In Vitro Coliform Resistance to Bioactive Compounds in Urinary Infection, Assessed in a Lab Catheterization Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Biological Materials
2.2. Solvent Extraction
2.3. Determination of Extract Cytotoxicity
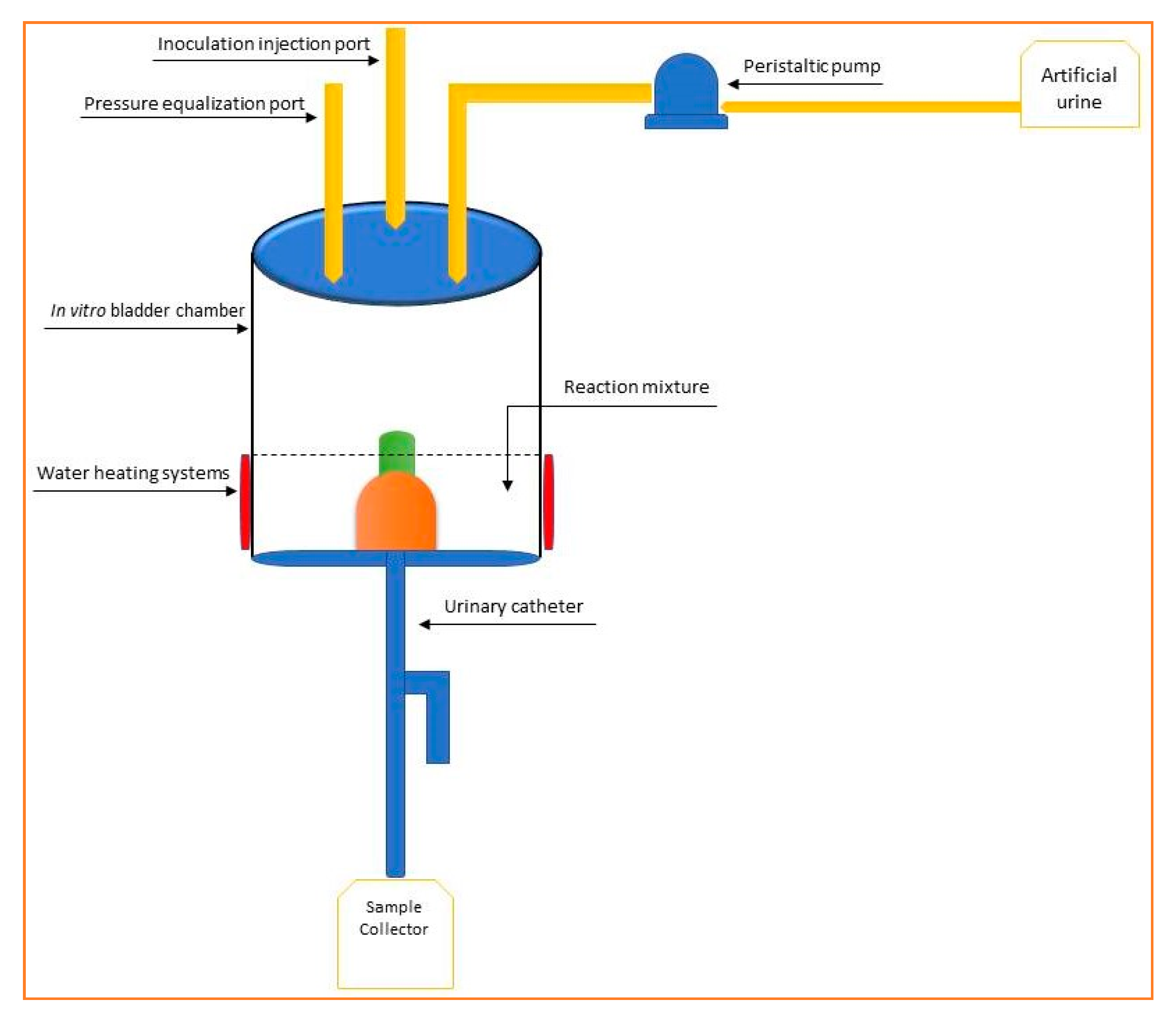
2.4. In Vitro Catheterization Model
- Duran vessel (minimum volume 1 L) provided with two orifices containing sterilized artificial urine [20];
- Peristaltic pump for regular administration of urine from the vessel of point 1, flow rate 0.4 mL/min, controlled by a timed outlet;
- Inoculation point with the tested strain-silicone septum (a stock culture with a minimum viability 1 × 105 CFU/mL, in 0.9% sterile NaCl);
- Theft made of silicone finished in a Millipore filter, diameter 0.45 µm;
- In vitro bladder simulation vessel (minimum volume 250 mL);
- Reaction mixture (simulated urine, the tested product, microbial strain);
- Temperature maintenance system—37 °C;
- Urinary catheter;
- Sterile sample collection vessel—Duran vessel provided with two orifices.
2.5. DNA Extraction and Quantitative Detection of E. coli Strains by qPCR
2.6. Determination of Bioactive Compounds
2.6.1. Total Polyphenolic Content
2.6.2. Procyanidins Content
2.6.3. HPLC Assay
2.7. In Vitro Antioxidant Activity-CUPRAC (CUPric Reducing Antioxidant Capacity) Assay
2.8. Statistical Analysis
3. Results and Discussion
3.1. Determination of Bioactive Compounds
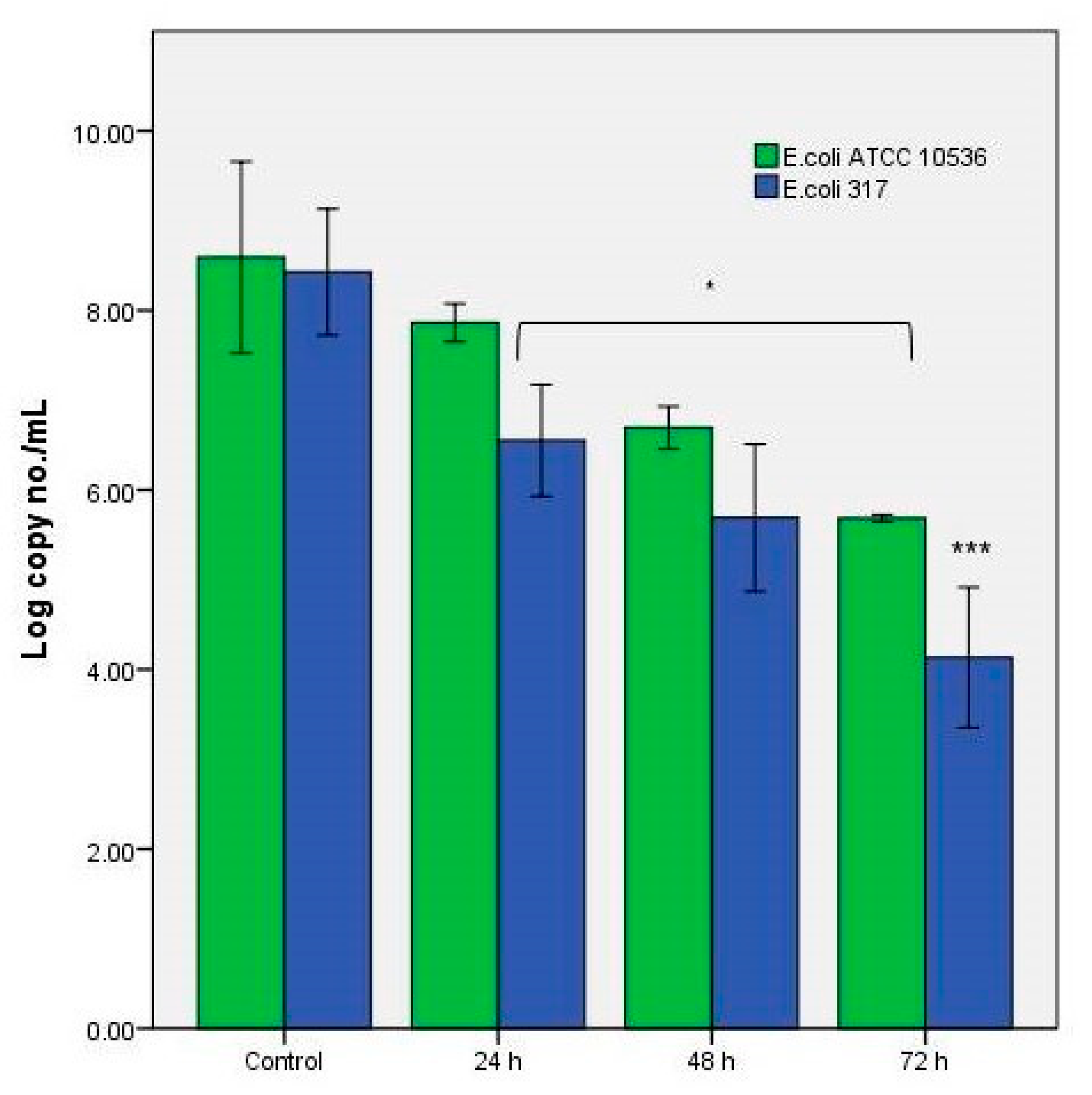
3.2. The Antibacterial Effect of the Extracts in the In Vitro System
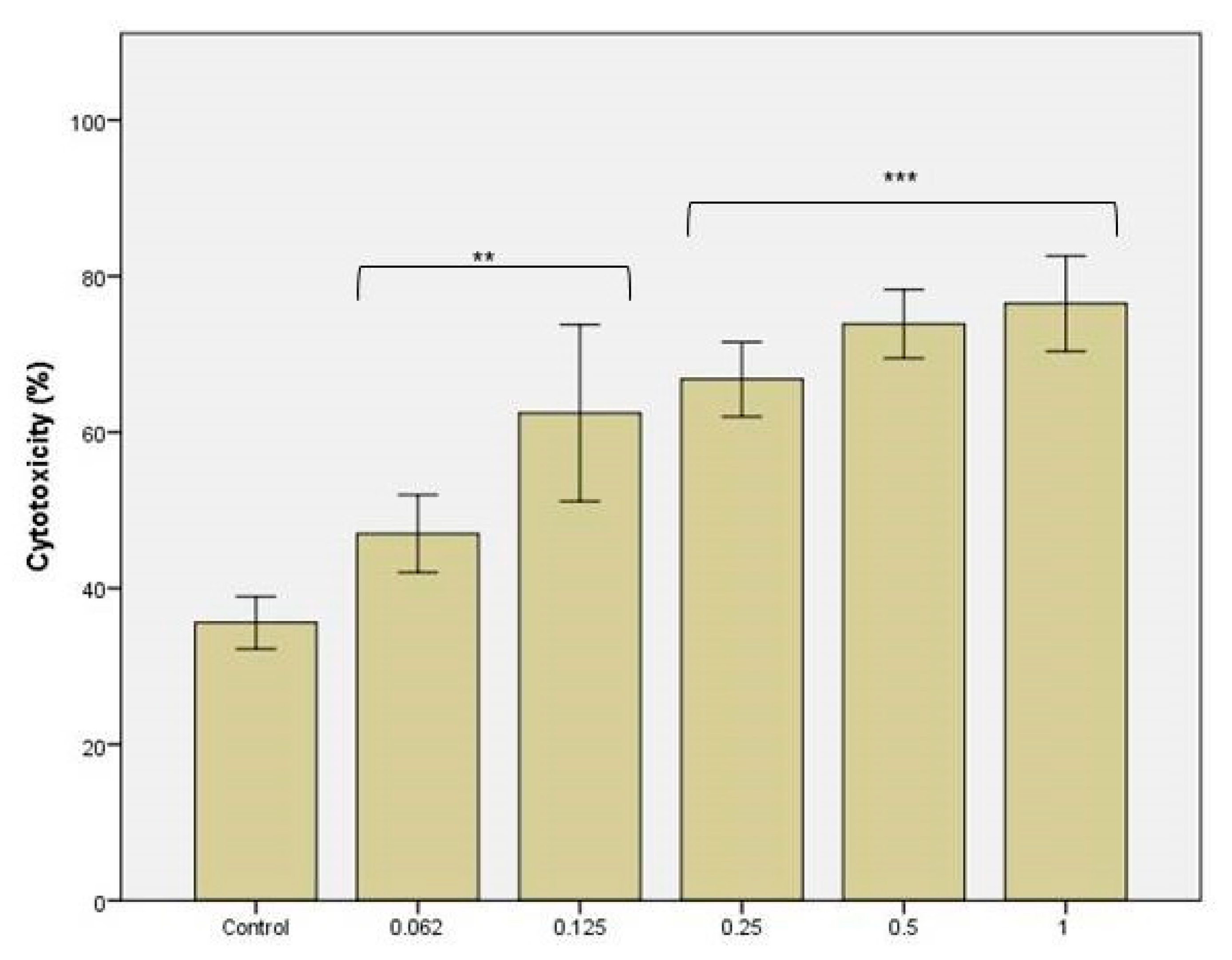
3.3. The Cytotoxicity Tests against C. Albicans
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef]
- van Seventer, J.M.; Hochberg, N.S. Principles of Infectious Diseases: Transmission, Diagnosis, Prevention, and Control. Int. Encyclop. Public Health 2017, 22–39. [Google Scholar] [CrossRef]
- Kwiecińska-Piróg, J.; Skowron, K.; Śniegowska, A.; Przekwas, J.; Balcerek, M.; Załuski, D.; Gospodarek-Komkowska, E. The impact of ethanol extract of propolis on biofilm forming by Proteus mirabilis strains isolated from chronic wounds infections. Nat. Prod. Res. 2019, 33, 3293–3297. [Google Scholar] [CrossRef]
- Azevedo, A.S.; Almeida, C.; Gomes, L.C.; Ferreira, C.; Mergulhão, F.J.; Melo, L.F.; Azevedo, N.F. An in vitro model of catheter-associated urinary tract infections to investigate the role of uncommon bacteria on the Escherichia coli microbial consortium. Biochem. Eng. J. 2017, 118, 64–69. [Google Scholar] [CrossRef][Green Version]
- Ceprnja, M.; Oros, D.; Melvan, E.; Svetlicic, E.; Skrlin, J.; Barisic, K.; Starcevic, L.; Zucko, J.; Starcevic, A. Modeling of Urinary Microbiota Associated With Cystitis. Front. Cell. Infect. Microbiol. 2021, 11, 643638. [Google Scholar] [CrossRef] [PubMed]
- Cortese, Y.J.; Wagner, V.E.; Tierney, M.; Devine, D.; Fogarty, A. Review of Catheter-Associated Urinary Tract Infections and In Vitro Urinary Tract Models. J. Health. Eng. 2018, 2018, 2986742. [Google Scholar] [CrossRef]
- Wagenlehner, F.M.; Abramov-Sommariva, D.; Höller, M.; Steindl, H.; Naber, K.G. Non-Antibiotic Herbal Therapy (BNO 1045) versus Antibiotic Therapy (Fosfomycin Trometamol) for the Treatment of Acute Lower Uncomplicated Urinary Tract Infections in Women: A Double-Blind, Parallel-Group, Randomized, Multicentre, Non-Inferiority Phase III Trial. Urol. Int. 2018, 101, 327–336. [Google Scholar] [PubMed]
- Vamanu, E.; Pelinescu, D.; Sarbu, I. Comparative Fingerprinting of the Human Microbiota in Diabetes and Cardiovascular Disease. J. Med. Food 2016, 19, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
- Leme, D.E.M.; Rodrigues, A.B.; de Almeida-Apolonio, A.A.; Gomes da Silva Dantas, F.; Norman Negri, M.F.; Svidzinski, T.I.E.; da Silva Mota, J.; Lima Cardoso, C.A.; Pires de Oliveira, K.M. In Vitro Control of Uropathogenic Microorganisms with the Ethanolic Extract from the Leaves of Cochlospermum regium (Schrank) Pilger. Evid. Based Complement. Alt. Med. 2017, 2017, 4687154. [Google Scholar] [CrossRef] [PubMed]
- Vamanu, E.; Pelinescu, D.; Avram, I.; Nita, S. An in vitro evaluation of antioxidant and colonic microbial profile levels following mushroom consumption. Biomed Res. Int. 2013, 2013, 289821. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Forsyth, V.S.; Armbruster, C.E.; Smith, S.N.; Pirani, A.; Springman, A.C.; Walters, M.S.; Nielubowicz, G.R.; Himpsl, S.D.; Snitkin, E.S.; Mobley, H.L.T. Rapid Growth of Uropathogenic Escherichia coli during Human Urinary Tract Infection. mBIO 2018, 9, e00186-18. [Google Scholar] [CrossRef]
- Chauhan, N.; Kruppa, M.D. Standard Growth Media and Common Techniques for Use with Candida albicans. In Candida albicans. Methods in Molecular Biology; Cihlar, R.L., Calderone, R.A., Eds.; Humana Press: Totowa, NJ, USA, 2009; Volume 499. [Google Scholar] [CrossRef]
- Dabulici, C.M.; Sârbu, I.; Vamanu, E. The Bioactive Potential of Functional Products and Bioavailability of Phenolic Compounds. Foods 2020, 9, 953. [Google Scholar] [CrossRef] [PubMed]
- Jurikova, T.; Skrovankova, S.; Mlcek, J.; Balla, S.; Snopek, L. Bioactive Compounds, Antioxidant Activity, and Biological Effects of European Cranberry (Vaccinium oxycoccos). Molecules 2018, 24, 24. [Google Scholar] [CrossRef]
- Uritu, C.M.; Mihai, C.T.; Stanciu, G.D.; Dodi, G.; Alexa-Stratulat, T.; Luca, A.; Leon-Constantin, M.M.; Stefanescu, R.; Bild, V.; Melnic, S.; et al. Medicinal Plants of the Family Lamiaceae in Pain Therapy: A Review. Pain Res. Manag. 2018, 2018, 7801543. [Google Scholar] [CrossRef] [PubMed]
- Olajide, O.A. Inhibitory effects of St. John’s Wort on inflammation: Ignored potential of a popular herb. J. Diet. Suppl. 2009, 6, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Ahangari, Z.; Naseri, M.; Vatandoost, F. Propolis: Chemical Composition and Its Applications in Endodontics. Iran. Endod. J. 2018, 13, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Parham, S.; Kharazi, A.Z.; Bakhsheshi-Rad, H.R.; Nur, H.; Ismail, A.F.; Sharif, S.; RamaKrishna, S.; Berto, F. Antioxidant, Antimicrobial and Antiviral Properties of Herbal Materials. Antioxidants 2020, 9, 1309. [Google Scholar] [CrossRef] [PubMed]
- Hudz, N.; Makowicz, E.; Shanaida, M.; Białoń, M.; Jasicka-Misiak, I.; Yezerska, O.; Svydenko, L.; Wieczorek, P.P. Phytochemical Evaluation of Tinctures and Essential Oil Obtained from Satureja montana Herb. Molecules 2020, 25, 4763. [Google Scholar] [CrossRef]
- Kabir, M.A.; Hussain, M.A.; Ahmad, Z. Candida albicans: A Model Organism for Studying Fungal Pathogens. ISRN Microbiol. 2012, 2012, 538694. [Google Scholar] [CrossRef]
- Wang, R.; Neoh, K.G.; Kang, E.T.; Tambyah, P.A.; Chiong, E. Antifouling coating with controllable and sustained silver release for long-term inhibition of infection and encrustation in urinary catheters. J. Biomed. Mater. Res. B 2015, 103, 519–528. [Google Scholar] [CrossRef]
- Vamanu, E. In Vitro System for Testing for Urinary Tract Infection with Escherichia coli and Test Method. Patent Application OSIM 00387/2020, 8 July 2020. [Google Scholar]
- Dinu, L.D.; Bach, S. Detection of viable but non-culturable Escherichia coli O157: H7 from vegetable samples using quantitative PCR with propidium monoazide and immunological assays. Food Control 2013, 31, 268–273. [Google Scholar] [CrossRef]
- Rusthen, S.; Kristoffersen, A.K.; Young, A.; Galtung, H.K.; Petrovski, B.É.; Palm, O.; Enersen, M.; Jensen, J.L. Dysbiotic salivary microbiota in dry mouth and primary Sjögren’s syndrome patients. PLoS ONE 2019, 14, e0218319. [Google Scholar] [CrossRef]
- Meroni, G.; Soares Filipe, J.F.; Martino, P.A. In Vitro Antibacterial Activity of Biological-Derived Silver Nanoparticles: Preliminary Data. Vet. Sci. 2020, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Xi, Z.Q.; Xiao, F.; Yuan, J.; Wang, X.F.; Wang, L.; Quan, F.Y.; Liu, G.W. Gene expression analysis on anterior temporal neocortex of patients with intractable epilepsy. Synapse 2009, 63, 1017–1028. [Google Scholar] [CrossRef] [PubMed]
- Hatami, T.; Emami, S.A.; Miraghaee, S.S.; Mojarrab, M. Total Phenolic Contents and Antioxidant Activities of Different Extracts and Fractions from the Aerial Parts of Artemisia biennis Willd. Iran. J. Pharm. Res. 2014, 13, 551–559. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4157030/ (accessed on 23 March 2021).
- Cheng, A.; Chen, X.; Wang, W.; Gong, Z.; Liu, L. Contents of Extractable and Non-extractable Polyphenols in the Leaves of Blueberry. Czech J. Food Sci. 2013, 31, 275–282. Available online: https://www.agriculturejournals.cz/publicFiles/92405.pdf (accessed on 23 March 2021). [CrossRef]
- Roman, M.C. Determination of catechins and caffeine in camillia sinensis raw materials, extracts, and dietary supplements by HPLC-uv: Single-laboratory validation. J. AOAC Int. 2013, 96, 933–941. [Google Scholar] [CrossRef]
- Vamanu, E.; Gatea, F.; Pelinescu, D.R. Bioavailability and Bioactivities of Polyphenols Eco Extracts from Coffee Grounds after In Vitro Digestion. Foods 2020, 9, 1281. [Google Scholar] [CrossRef] [PubMed]
- Al-Rimawi, F.; Rishmawi, S.; Ariqat, S.H.; Khalid, M.F.; Warad, I.; Salah, Z. Anticancer Activity, Antioxidant Activity, and Phenolic and Flavonoids Content of Wild Tragopogon porrifolius Plant Extracts. Evid. Based Complement. Alt. Med. 2016, 2016, 9612490. [Google Scholar] [CrossRef]
- Ramírez-Castillo, F.Y.; Moreno-Flores, A.C.; Avelar-González, F.J.; Márquez-Díaz, F.; Harel, J.; Guerrero-Barrera, A.L. An evaluation of multidrug-resistant Escherichia coli isolates in urinary tract infections from Aguascalientes, Mexico: Cross-sectional study. Ann. Clin. Microbiol. Antimicrob. 2018, 17, 34. [Google Scholar] [CrossRef]
- Dusane, D.H.; Hosseinidoust, Z.; Asadishad, B.; Tufenkji, N. Alkaloids Modulate Motility, Biofilm Formation and Antibiotic Susceptibility of Uropathogenic Escherichia coli. PLoS ONE 2014, 9, e112093. [Google Scholar] [CrossRef]
- Sienkiewicz, M.; Łysakowska, M.; Pastuszka, M.; Bienias, W.; Kowalczyk, E. The potential of use basil and rosemary essential oils as effective antibacterial agents. Molecules 2013, 18, 9334–9351. [Google Scholar] [CrossRef] [PubMed]
- Khamael, L.S.; Hassan, M.R.; Entissar, F.A. Study of rosemary essential oil antibacterial effect on bacteria isolated from urinary tract infection in some hospital of Baghdad. Curr. Res. Microb. Biotech. 2018, 6, 1490–1495. Available online: http://crmb.aizeonpublishers.net/content/2018/1/crmb1490-1495.pdf (accessed on 23 March 2021).
- Loose, M.; Pilger, E.; Wagenlehner, F. Anti-Bacterial Effects of Essential Oils against Uropathogenic Bacteria. Antibiotics 2020, 9, 358. [Google Scholar] [CrossRef]
- Huo, M.; Cui, X.; Xue, J.; Chi, G.; Gao, R.; Deng, X.; Guan, S.; Wei, J.; Soromou, L.W.; Feng, H. Anti-inflammatory effects of linalool in RAW 264.7 macrophages and lipopolysaccharide-induced lung injury model. J. Surg. Res. 2013, 180, e47–e54. [Google Scholar] [CrossRef]
- Nieto, G.; Ros, G.; Castillo, J. Antioxidant and Antimicrobial Properties of Rosemary (Rosmarinus officinalis, L.): A Review. Medicines 2018, 5, 98. [Google Scholar] [CrossRef] [PubMed]
- Lyu, F.; Xu, X.; Zhang, L. Natural polysaccharides with different conformations: Extraction, structure and anti-tumor activity. J. Mater. Chem. B 2020, 8, 9652–9667. [Google Scholar] [CrossRef] [PubMed]
- Lone, S.A.; Wani, M.Y.; Fru, P.; Ahmad, A. Cellular apoptosis and necrosis as therapeutic targets for novel Eugenol Tosylate Congeners against Candida albicans. Sci. Rep. 2020, 10, 1191. [Google Scholar] [CrossRef]
- Chua, R.Y.R.; Lim, K.; Leong, S.S.J.; Tambyah, P.A.; Ho, B. An in-vitro urinary catheterization model that approximates clinical conditions for evaluation of innovations to prevent catheter-associated urinary tract infections. J. Hosp. Inf. 2017, 97, 66e73. [Google Scholar] [CrossRef]
- Terlizzi, M.E.; Gribaudo, G.; Maffei, M.E. UroPathogenic Escherichia coli (UPEC) Infections: Virulence Factors, Bladder Responses, Antibiotic, and Non-antibiotic Antimicrobial Strategies. Front. Microbiol. 2017, 8, 1566. [Google Scholar] [CrossRef]
- Roy, R.; Tiwari, M.; Donelli, G.; Tiwari, V. Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action. Virulence 2018, 9, 522–554. [Google Scholar] [CrossRef]
- Arita-Morioka, K.; Yamanaka, K.; Mizunoe, Y.; Tanaka, Y.; Ogura, T.; Sugimoto, S. Inhibitory effects of Myricetin derivatives on curli-dependent biofilm formation in Escherichia coli. Sci. Rep. 2018, 8, 8452. [Google Scholar] [CrossRef]
- Nicu, I.; Pîrvu, L.; Vamanu, A. Antibacterial activity of ethanolic extracts from Agrimonia eupatoria L. and Epilobium hirsutum L. HERBA. Sci. Bull. Ser. F. Biotechnol. 2017, XXI, 127–132. Available online: http://biotechnologyjournal.usamv.ro/pdf/2017/Art22.pdf (accessed on 6 May 2021).
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simões, M. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.; Saavedra, M.J.; Simões, M. The activity of ferulic and gallic acids in biofilm prevention and control of pathogenic bacteria. Biofouling 2012, 28, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Du, C.; Beaman, H.T.; Monroe, M.B.B. Characterization of Phenolic Acid Antimicrobial and Antioxidant Structure–Property Relationships. Pharmaceutics 2020, 12, 419. [Google Scholar] [CrossRef]
- Choi, J.H.; Park, J.K.; Kim, K.M.; Lee, H.J.; Kim, S. In vitro and in vivo antithrombotic and cytotoxicity effects of ferulic acid. J. Biochem. Mol. Toxicol. 2018, 32, 1. [Google Scholar] [CrossRef] [PubMed]
- Nzakizwanayo, J.; Pelling, H.; Milo, S.; Jones, B.V. An In Vitro Bladder Model for Studying Catheter-Associated Urinary Tract Infection and Associated Analysis of Biofilms. Methods Mol. Biol. 2019, 2021, 139–158. [Google Scholar] [CrossRef]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic resistance: A rundown of a global crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef] [PubMed]
- Davenport, M.; Mach, K.E.; Shortliffe, L.M.D.; Banaei, N.; Wang, T.H.; Liao, J.C. New and developing diagnostic technologies for urinary tract infections. Nat. Rev. Urol. 2017, 14, 296–310. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vamanu, E.; Dinu, L.D.; Luntraru, C.M.; Suciu, A. In Vitro Coliform Resistance to Bioactive Compounds in Urinary Infection, Assessed in a Lab Catheterization Model. Appl. Sci. 2021, 11, 4315. https://doi.org/10.3390/app11094315
Vamanu E, Dinu LD, Luntraru CM, Suciu A. In Vitro Coliform Resistance to Bioactive Compounds in Urinary Infection, Assessed in a Lab Catheterization Model. Applied Sciences. 2021; 11(9):4315. https://doi.org/10.3390/app11094315
Chicago/Turabian StyleVamanu, Emanuel, Laura Dorina Dinu, Cristina Mihaela Luntraru, and Alexandru Suciu. 2021. "In Vitro Coliform Resistance to Bioactive Compounds in Urinary Infection, Assessed in a Lab Catheterization Model" Applied Sciences 11, no. 9: 4315. https://doi.org/10.3390/app11094315
APA StyleVamanu, E., Dinu, L. D., Luntraru, C. M., & Suciu, A. (2021). In Vitro Coliform Resistance to Bioactive Compounds in Urinary Infection, Assessed in a Lab Catheterization Model. Applied Sciences, 11(9), 4315. https://doi.org/10.3390/app11094315






