Multifunctional Ingredient from Aqueous Flavonoidic Extract of Yellow Onion Skins with Cytocompatibility and Cell Proliferation Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Flavonoid Extraction from Yellow Onion Skins
2.3. Powder Formula
2.4. Extract and Powder Characterization
2.5. In Vitro Digestion
2.6. Thermal and pH Stability of the Extracted Phytochemicals
2.7. Cell Culture and Treatment
2.8. Cell Viability
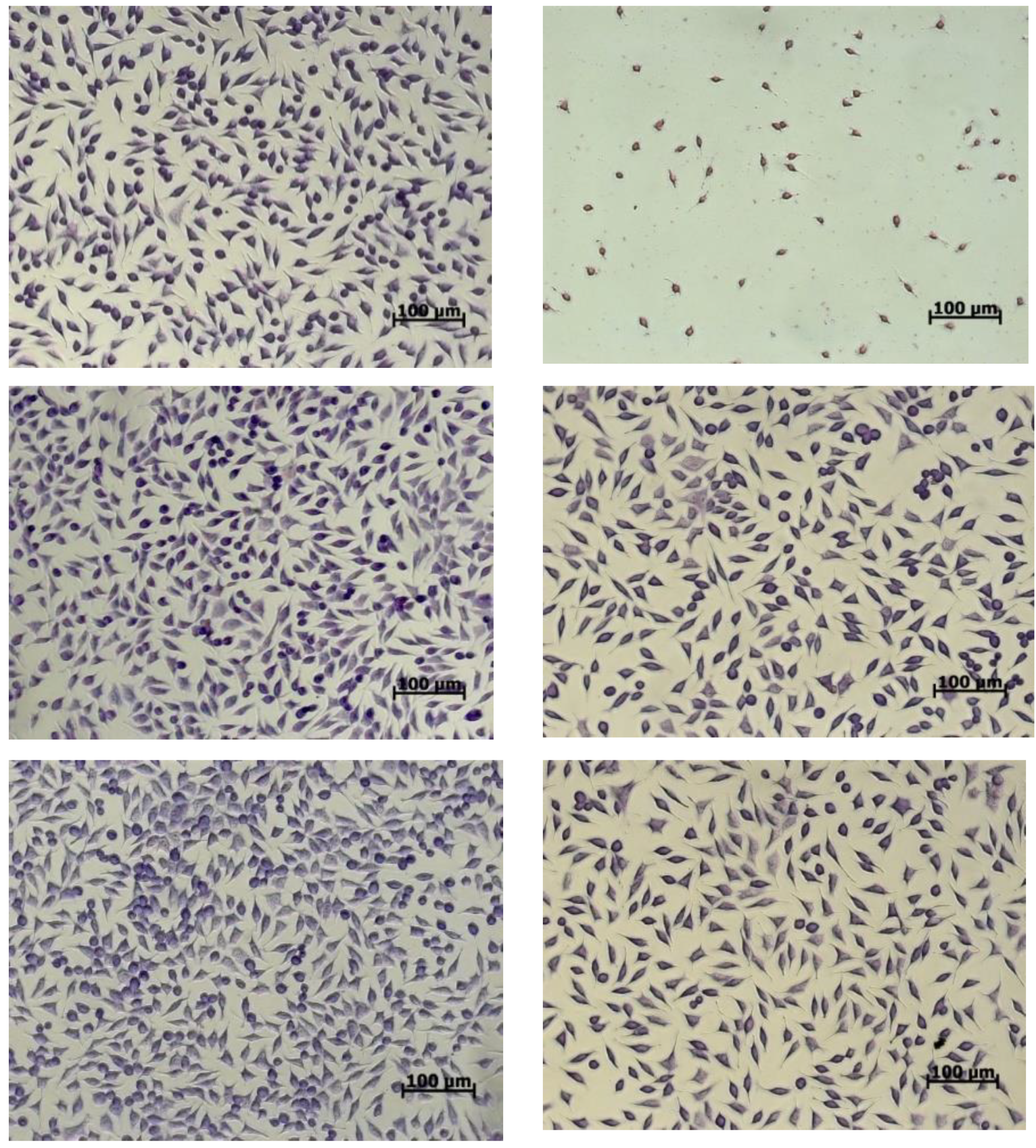
2.9. Cell Morphology
2.10. Confocal Laser Microscope Spectroscopy
2.11. Statistical Analysis
3. Results
3.1. Yellow Onion Skin Extract and Powder Characterization
3.2. The Microstructural Design of the Powders by Confocal Microscopy
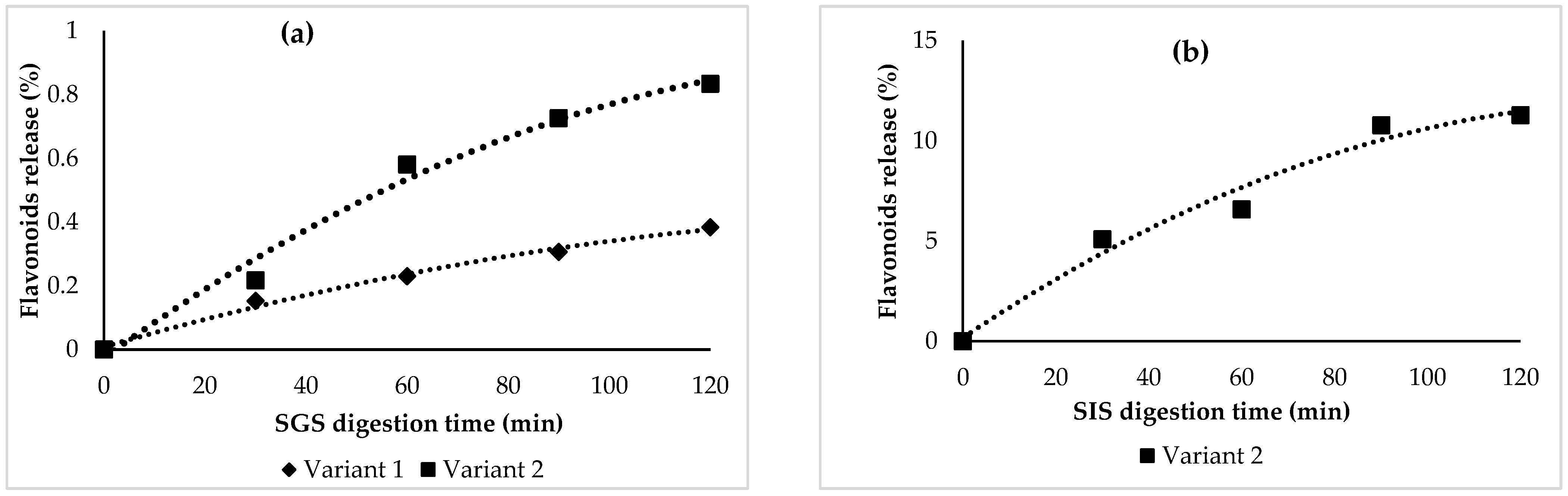
3.3. In Vitro Digestion of Flavonoids
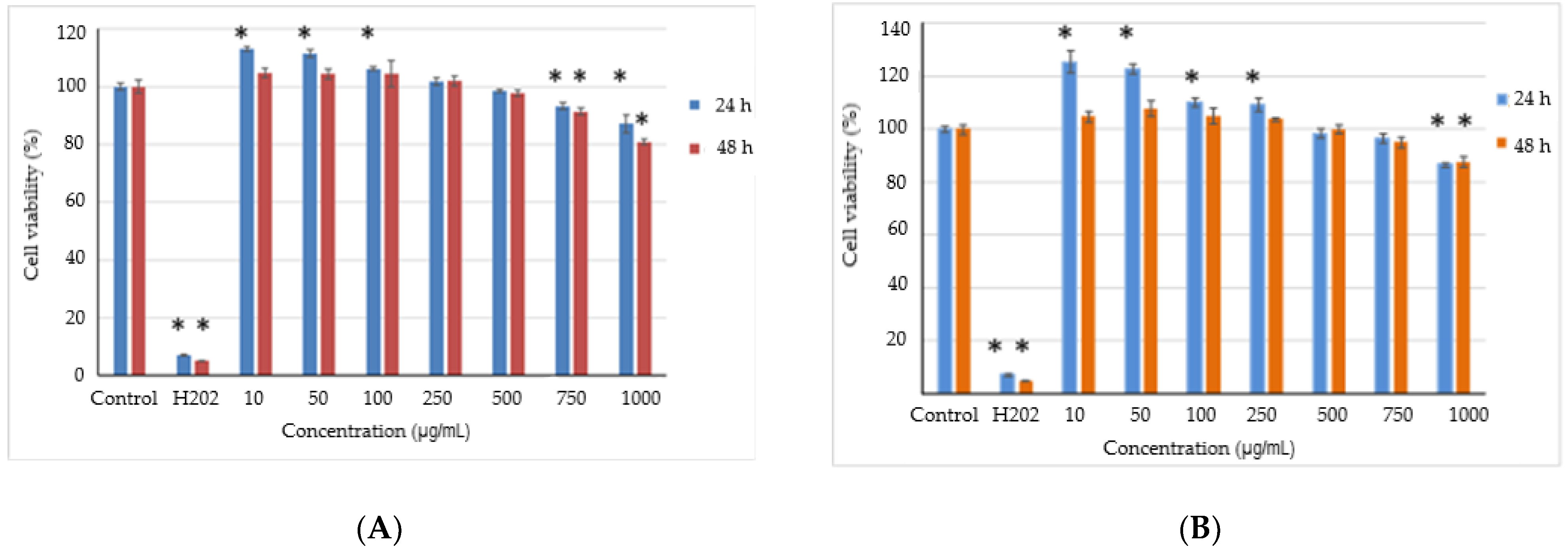
3.4. In Vitro Cytotoxicity of the Microencapsulated Powders
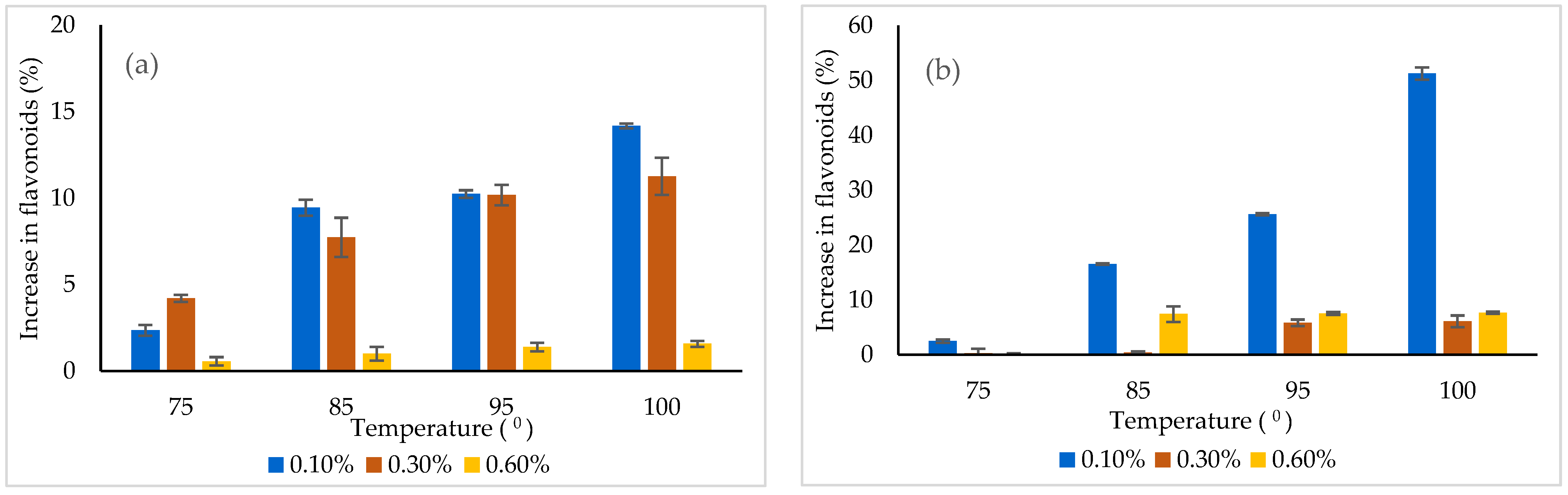
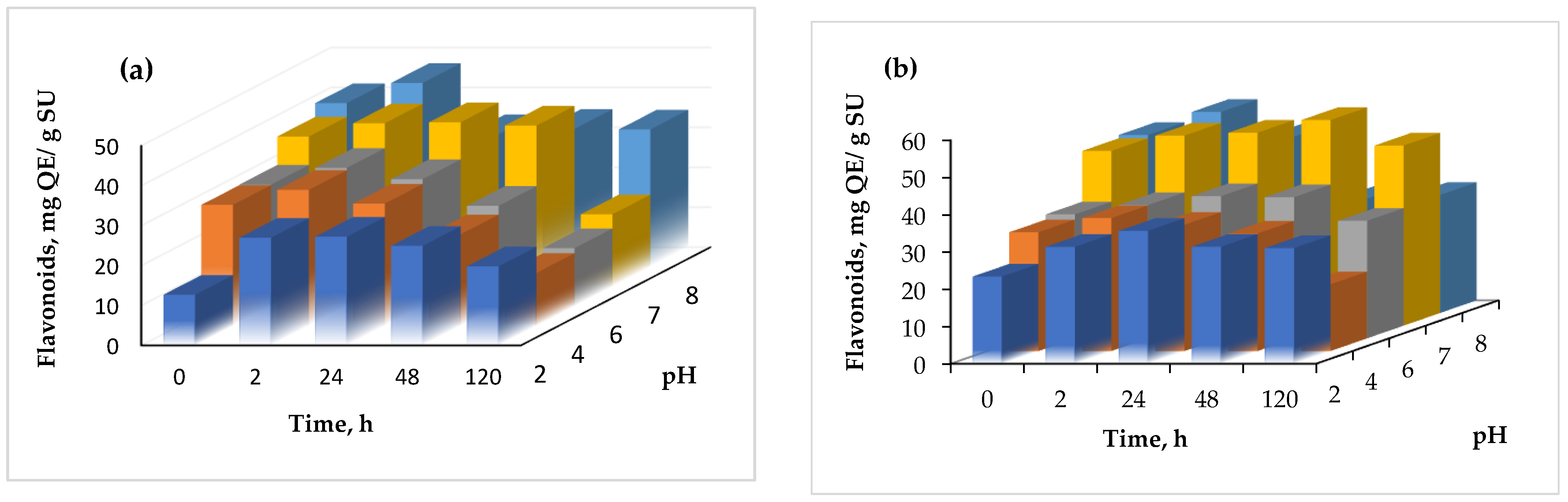
3.5. Thermal and pH Stability
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ren, F.; Niana, Y.; Perussello, C.A. Effect of storage, food processing and novel extraction technologies on onions flavonoid content: A review. Food Res. Int. 2020, 132, 108953. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.; Cho, E.; Moon, J.; Bae, H. Onion skin waste as a valorization resource for the by-product’s quercetin and biosugar. Food Chem. 2015, 188, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Li, Y.; Yang, Z.; Pu, X.; Du, J.; Yang, X.; Yang, T.; Yang, S. Therapeutic Role of Functional Components in Alliums for Preventive Chronic Disease in Human Being. Evid. Based Complement. Alternat. Med. 2017, 2017, 9402849. [Google Scholar] [CrossRef] [PubMed]
- Tsao, R.; Yang, R. Optimization of a new mobile phase to know the complex and real polyphenolic composition: Towards a total phenolic index using high-performance liquid chromatography. J. Chromat. A 2003, 1018, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Akdeniz, N.; Sumnu, G.; Sahin, S. Microencapsulation of phenolic compounds extracted from onion (Allium cepa) skin. J. Food Process. Preserv. 2018, 42, e13648. [Google Scholar] [CrossRef]
- Panja, P. Green extraction methods of food polyphenols from vegetable materials. Curr. Opin. Food Sci. 2018, 23, 173–182. [Google Scholar] [CrossRef]
- Cui, Q.; Liu, J.-Z.; Wang, L.-T.; Kang, Y.-F.; Meng, Y.; Jiao, J.; Fu, Y.-J. Sustainable deep eutectic solvents preparation and their efficiency in extraction and enrichment of main bioactive flavonoids from sea buckthorn leaves. J. Clean. Prod. 2018, 184, 826–835. [Google Scholar] [CrossRef]
- Meng, Z.; Zhao, J.; Duan, H.; Guan, Y.; Zhao, L. Green and efficient extraction of four bioactive flavonoids from Pollen Typhae by ultrasound-assisted deep eutectic solvents extraction. J. Pharmaceut. Biomed. Anal. 2018, 161, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Cheynier, V. Polyphenols in foods are more complex than often thought. Am. J. Clin. Nutr. 2005, 81, 223S–229S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saiki, S.; Kumar, N.; Charu, M.; Mahanta, L. Optimisation of phenolic extraction from Averrhoa carambola pomace by response surface methodology and its microencapsulation by spray and freeze-drying. Food Chem. 2015, 171, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Çam, M.; Cihatİçyer, N.; Erdoğan, F. Pomegranate peel phenolics: Microencapsulation, storage stability and potential ingredient for functional food development. LWT 2014, 55, 117–123. [Google Scholar] [CrossRef]
- Milea, A.S.; Aprodu, I.; Vasile, A.M.; Barbu, V.; Râpeanu, G.; Bahrim, G.E.; Stănciuc, N. Widen the functionality of flavonoids from yellow onion skins through extraction and microencapsulation in whey proteins hydrolysates and different polymers. J. Food Eng. 2019, 251, 29–35. [Google Scholar] [CrossRef]
- Milea, Ș.A.; Vasile, M.A.; Crăciunescu, O.; Prelipcean, A.M.; Bahrim, G.E.; Râpeanu, G.; Oancea, A.; Stănciuc, N. Co-microencapsulation of flavonoids from yellow onion skins and lactic acid bacteria lead to a multifunctional ingredient for foods and pharmaceutics applications. Pharmaceutics 2020, 12, 1053. [Google Scholar] [CrossRef] [PubMed]
- Oancea, A.M.; Hasan, M.; Vasile, A.M.; Barbu, V.; Enachi, E.; Bahrim., G.; Rapeanu, G.; Silvi, S.; Stănciuc, N. Functional evaluation of microencapsulated anthocyanins from sour cherries skins extract in whey proteins isolate. LWT 2018, 95, 129–134. [Google Scholar] [CrossRef]
- Craciunescu, O.; Gaspar, A.; Toma, L.; Utoiu, E.; Moldovan, L. Evaluation of antioxidant and cytoprotective activities of Arnica montana L. and Artemisia absinthium L. ethanolic extracts. Chem. Central J. 2012, 6, 97–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piechowiak, T.; Grzelak-Błaszczyk, K.; Bonikowski, R.; Balawejder, M. Optimization of extraction process of antioxidant compounds from yellow onionskin and their use in functional bread production. LWT 2020, 117, 108614. [Google Scholar] [CrossRef]
- Thakur, H.N.S.; Thakur, A. Microencapsulation of wild pomegranate flavedo phenolics by lyophilization: Effect of maltodextrin concentration, structural morphology, functional properties, elemental composition, and ingredient for development of functional beverage. LWT 2020, 133, 110077. [Google Scholar] [CrossRef]
- Horincar, G.; Aprodu, I.; Barbu, V.; Râpeanu, G.; Bahrim, G.E.; Stănciuc, N. Interactions of flavonoids from yellow onion skins with whey proteins: Mechanisms of binding and microencapsulation with different combinations of polymers. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 215, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.Q.; Yang, J.; Liu, J.; Liu, S.N.; Song, H.X.; Zhao, W.E.; Liu, Y.Q. Isolation of flavonoids from onion skin and their effects on K562 cell viability. Bangladesh J. Pharmacol. 2016, 11, S18–S25. [Google Scholar] [CrossRef] [Green Version]

| Variants | Entrapping Efficiency, % | Total Flavonoids, mg QE/g DW | Total Polyphenols, mg GAE/g DW | Antioxidant Activity, mM Trolox/g DW |
|---|---|---|---|---|
| 1 | 76.55 ± 2.62 *,b | 26.91 ± 2.93 a | 21.24 ± 0.57 a | 102.76 ± 0.82 b |
| 2 | 83.51 ± 1.81 a | 20.75 ± 0.78 b | 18.57 ± 0.32 b | 109.28 ± 0.12 a |
| Variants | Total Flavonoids, mg QE/g DW | Total Polyphenols, mg GAE/g DW | Antioxidant Activity, mM Trolox/g DW |
|---|---|---|---|
| 1 | 22.35 ± 0.60 *,a | 19.56 ± 0.05 a | 82.23 ± 0.92 b |
| 2 | 22.74 ± 0.18 a | 18.91 ± 0.18 b | 86.99 ± 1.15 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milea, Ș.A.; Crăciunescu, O.; Râpeanu, G.; Oancea, A.; Enachi, E.; Bahrim, G.E.; Stănciuc, N. Multifunctional Ingredient from Aqueous Flavonoidic Extract of Yellow Onion Skins with Cytocompatibility and Cell Proliferation Properties. Appl. Sci. 2021, 11, 7243. https://doi.org/10.3390/app11167243
Milea ȘA, Crăciunescu O, Râpeanu G, Oancea A, Enachi E, Bahrim GE, Stănciuc N. Multifunctional Ingredient from Aqueous Flavonoidic Extract of Yellow Onion Skins with Cytocompatibility and Cell Proliferation Properties. Applied Sciences. 2021; 11(16):7243. https://doi.org/10.3390/app11167243
Chicago/Turabian StyleMilea, Ștefania Adelina, Oana Crăciunescu, Gabriela Râpeanu, Anca Oancea, Elena Enachi, Gabriela Elena Bahrim, and Nicoleta Stănciuc. 2021. "Multifunctional Ingredient from Aqueous Flavonoidic Extract of Yellow Onion Skins with Cytocompatibility and Cell Proliferation Properties" Applied Sciences 11, no. 16: 7243. https://doi.org/10.3390/app11167243
APA StyleMilea, Ș. A., Crăciunescu, O., Râpeanu, G., Oancea, A., Enachi, E., Bahrim, G. E., & Stănciuc, N. (2021). Multifunctional Ingredient from Aqueous Flavonoidic Extract of Yellow Onion Skins with Cytocompatibility and Cell Proliferation Properties. Applied Sciences, 11(16), 7243. https://doi.org/10.3390/app11167243









