In reality, the action of the PDT against
H. pylori was tested by Bedwell as early as 1990 [
12]. Further experiments, aimed at detecting the action of different PS, reported interesting results following the application of Protoporphyrin IX in the treatment of
H. pylori [
13]. The determination of the ability, by various strains of
H. pylori, to spontaneously synthesize porphyrins was the real step forward in considering PDT as an alternative treatment to the antibiotic. The bacterium, cultured in an aqueous medium, has, in fact, shown the ability to synthesize a quantity of protoporphyrins IX and coprorphyrins sufficient to kill even the most virulent and antibiotic resistant species, simply by using visual light. All strains involved in the experiment showed 99.9% eradication following irradiation at 20 J/cm
2 with a wavelength of 405 nm [
14]. The absorption spectrum of the bacterium’s endogenous porphyrins varies in relation to the relative concentration between PPIX (protoporphyrin IX), and CP (coproporphyrin I). However, this spectrum is characterized by a maximum absorption peak around 400 nm followed by a second peak, approximately 10 times lower in intensity, located between 500 nm and 650 nm. Porphyrin extracted from various strains of
H. pylori shows, in fact, the same fluorescence spectrum as commercial porphyrin [
6].
Lembo and co. created a device capable of supplying 12 W to the entire surface of the stomach with a wavelength of 408 nm. The comparison between the samples taken before and after the treatment shows a significantly different bacterial load in the antrum and in the body, but not in the fundus of the stomach. The long-term results of the urea breath test show no changes following treatment, indicating the failure of the proposed therapy. One result could be derived from the presence of bacterial colonies undermined in depth in the wrinkles or in the crypts of the mucosa, difficult to reach by the wavelength used, regardless of the duration of irradiation. The result also indicates that a small residual bacterial fraction is enough to re-establish the colonization of H. pylori.
2.1. Importance of Actuation
The pre-industrial prototype created due to dimensional and, consequently, energy limitations, manages to integrate only 8 LEDs at 625 nm, ending up delivering an average dose of 12 J/cm
2 compared to the 16 J/cm
2 necessary for eradication of the bacterium with red light [
11]. The device is shown in
Figure 1. In order to deliver the minimum dose necessary for eradication, it is therefore necessary to ingest two capsules in the case of a collapsed stomach and 11 capsules in the case of a distended stomach. The LEDs at 405 nm, on the other hand, although we have shown their efficiency to be similar to red LEDs, they were integrated only into an experimental prototype powered by batteries not certified for clinical use. Theoretical calculations indicate that three capsules are needed in the case of a collapsed stomach and ten with a distended stomach (capsules of 8 LEDs at 405 nm to reach a total of 9 J/cm
2 necessary for the eradication of the bacterium with blue light) [
17].
Given the limitation of the current device, the aim of this paper is the introduction of an actuation mechanism for improving the overall performance of the device. The realization of the implementation of a capsule can follow two approaches defined, respectively, as internal locomotion and external locomotion. In internal locomotion systems, an attempt is made to integrate a miniaturized locomotion system inside the capsule, while external locomotion is generally entrusted to sources of magnetic fields. There are several approaches to building an internal locomotion system [
18]. Tortora and co. developed an interesting actuation system based on the integration of four miniaturized propellers, placed on the back of the capsule, independently actuated by means of DC brushed motors [
19]. The performance in the capsule was improved with the work by De Falco and co. [
20], which, by integrating a vision system and a wireless transmission system, created an advanced version of the same capsule. The simultaneous powering of the motors and the vision system, however, provides the capsule with only 13 min of autonomy. Several endoscopic capsules integrate bio-inspired locomotion systems. The locomotion system devised by Kim and co., for example, is inspired by the movement of the earthworm [
21]. The implementation of the capsule derives from the compression and elongation cycles of an SMA spring in combination with an anchoring system with directional micro needles. However, the power required by the system is too high to be delivered by means of batteries that can be dimensionally integrated inside the capsule [
22]. Not even the development of a special piezoelectric spring actuator, in a second version of the capsule, solved the problem related to the power supply [
22].
The capsule made by Li and co. inspired by the movement of the cilia of the gastric mucosa. The device consists of six cilia-like units, each of which is connected to two SMA actuators to ensure bi-directional movement. The capsule does not integrate an on-board power system. It is powered by cable [
23].
Park and co. proposed a solution inspired by the movement of canoes [
24]. The capsule is equipped with six legs activated simultaneously by a single step motor associated with a screw, in order to guarantee linearity of the movement. Glass and co. created a three-legged capsule inspired by the locomotion of geckos and cockroaches [
25]. The system is able to withstand peristaltic loads, allowing the capsule to remain anchored to the intestinal wall. The system based on three SMA legs with strain gage was proposed by Tognarelli and co. for anchoring the capsule in the esophageal tract [
26].
At the biorobotics institute of the Sant’Anna School, several capsules with legs were developed [
18]. However, both the capsule with four superelastic legs connected to a single DC motor and the one with eight legs, organized in two groups of four implemented separately, need to be powered by cable [
27,
28].
Valdastri and co. created a prototype with 12 legs capable of improving the distension of the lumen and, probably, anchoring in the event of reflux. In the experimental phase, the capsule was fed by wire [
29].
Another example of internal locomotion is presented by a progression of the capsule obtained by means of a series of vibrations [
30]. The system is very simple but does not allow directional changes, capsule anchoring or active orientation control.
2.2. Overall Characteristics of the Device
The work is aimed at the design and construction of a device capable of providing an attractive, alternative and universally applicable approach for the eradication of H. pylori.
It was established that an acceptable therapeutic solution must exhibit an eradication rate of no less than 90%. Some pharmacological therapies traditionally used against H. pylori infection show a failure rate of 32% and the ineffectiveness is clearly increasing due to the progressive increase in antibiotic resistance. The latest therapies based on bismuth and non-bismuth-based quadruple therapy administered for 14 days have > 90% eradication rate. However, they are still based on a pure drug approach.
The recent literature shows preliminary experimental tests that show the good photo-killing capacity of H. pylori by specific wavelengths in the visible range. The bacterium, in fact, has endogenous porphyrins that allow the administration of photodynamic therapy without the need to introduce exogenous photosensitizers. The application of PDT to H. pylori, therefore, simply translates into the illumination of the bacterium with appropriate wavelengths.
The thesis developed a hypothetical approach for photodynamic therapy against H. pylori administered via a spun capsule.
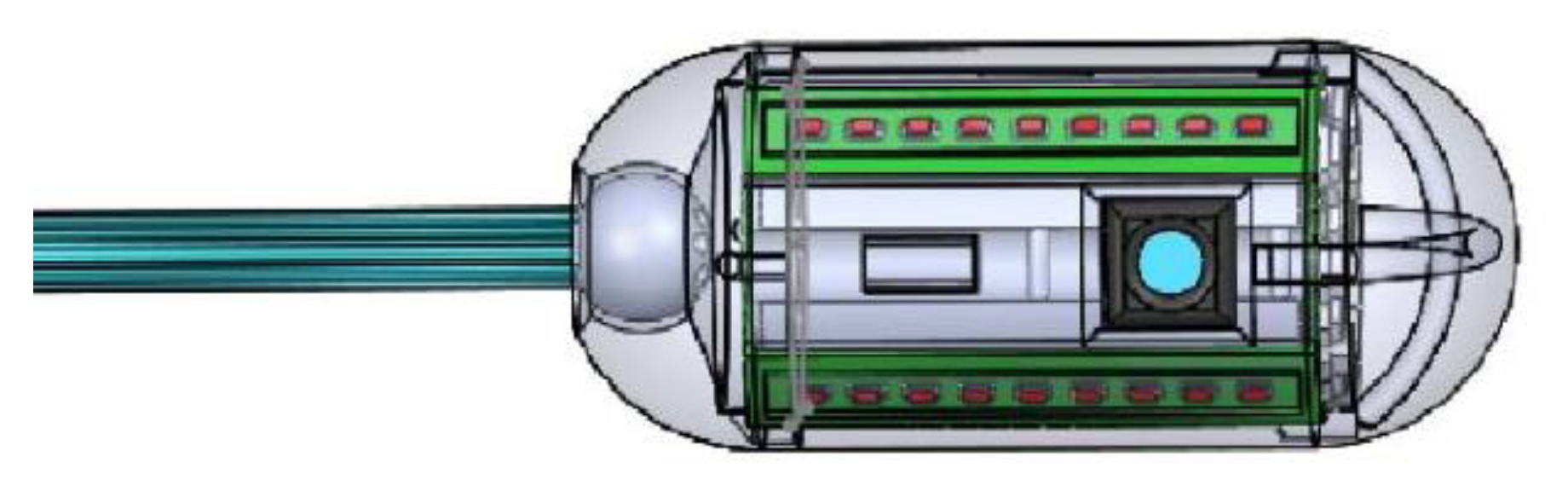
The device has the objective of carrying out an effective and targeted therapeutic treatment. In fact, the aim is to focus the administration only in the areas affected by the bacterium in order to maximize therapeutic efficiency, reducing unnecessary energy dissipation and minimizing the overheating of healthy tissues. The device, therefore, consists of the integration of two distinct but complementary modules that work synergistically to achieve the set goal.
The therapeutic module, as the name itself suggests, is aimed at administering the actual therapeutic treatment. It is therefore based on the release of light energy at appropriate wavelengths, considered optimal for the eradication of the bacterium. Given the typical dimensional constraints associated with the creation of a capsule for the gastrointestinal tract, only LED energy sources were considered and integrated. In fact, thanks also to the developments in microelectronics, the market offers small-sized LEDs capable of delivering high power, which are ideal for the application in question. The module can be powered by batteries approved for clinical use that can be integrated inside the capsule. It was, in fact, conceived and designed as a energetically and functionally autonomous system, which can be integrated and used without the need for additional components. It could, in fact, be considered the realization of a therapeutic capsule constituted solely by the therapeutic module in question. This capsule would still be able to administer PDT against H. pylori but would be characterized by a low energy content and passive locomotion by peristalsis. Basically, you would have a capsule with little energy randomly released inside the stomach. The device, therefore, would be inefficient and, with great probability, ineffective.
The active locomotion module has the objective of focusing the treatment in the area of interest, maximizing its efficiency. It is based on the combined action of vision and actuation, which guarantees movement toward the target area after having visually identified it.
The implementation allows the active movement of the capsule, allowing it to move in a targeted manner. Considering the various problems related to the different implementation proposals for capsules and the limitations presented by the therapeutic module, deriving from battery power, a cable actuation mechanism is proposed that does not require additional energy requirements. The mechanism introduces a minimal invasiveness in the treatment, which is amply compensated by the increase in efficiency.
The introduction of a cannula, functional to the actuation mechanism, justifies the integration of a commercial video camera with wire. The camera, being fully integrated into the active locomotion system, does not increase the invasiveness of the device but certainly increases its efficiency, allowing the identification of the target area.
The components aimed at carrying out the implementation and the camera are harmoniously integrated within the dimensions, 11 mm in diameter by 26 mm in length, of the capsule.
However, it must be emphasized that the active locomotion system, like the therapeutic module, is capable of operating autonomously. It could, in fact, be implemented separately within a device that performs screening or diagnostic tests.
The two modules used separately would, however, give rise to inefficient devices and, probably, poor functionality. Only the combined action of the two allows for good performance and a targeted treatment of desirable effectiveness.
2.2.1. Therapeutic Module
The therapeutic module is presented as the main component of the capsule to eradicate the bacterium. As the name itself suggests, in fact, it is the functional module for administering the treatment of PDT. To ensure effective photodynamic therapy, careful selection of the wavelengths that are optimal for photo-killing is essential.
The most effective wavelengths, at least intuitively, should be those at which the endogenous porphyrins of H. pylori show increased sensitivity.
The number of ROS generated, in fact, can be considered, as a first approximation, directly proportional to the quantity of photons absorbed by the PS.
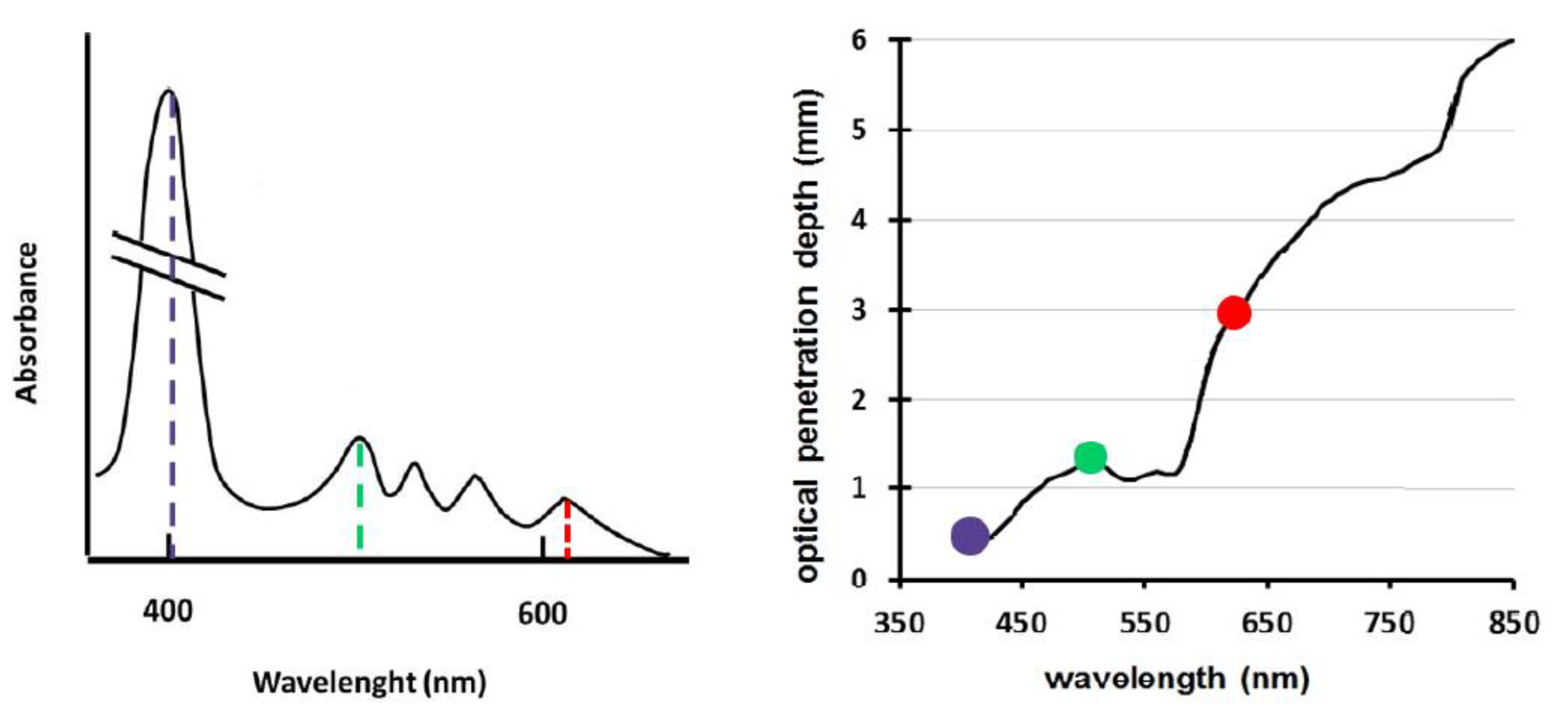
By analyzing the absorption spectrum of the endogenous porphyrins of
H. pylori, there is an important peak around 400–410 nm. The spectrum reveals, in reality, other peaks in the visible range, which, however, are clearly lower than the main one and less and less important proceeding in the direction of the infrared. For this reason, it would seem that the most effective wavelength for photo-killing is in the violet range. However, the first therapeutic solutions based on the use of this wavelength have found a high rate of long-term reinfection. Although, in fact, the eradication rate manifested immediately after the administration of the treatment was 99%, the results of the breath test revealed the reappearance of the infection already after 5 days [
11]. Additionally, taking into account the 99.9% eradication detected by in vitro experimentation, it is assumed that the reappearance of the infection is due to the rapid proliferation of bacterial colonies nested in the innermost layers of the gastric mucosa that cannot be reached by photons at 400 nm.
In fact, if the penetration depth of visible light inside the gastric mucosa is also taken into consideration, the graph on the right in
Figure 2, it can be seen that wavelengths around 400 nm have low penetrating power. Penetration is optimal in the red range. A small peak of penetrating power is also recorded near 500 nm.
Comparing the two graphs in
Figure 2, it is clear that photons at 400 nm are ideal for the excitation of PS but not very suitable for penetrating into tissues. On the other hand, in the red range, characterized by maximum penetrating power within visible light, the absorption spectrum of the porphyrins endogenous to
H. pylori has a small spike close to 630 nm. It could, therefore, be assumed that the wavelength of 630 nm, combining the high penetration into the mucosa with the discrete ability to be absorbed by the PS, could somehow compensate for the inefficient penetrating capacity of blue, optimizing the outcome of the therapy.
In the same vein, the wavelength of 500 nm attracted attention. At 500 nm, in fact, there is an average penetrating power inside the mucosa and a medium absorption capacity by porphyrins. The combination of the two actions of medium intensity could have an even better effect than that of red and blue, which tend to excel in one aspect but are mediocre in the other.
By virtue of the provisions and also taking into account the results obtained in the simulation of Romano et al. on the effectiveness of photo-killing, it was decided to integrate three wavelengths into the therapeutic module: 405 nm, 630 nm and 500 nm.
Having to administer a therapeutic treatment in addition to selecting the wavelengths, it is also essential to consider the emission of the light source. The energy released by the therapeutic module per unit of surface in the unit of time, otherwise identified as a dose, must be suitable for the eradication of the bacterium. It would be preferable to be able to carry out the treatment in the shortest possible time in order to provide an efficient therapy without reducing effectiveness.
The purposes of the therapeutic module in fact require great attention in the selection of an appropriate light source.
To verify the photo-killing capacity of the selected wavelengths and determine the amount of dose necessary to obtain a possible therapeutic eradication rate, a special experimental setup was developed.
2.2.2. Experimental Setup for Preliminary Evaluation of the Effectiveness of the Chosen Wavelengths on the Eradication of H. pylori
The design of the experimental setup is aimed at determining the effectiveness of the chosen wavelengths on the eradication of H. pylori. It basically looks like a device that allows the lighting of the bacterium and is capable of varying the light energy emitted by variations in the duration and/or intensity of the emission.
We tried to create a solid and reliable tool that guarantees high reproducibility of the tests in order to obtain results that are valid not only for the work in question, but for the scientific community in general.
Conventional microbiology bags placed inside an incubator at 37 °C were used to carry out the tests.
Being that H. pylori is a microaerophilic bacterium, it is necessary that the tests are carried out in a controlled environment. To recreate optimal experimental conditions, the typical bags for microbiological experimentation are used, placed inside an incubator at 37 °C.
Therefore, it is also necessary that the resulting device, the illuminator for bacterial culture, is entirely contained within the 33 cm length and 15 cm width of the bag used. It is essential to introduce a battery power supply, as it does not have the ability to introduce cables inside the bag. The energy supplied by the battery must be released in a relatively short time. The time of experimental interest is set at 1 h and represents the maximum established duration of the experiment, that is, of the lighting. This is quite a short time, compared to the 3.5 h it takes for the bacterium to replicate. It is important that the emitted power remains constant throughout the duration of the experiment and that it is almost entirely transferred to the bacterium.
To allow the setup to work optimally, the components it consists of must be carefully selected. The experimental setup, having to test the photo-killing effect of the various wavelengths, must be adequate to ensure homogeneous and uniform illumination of the Petri dish, avoiding the interaction of the light emission with other components before reaching the bacteria. To achieve the purpose, it is therefore essential to use a photo-absorbent material to create the support in order to avoid scattering, reflection of light or other phenomena that could generate indirect illumination of
H. pylori. To achieve the purpose, the device was built according to a modular architecture, shown in
Figure 3.
The fundamental component is certainly the electronic board. The electronic board is in fact the component that provides the lighting, adapting it to the experimental needs. It is good to carefully consider the electronic components used in order to reuse the therapeutic module of the capsule in the electronics.
2.2.3. Manufacturing of the Illuminator Boards
The configurations used for the last three types of LEDs described above can be superimposed both with respect to the position and the very similar size of the LEDs.
It is, therefore, possible to create all the electronic boards you need by standardizing them to only two schematic types, called Type A and Type B, shown in the following figure.
To allow adequate light output, in addition to the careful selection and reasoned positioning of the LEDs, the use of electronic components capable of programming and modulating the operation of the LEDs and ensuring the stability of the power supply during the experiments is essential. While the upper board is dedicated to the location of the light source, the lower board houses the electronic components aimed at ensuring reliable and repeatable delivery. The lower board, in fact, can be considered, at least structurally, identical for all LED types. All the boards all shown in
Figure 4.
2.2.4. Outcomes from Preliminary Testing of the Illumination Module
The illumination module was extensively tested in previous articles [
7,
8,
9]. The effectiveness of the illumination module was already demonstrated and reported here for the sake of clarity. However, basing on the scientific outcomes, it was calculated the number of capsules a patient should swallow for achieving complete eradication.
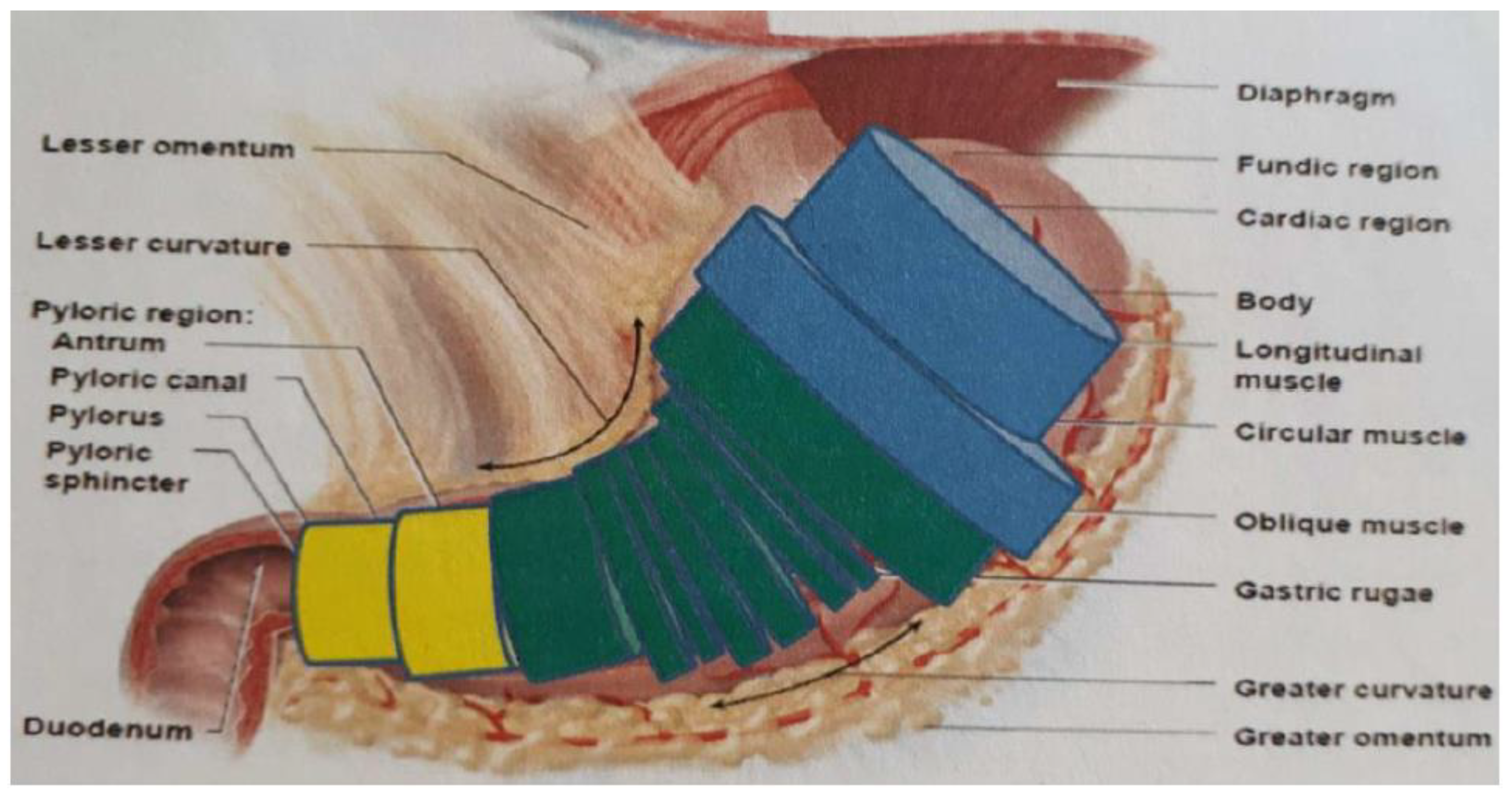
If a capsule with the calculated light output was static, the time required for the delivery of a therapeutic dose could be determined by carrying out a simple modeling of the regions most affected by
H. pylori within the stomach. This was calculated with the aim of a simplified model of the stomach, approximated by means of a series of equivalent cylinders (
Figure 5).
For the purposes of this analysis, it is advantageous and plausible to further approximate the model series in overall three cylinders, which respectively represent the equivalent volume of the bottom, the body and the antrum. Considering that the volume of the slightly distended stomach is around 0.5 L and that, from the literature, it is indicated that the volumes occupied by the antrum and the body are, respectively, 20% and 65% of the total, it can be considered a volume of the cave equal to 100 cm3 and that of the body equal to 325 cm3. The antrum generally has a diameter of 1–2 cm, while the diameter of the body, in conditions of slight expansion, can reach 6 cm. Taking as a reference a diameter of 2 cm for the cave and 6 cm for the body, a surface to be treated of 100 cm2 and 325 cm2, respectively, are obtained. The average power density transmitted by the capsule is therefore equal to 3.15 mW/cm2 in the cave and 1.49 mW/cm2 in the body. As no experimental data are available on the effective dose at 500 nm and with different wavelengths in the capsule, we are in an intermediate situation between the efficacy of red and that of blue, considering as a reference a therapeutic dose of 18 J/cm2. The eradication, according to the theoretical calculations, would be obtained in 1.5 h in the cavern and in 3.3 h in the body, a value not compatible with the power in one single swallowable capsule.
The capsule conceived, however, having an edge with an active locomotion system, aims to focus the treatment exclusively on the areas affected by the bacterium. It therefore offers the great advantage of considerably increasing the local power times by greatly reducing treatment. Treatment times could be reduced by creating a future version of the therapy module which, by presenting the cable power, integrates equally small but more powerful LEDs. The use of an active intelligent locomotion module will allow to reduce the invasiveness of the approach. Although the single capsule can be still considered not invasive, ingesting more than one capsule can result in additional risks and an impact on the environment. In fact, although a recovery of the capsule was foreseen, the fact that the patient recovers the capsule after expulsion cannot be guaranteed.
2.3. Design of the Therapeutic Module of the Intelligent Capsule
We set ourselves the goal of creating a capsule capable of releasing the illumination uniformly in order to maintain a constant amount of light energy delivered to the unit of surface in the unit of time. In fact, an attempt is made to design a device with reliable behavior that does not add to the subjectivity variability of each individual uncertainty linked to variations in the performance or operation of the device.
The uniformity of the lighting is obtained by distributing the chosen LEDs as evenly as possible and by adjusting the lighting parameters using appropriate electronic components. An attempt is made to evenly distribute the selected types of LEDs along the perimeter of the capsule. Stringent dimensional constraints, linked above all to the size of the battery power supply and the characteristics of the casing, require that the surface of the capsule is not entirely covered with LEDs.
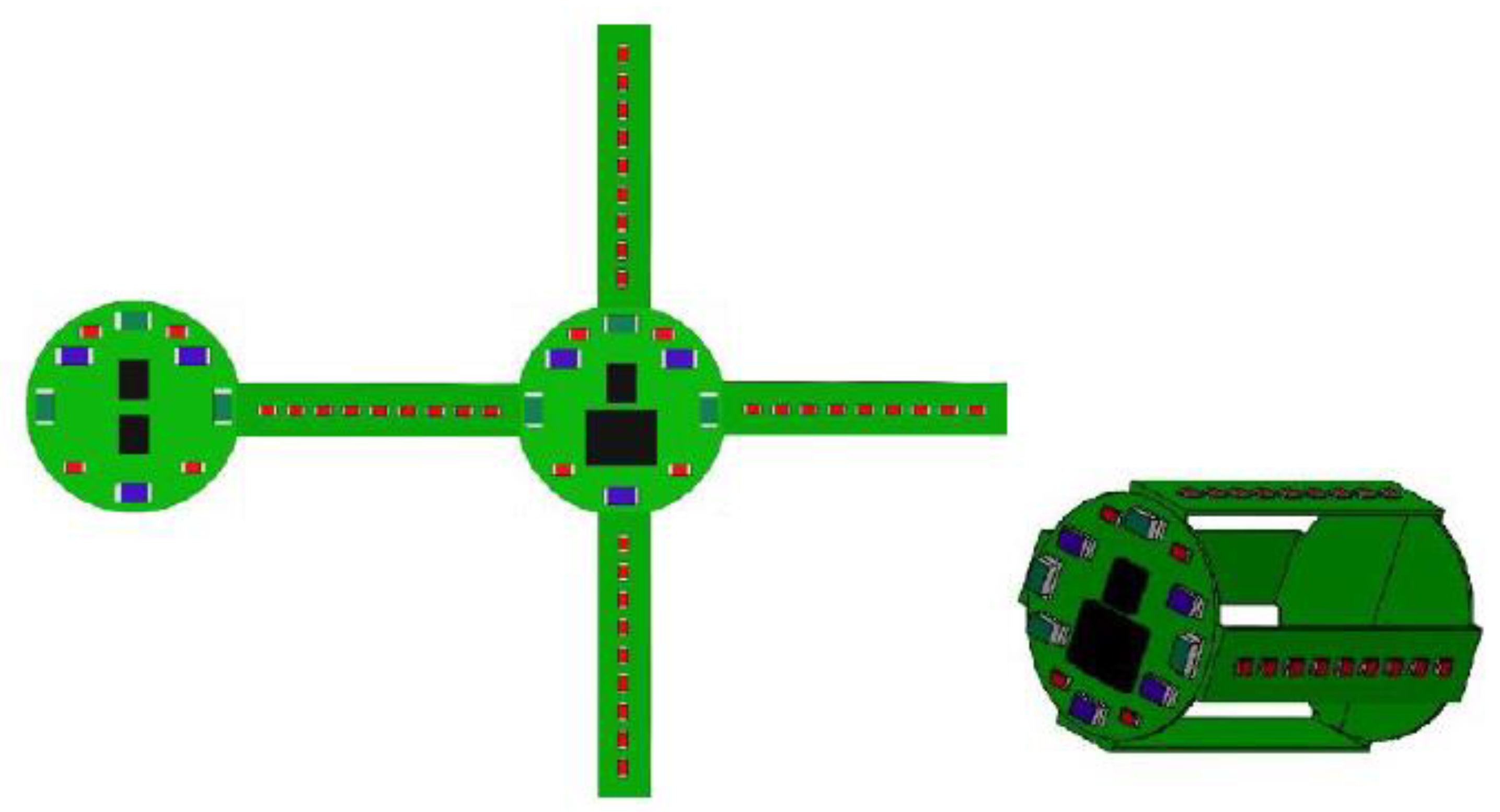
The design of the electronic card, shown in
Figure 4, is articulated on two identical round cards from which four arms branch off. The distribution of the LEDs in the various areas tries to recreate an equal light intensity of the three integrated wavelengths. The two round boards, in fact, are less tied to dimensional constraints, and each have ten LEDs, of which three are Bivar, SM0603UV-40555, three are Everlight, EL-19-21UBGC/TR856 and four are Panasonic, LNJ247W82RA57. In the side arms, on the other hand, due to the limited space available, it is possible to place only the Panasonic, LNJ247W82RA57, having a size equal to 1 × 0.5 × 0.2 mm. The other two types of LEDs, having similar overall dimensions, precisely equal to 1.6 × 0.8 × 0.6 mm for the Bivars, SM0603UV-40555, and 1.6 × 0.8 × 0.8 mm for the Everlight, EL-19-21UBGC/TR856, have submillimetric heights, which are, in any case, too high to be integrated into the lateral areas of the capsule. Even by decentralizing the electronics with respect to the external casing and halving the number of side arms, it is possible to insert the Bivar, SM0603UV-40555, or the Everlight, EL-19-21UBGC/TR856, laterally without increasing the 11 mm diameter and 26 mm capsule height.
Nine Panasonic, LNJ247W82RA57, are inserted into each side arm. By adding the LEDs inserted in the six parts into which the board is divided, a total of 56 LEDs on board the capsule are obtained of which, however, 44 emit a wavelength of 630 nm. It is clearly evident that Panasonic’s number, LNJ247W82RA57, is decidedly greater than that of the other two types. This imbalance between the types of LEDs on board the capsule is not simply linked to reasons of space. Wanting to create a module capable of operating autonomously, the electronics are enabled for housing the battery power supply by inserting the appropriate contacts. Given the size and the desire to have the greatest possible amount of energy on board the capsule, the series of four Renata batteries, 380 SR936W, is considered a hypothetical power supply for the therapeutic module. The capsule electronic board design is shown in
Figure 6 while the complete electronics embedded with batteries is shown in
Figure 7.
2.4. Active Locomotion of the Intelligent Capsule
Active locomotion is aimed at focusing the treatment on the regions affected by the bacterium, avoiding the transfer of light energy to healthy tissues. Although the wavelengths used do not induce the onset of tissue damage, it is still desirable not to carry out unjustified administration of doses to avoid energy waste and, potentially, tissue overheating.
The focus of the treatment on the areas affected by the infection requires the identification of the same areas and the possibility of placing oneself in the vicinity of the areas to be treated. The integration of an active locomotion system based on the interaction between vision and implementation is, therefore, essential. In fact, vision allows to identify the target areas, while locomotion offers the possibility of optimizing the position of the capsule for the administration of the treatment.
Actuation System
Based on the energy limitations that characterize the capsules implemented and taking into account the limits related to the battery power supply of the therapeutic module, the best solution for the implementation of the capsule seems to be the one with cables.
The cable actuation system designed is energetically very convenient, as it does not require the use of active power sources. The position and orientation of the capsule, in fact, are varied through the application of external forces by the user.
The realization of an actuation system for endoscopic capsules without energy limitations is very advantageous since the main limitation in the realization of implemented capsules is precisely the insufficient energy made available by the use of batteries that can be integrated inside the capsule.
The realization of the cable implementation, however, involves the generation of a spun device from which a slight invasiveness is derived. The introduction of minimal invasiveness is the main flaw of the proposed implementation system. However, it is necessary to underline that the level of invasiveness linked to the implemented actuation system is far lower than that deriving from traditional endoscopy practices of usual clinical use. The introduction of the system inside therapeutic capsules made to carry out treatments normally performed using an endoscope would, however, generate less invasive treatment alternatives than those in use.
In fact, it should be emphasized that the implementation system, as well as the therapeutic module, was conceived and implemented as an independent component. As such, it can be integrated and used in any other device compatible with cable actuation mechanisms.
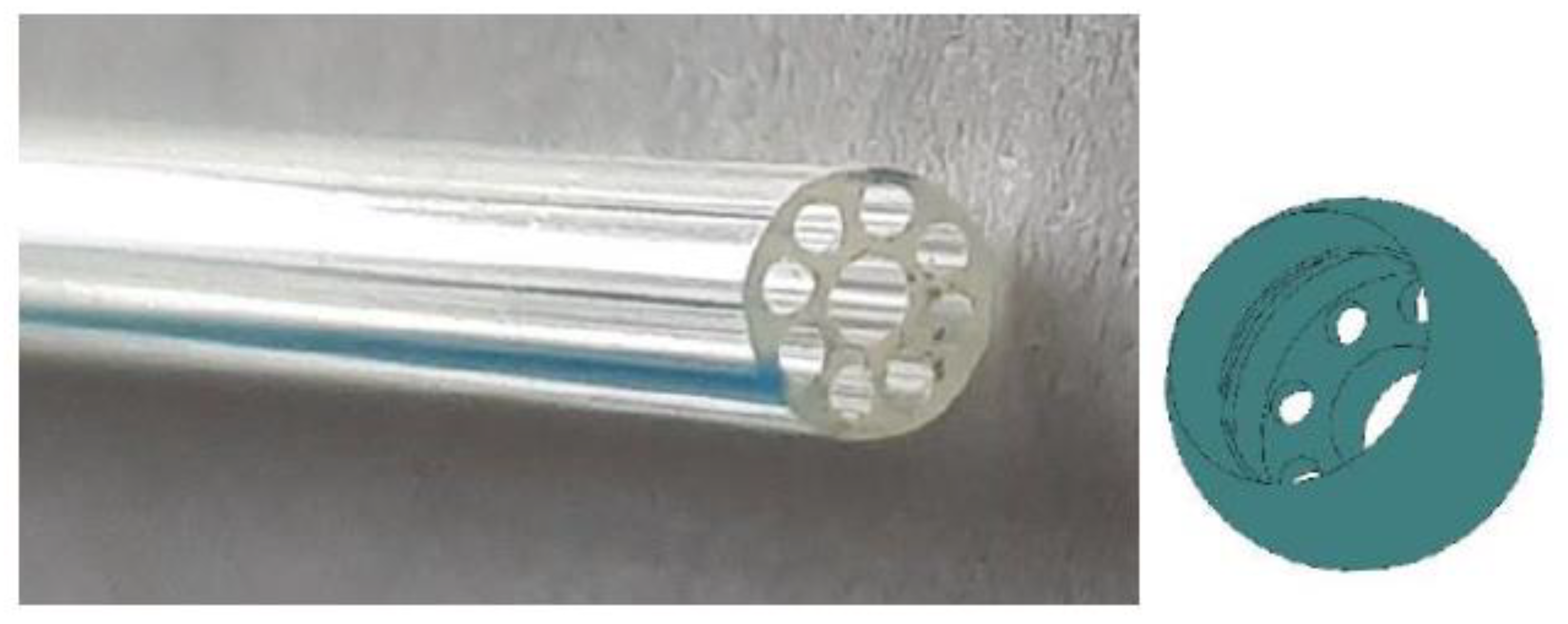
The actuation system is based on a very simple design and operating mechanism. At the base of the whole, there is an appropriate modification of the casing, aimed at enabling the passage of the actuation cables. The cables through the gastrointestinal tract connecting the capsule to the user using a multilumen cannula, shown on the left in
Figure 8, with an external diameter of 2.2 mm from the Microspec Corporation. The cannula has a main central hole inside it, with a diameter of 0.9 mm, around which eight smaller through holes with a diameter of 0.4 mm extend radially.
To enable angular movements, a suitable ball joint was designed and built in rapid prototyping, shown on the right in
Figure 8. The joint was created ad hoc to optimize the interaction with the multilumen cannula used. The goal was to obtain, following the assembly of the two parts, a joint–cannula system that operates as a single part, optimizing the displacements resulting from the application of forces.
The joint is hollow, and the architecture of the lower surface exactly reproduces the morphology of the multilumen cannula. On the lower cap of the joint, in fact, a central hole with a diameter of 1.2 mm was designed, surrounded by eight equally spaced holes, with a diameter of 0.4 mm, arranged circularly.
The housing, with a depth of 0.8 mm, for the cannula was prepared in the upper part of the joint. The cannula is inserted by exploiting the compliance of the material it is made of in order to avoid future detachments after assembly.
A special hemispherical housing was obtained on the casing to contain the joint, guaranteeing complete rotational freedom. While the bidirectional translation in height and the bidirectional axial rotation on 360° of the capsule were trivially obtained by translating and rotating, respectively, the multilumen cannula, to enable angular rotations with respect to the central axis, it is necessary to have a joint that disrupts the movement.
Through the holes of the joint–cannula coupling, the implementation wires on which the user has to operate are brought to the outside to generate the angular movement of the capsule with respect to the central axis. The need for a sturdy, thin and low-friction cable led to the use of the 0.18 mm diameter Fladen, Vantage.
The actuation wires are appropriately associated with the casing in order to create a mechanism based on the generation of angular moments.
The first version of the devised mechanism is based on the generation of very small slots inside the thickness of the casing. The slots are positioned, as in
Figure 9, so as to ensure the sliding of a single thread, for each direction, along the perimeter of the capsule. The mechanism therefore provides for a thread that crosses the capsule from right to left and another that crosses it from front to back. Both ends are available for each thread. To tilt the capsule in one of the two directions, it is necessary to hold one end of the wire still and pull the other.
The mechanism was implemented in two configurations. The configuration visible on the left in
Figure 9 is more congenial to integration with the therapeutic module, as it takes full advantage of the free spaces between one radial arm and the other of the electronic board. It involves the creation of a special housing in which to insert the joint. Given the limited space available for the creation of the housing, the joint cannot have a radius greater than 3 mm. The position of the cannula in this configuration seems rather inconvenient, even for the ingestion of the capsule.
The other configuration of the mechanism appears much less invasive, on the left in
Figure 9. The housing of the joint can exploit the material already present at the end of the capsule. Thus, there is the opportunity to create a joint with a radius of 4 mm.
Starting from the limitations encountered in the first version, the second version of the mechanism was designed. We tried not to completely eliminate the system based on the sliding of a single wire along the same perimeter direction. This system is aimed at the distribution of stresses avoiding the formation of points of application of the forces that could cause cracks or breaks in the millimeter thickness of the casing.
The slots are replaced by more bulky housings that extend from the inner surface of the shell toward the inside of the capsule. The housings, on the right in 8, have a hole for sliding the wire. In reality, the morphology of the housing allows for the implementation of two different implementation mechanisms on the same design. The version is implemented using two configurations, shown as the graph on the left and in the center in 9, following the architectural line already used in the first version of the implementation. Therefore, four different configurations are obtained, the operating mechanism of which is illustrated in 9. The first two boxes implement a mechanism that is completely similar to that used in the first version. They have two wires which, passing through the housings, run along the perimeter of the capsule.
The other actuation mechanism, visible in the two boxes, requires the use of four separate wires, one for each side. Each wire is tied to only one housing. The inclination on each side can be impressed by pulling the corresponding wire.