but it is assumed that there are not strong symptoms of disease. Even in an early phase after immunization, a stronger reaction of the protected body might take place if it is confronted by a massive viral exposition. In this early phase the body is able to suppress the infection quickly (Figure 1b). After a longer time, a massive viral impact can result in the propagation of infection. In this case, the body can overcome the infection by means of the residual protection from the original immunization (e.g., vaccination) in combination with the virus-induced re-activation of the immune system (Figure 1c). The disease is obvious as there are significant symptoms, but the course of disease is less serious than without the early immunization. However, after a longer time span between vaccination and a massive viral impact, the common power of residual specific immunity, general immunity and instant immune reaction might be too small for overcoming the infection, and the virus wins the battle (Figure 1d).
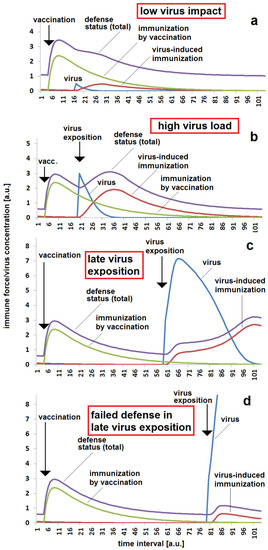
Figure 1.
Immunization status with respect to time between vaccination and virus exposition: (a) low viral impact a short time after vaccination, meaning successful protection by vaccination, small additional stimulation of immune system by the viral impact; (b) high viral impact a short time after vaccination, meaning successful protection by vaccination, considerable additional stimulation of immune system by the viral impact; (c) strong reaction after a longer time between vaccination and virus exposition, but survived illness; (d) non-sufficient defense reaction when there is a long time between vaccination and virus exposition, resulting in serious illness. The same parameter set was used for all four simulations (a–d): kvir = 0.3; kr = 0.12; kst = 0.12; Dvacc = 0.05; DAB = 0.05; cBGim = 0.5; voo = 30; stoo = 10.
Besides the time since vaccination, the reaction rate between the virus and the immune system kr is important for the estimation of the seriousness of a massive viral exposition. The reaction rate is probably dependent on the individual immune system on the one hand and the properties of the antigen (virus) on the other hand. The simulation shows a low reaction in the case of a high infection rate (Figure 2a). Even, the re-activation of the immune system is low under these conditions. Much higher risks of illness and heavier symptoms have to be expected in the case of lower reaction rates (Figure 2b,c). However, such a high reaction is connected with stronger activation of the immune system, resulting in higher protection after the disease has passed by.
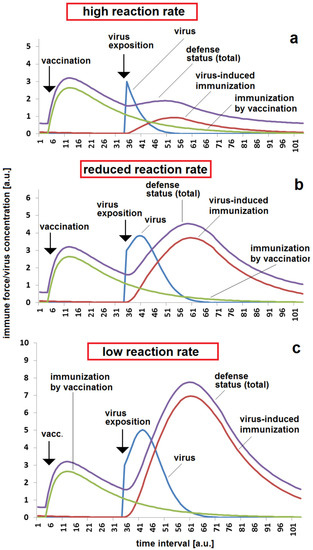
Figure 2.
Immunization status, reaction and re-activation by infection with respect to reaction rate in case of a moderate time span between vaccination and virus exposition: (a) low immune response in the case of a high reaction rate kr = 0.2; (b) enhanced response in the case of a moderate reaction rate, kr = 0.06; (c) high response to virus exposition and strong additional immune response in the case of a low reaction rate, kr = 0.03. The set of other parameters for all three simulations (a–c): kvir = 0.3; kst = 0.12; Dvacc = 0.05; DAB = 0.05; cBGim = 0.5; voo = 30; stoo = 10.
A very important aspect is the viral replication rate kvir. This parameter is a specific property of a virus with a certain molecular furnishing. It is dependent on variations in the virus’s genome and can vary between different mutants. It is easy for a virus-exposed body to overcome the virus if its replication rate is low (Figure 3a). A slightly enhanced virus replication rate might cause the appearance of symptoms. A fast breakthrough and, therefore, a heavy infection with serious risks has to be expected in case of fast viral replication (Figure 3c).
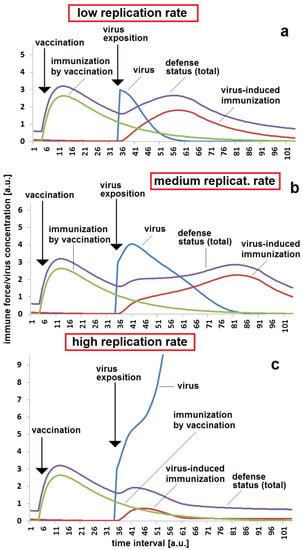
Figure 3.
Immunization status, reaction and breakthrough of infection with respect to the virus replication rate in the case of a moderate time span between vaccination and virus exposition: (a) sufficient protection in the case of a low virus replication rate, kvir = 0.3; (b) stronger reaction and re-activation of immune response in the case of a medium replication rate, kvir = 0.5; (c) non-sufficient protection in the case of a high virus replication rate, kvir = 0.06. The set of other parameters for all three simulations (a–c): kr = 0.12; kst = 0.12; Dvacc = 0.05; DAB = 0.05; cBGim = 0.5; voo = 30; stoo = 10.
From the side of a concerned body, the ability to react to a virus impact by activation of a specific immune response is another important factor for estimating the risk of a breakthrough in a certain temporal distance after vaccination. At a higher stimulation rate kst, the attack caused by a massive viral exposition can be fended off, even in the case where the protective effect of a vaccination is reduced by antibody decay after a certain time (Figure 4a). In contrast, the virus can easily breakthrough if the stimulation rate is too low and the specific re-activation of the immune response is slower than the virus replication (Figure 4b).
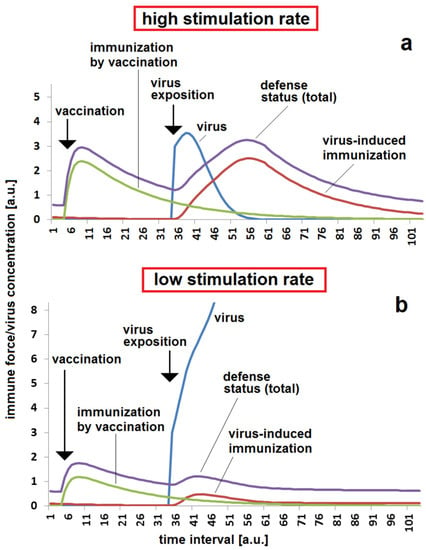
Figure 4.
Immunization status, reaction and breakthrough of infection with respect to the stimulation of a specific immune response by virus exposition in the case of a moderate time span between vaccination and virus exposition: (a) sufficient protection by a combination of vaccination and strong stimulation of immune response by viral infection, kst = 0.12; (b) non-sufficient protection due to decay of immunization after vaccination and low stimulation rate of immune response by viruses, kst = 0.06. The set of other parameters for both simulations (a,b): kr = 0.12; kvir = 0.3; Dvacc = 0.05; DAB = 0.05; cBGim = 0.5; voo = 30; stoo = 10.
3.2. Secondary Vaccination and Immunity by Infection
It is well known that the risk of re-infection can be drastically lowered by a second or third vaccination. The second vaccination has to be applied before the concentration of specific antibodies falls below a critical concentration. If the second vaccination is timely, than the outbreak of disease after a massive viral impact can be avoided (Figure 5a). If this second vaccination is missed, there is a high risk that a massive viral exposition cannot be controlled by the immune system and a serious illness results (Figure 5b). Under the same conditions, the outbreak of disease can also be avoided if the stimulation effect of a virus on the specific immune response is strong (Figure 5c). The higher stimulation rate kst enhances the effect of the first and second vaccinations as well as the reaction when the virus attacks the body. It is to kept in mind that the second or third vaccination probably does not protect the body forever. The immune protection also decays after the second or third immunization. An earlier infection can be fended off (Figure 6a), but a later massive viral exposition can cause a serious illness (Figure 6b).
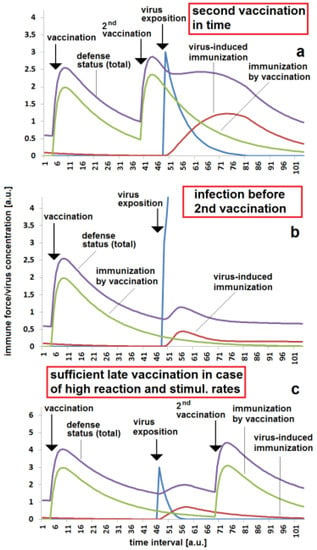
Figure 5.
Importance of second immunization date with respect to reaction and stimulation rate: (a) safe protection due to second vaccination being given in time, kr = 0.1 and kst = 0.05; (b) disease breakthrough in the case of a low virus-induced immunization effect and second vaccination not being given in time, kr = 0.1 and kst = 0.05; (c) sufficient late vaccination due to higher reaction and stimulation rates, kr = 0.3 and kst = 0.15. The set of other parameters for the simulations (a–c): kvir = 0.3; Dvacc = 0.05; DAB = 0.05; cBGim = 0.5; voo = 30; stoo = 10.
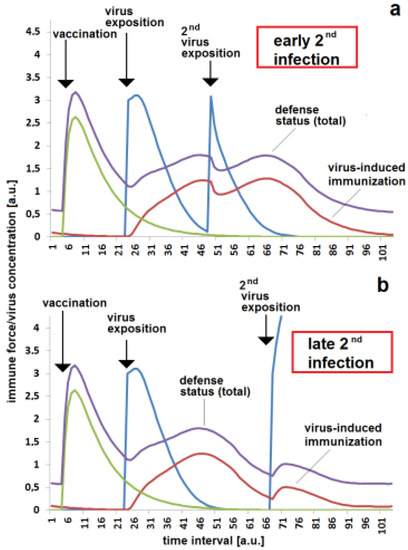
Figure 6.
Parameter-dependent requirement of third immunization—either by virus contact or by vaccination— given in time: (a) successful defense of infection shortly after second activation of immune response by virus exposition; (b) disease breakthrough after first immunization by vaccination and re-activation of immune response by a virus exposition, resulting in a fended-off infection due to missing third immunization by vaccination. The same set of parameters were used for all simulations (a,b): kvir = 0.3; kr = 0.2; kst = 0.15; Dvacc = 0.4; DAB = 0.1; cBGim = 0.5; voo = 30; stoo = 10.
3.3. Role of Vaccine Decay Rate
A special situation for kinetics in immunization and infection mechanisms is present for the m-RNA and related vector vaccinations. This type of vaccination is marked by two main decay mechanisms. On the one hand, the concentration of produced antibodies decreases over time, such as in the case of conventional vector immunization. On the other hand, the primary vaccine causing the production of immune-stimulating virus proteins is also decaying. This process is much faster than the antibody decay leading to a drastic reduction in the efficacy of the primary vaccine in only a few days or some hours. A high degradation rate Dvacc means that a lower concentration of stimulating virus protein is produced, which results in a low efficiency of vaccination (Figure 7a). The vaccination effect is much higher when the decay is slower. Then, the body has more time for producing the immune-stimulating virus protein and obtains, therefore, a higher total of immune stimulation, resulting into higher production of specific antibodies. This supplies a high protection for a later viral attack (Figure 7b). Individual differences in the degradation rate of the vaccine could have an important impact on a later breakthrough infection (Figure 7c).
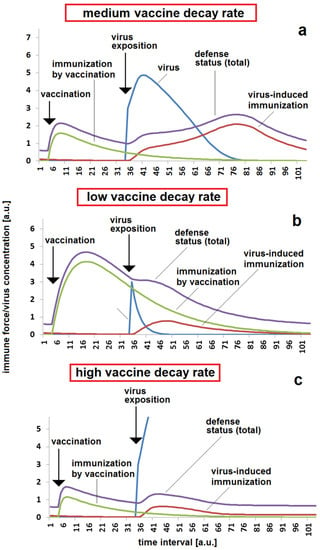
Figure 7.
Effect of vaccine decay rate in the response to a massive viral impact after a moderate time between vaccination and virus exposition: (a) moderate protection by vaccination, meaning a significant reaction, but there is protection against a heavy illness in the case of a moderate vaccine decay rate, Dvacc = 0.4; (b) safe protection and low reaction to virus exposition in the case of a low vaccine decay rate, Dvacc = 0.1; (c) low protection by vaccination due to fast vaccine decay rate resulting in breakthrough in the case of a viral exposition, Dvacc = 0.6. The same set of other parameters were used for all simulations (a–c): kvir = 0.3; kr = 0.12; kst = 0.08; DAB = 0.05; cBGim = 0.5; voo = 30; stoo = 10.
3.4. Unspecific Background Immunity
Besides the specific immune defense, the contribution of non-specific immune forces and immune forces of lower specificity are important for the reaction of a body against a viral impact. It can be assumed that expositions against other viruses, in particular viruses belonging to the same virus class, could be accompanied by the production and availability of antibodies with a reduced specificity. They contribute, together, to the general defense mechanism of “background immunity”. In the illustrated model presented here, it is assumed that this background immunity simply supplies an additive contribution. Figure 8 demonstrates the importance of this contribution. After a certain decay of specific immunization, the background immunity ensures that the critical threshold for a defense against virus attack is achieved (Figure 8a). An infection at the same time after immunization, and with the same other kinetic parameters, results in a breakthrough infection (Figure 8b).
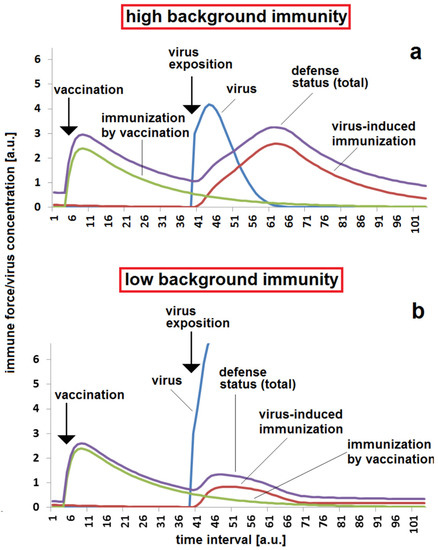
Figure 8.
Effect of less specific and unspecific background immunity: (a) sufficient protection after a moderate time span between vaccination and virus exposition in the case of moderate unspecific background immunity cBGim = 0.5; (b) infection breakthrough in the case of low background immunity, cBGim = 0.15. The same set of other parameters were used for all simulations (a,b): kvir = 0.3; kr = 0.12; kst = 0.08; DAB = 0.05; Dvacc = 0.4; voo = 30; stoo = 10.
Whereas the concentration of antibodies in the blood can be regarded as a constant parameter (at small time scales) or as a monotonously decreasing parameter (at longer time scales), components of non-specific defense forces might vary with different amplitudes and frequencies over time. These changes can be due to local hyperthermia, physical exhaustion or to additional impacts of other viruses or bacteria, for example. The variations in the resulting total of defense forces are particularly critical if a massive virus exposition takes place in a certain phase of decay of vaccination (Figure 9a). In the lucky case, of a period with more positive elongations of non-specific background immune forces, the viral impact can be fended off quickly, on the one side. However, the same kinetic parameters and the same noise in fluctuations of background immunity can result in a breakthrough infection if the fluctuation drives the immune system in the wrong direction during the critical phase of viral impact (Figure 9c). These critical effects of fluctuation of background immunity should be taken into account in a decision regarding the proper time for a second or third vaccination.
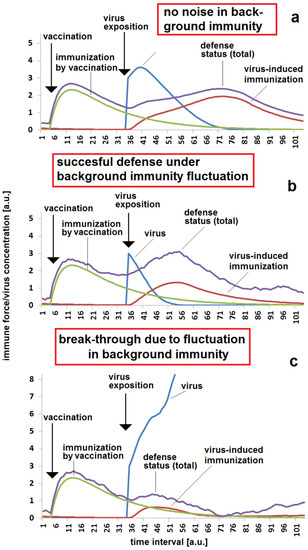
Figure 9.
Effect of fluctuations in unspecific background immunity with different amplitudes: (a) sufficient protection for a certain time after vaccination in the case of very low or no fluctuations in background immunity, noise = 0; (b) successful defense against infection in the case of a higher fluctuation amplitude, but with a virus exposition in a phase of high unspecific background immunity; (c) breakthrough infection in the case of a higher fluctuation amplitude, but with a virus exposition in a critical phase of low unspecific background immunity. (b,c) have the same noise = 0.2 and the same set of other parameters were used for all simulations (a–c): kvir = 0.3; kr = 0.12; kst = 0.07; DAB = 0.05; Dvacc = 0.4; cBGim = 0.3; voo = 30; stoo = 10.
4. Conclusions
The results of the simple simulations shown above are able to reflect, qualitatively, the presence of gradual and time-dependent immune protection and the possibility of a gradual immune response with respect to a small set of kinetic parameters. It gives hints as to the importance of individual differences in the robustness and responsivity of the immune defense. The individual properties and state of the target body after immunization, as well as virus properties, have to be considered in assumptions about the probability of outbreak of disease after exposure to a virus. Quantitative factors, such as the replication rate of viruses, its stimulation effect on the immune system, the reaction rate between viruses and antibodies as well as the decay rates of the primary applied vaccines and the antibodies, play a role in the competition between the immune system and viruses after infection. In principle, the discussed factors are also relevant to other diseases besides COVID-19. However, the importance of single factors being strongly dependent on the type of virus has to be considered. Thus, the decay of antibodies, and the overall immune response, is very different for different diseases, for example.
In addition, the background status of the body and the situation-dependent fluctuations in “background immunity” can play a significant role for the reliable protection by vaccination or by an earlier passing of the disease. The spectrum of individual responses to vaccination, and on virus exposition, is an important boundary condition for the simulation of the development of epidemics. The modeling of the spread of infection and achieving herd immunity has to consider the individual situations for infection pathways and the very different individual infection-susceptibility statuses and their temporal changes. It is necessary to respect the very different individual health conditions and the very different situations in schools and companies, nursing homes and restaurants. Individual factors strongly modulate the probability of transfer of infection in the case of contacts and of the probability of infection in the case of decaying protection after a survived illness or vaccination. All these effects have to be taken into account in decisions on strategies for re-vaccination and for achieving and sustaining herd immunity.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/app12010031/s1, Table S1: Example for a model calculation by an Excel table.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
We would like to thank Stefan Wölfl, Heidelberg for very helpful discussions and advices.
Conflicts of Interest
The author declares no conflict of interest.
References
- Fine, P.; Eames, K.; Heymann, D.L. Herd immunity: A rough guide. Clin. Infect. Dis. 2011, 52, 911–916. [Google Scholar] [CrossRef] [PubMed]
- Daley, D.J.; Gani, J. Epidemic Modeling: An Introduction; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Brauer, F.; Castillo-Chávez, C. Mathematical Models in Population Biology and Epidemiology; Springer: Berlin/Heidelberg, Germany, 2001. [Google Scholar]
- Fonseca I Casa, P.; Garcia I Carrasco, V.; Garcia I Subirana, J. SEIRD COVID-19 formal characterization and model comparison validation. Appl. Sci. 2020, 10, 5162. [Google Scholar] [CrossRef]
- Webb, G. A COVID-19 epidemic model predicting the effectiveness of vaccination in the US. Infect. Dis. Rep. 2021, 13, 654–667. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, Y.; Alqahtani, A.; Albalawi, O.; Bakouri, M. Epidemological modeling of COVID-19 in Saudi Arabia: Spread projection, awareness, and impact of treatment. Appl. Sci. 2020, 10, 5895. [Google Scholar] [CrossRef]
- Trivedi, A.; Sreenivas, N.K.; Rao, S. Modeling the spread and control of COVID-19. Systems 2021, 9, 53. [Google Scholar] [CrossRef]
- Reyes-Silveyra, J.; Mikler, A.R. Modeling immune response and its effect on infectious disease outbreak dynamics. Theor. Biol. Med. Model. 2016, 13, 10. [Google Scholar] [CrossRef] [Green Version]
- Nag, D.S.; Chaudry, R.; Mishra, M.; Rai, S.; Gupta, M. A prospective on rapid declining SARS-Cob-2 IgG antibodies within one to three month of testing IgG positive: Can it lead to potential reinfections? Cureus 2020, 12, e11845. [Google Scholar] [PubMed]
- Ivanov, A.; Semenova, E. Long-term monitoring of the development and extinctionof IgA and IgG responses to SARS-cov-2 infection. J. Med. Virol. 2021, 93, 5953–5960. [Google Scholar] [CrossRef] [PubMed]
- Zamir, M.; Nadeem, F.; Abdeljawad, T.; Hammouch, Z. Threshold condition and non-pharmaceutical interventions’s control strategies for elimination of COVID-19. Results Phys. 2021, 20, 103698. [Google Scholar] [CrossRef]
- Biswas, M.H.A.; Islam, M.A.; Akter, S.; Mandal, S.; Khatum, M.S.; Samad, S.A.; Paul, A.K.; Khatum, M.R. Modelling the effect of self-immunity and the impacts of asymptomatic and symptomatic individuals on COVID-19 outbreak. CMES 2020, 125, 1033–1060. [Google Scholar] [CrossRef]
- Tomochi, M.; Kono, M. A mathematical model for COVID-19 pandemic-SIIR model: Effects of asymptomaic individuals. J. Gen. Fam. Med. 2021, 22, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Lipsitch, M.; Dean, N.E. Understanding COVID-19 vaccine efficacy. Science 2020, 370, 763–765. [Google Scholar] [CrossRef] [PubMed]
- Swan, D.A.; Goyal, A.; Bracis, C.; Moore, M.; Krantz, E.; Brown, E.; Cardozo-Ojeda, F.; Reeves, D.B.; Gao, F.; Gilbert, P.B.; et al. Mathematical moceling of vaccines that prevent SARS-CoV-2 Transmission. Viruses 2021, 13, 1921. [Google Scholar] [CrossRef] [PubMed]
- MacIntyre, C.A.; Costantino, V.; Trent, M. Modelling of COVID-19 vaccination strategies and herd immunity, in scenarios of limited and full vaccine supply in NSW, Australia. Vaccine 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Bauch, C.T.; Bhattacharyya, S.; D’Onofrio, A.; Manfredi, P.; Perc, M.; Perra, N.; Salathé, M.; Zhao, D. Statistical physics of vaccination. Phys. Rep. 2016, 664, 1–113. [Google Scholar] [CrossRef]
- Großmann, G.; Backenköhler, M.; Wolf, V. Heterogeneity matters: Contact structure and individual variation shape epidemic dynamics. PLoS ONE 2021, 16, e0250050. [Google Scholar] [CrossRef] [PubMed]
- Rzymski, P.; Camargo, C.A., Jr.; Fal, A.; Flisiak, R.; Gwenzi, W.; Kelishadi, R.; Leemans, A.; Nieto, J.J.; Ozen, A.; Perc, M.; et al. COVID-19 Vaccine Boosters: The good, the bad, and the ugly. Vaccines 2021, 9, 1299. [Google Scholar] [CrossRef] [PubMed]
- Köhler, J.M. Infection-immunity competition: A simple model for illustrating the background of individual response on herd immunity. Appl. Sci. 2020, 10, 3078. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).