Modifications of Wound Dressings with Bioactive Agents to Achieve Improved Pro-Healing Properties
Abstract
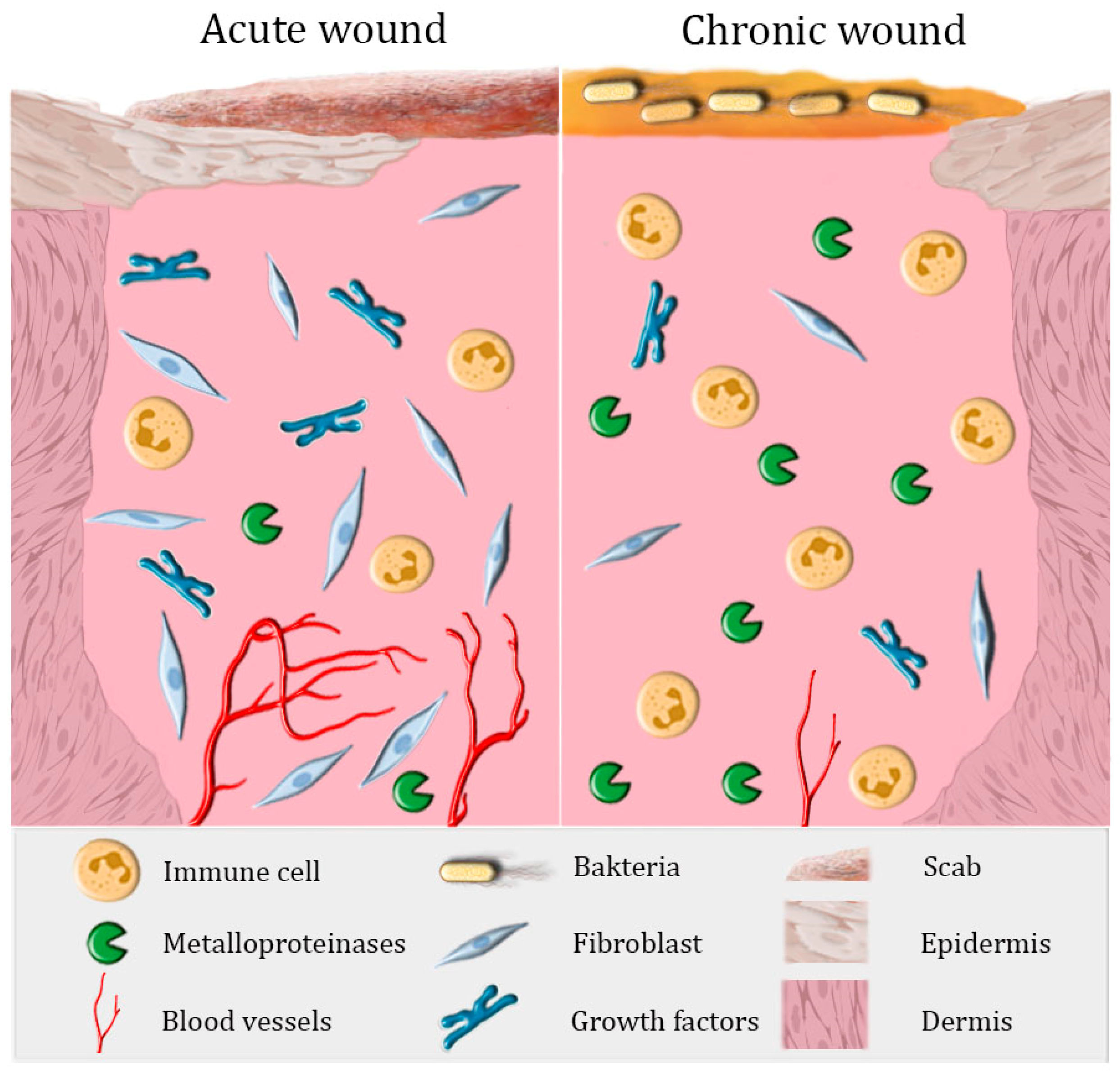
:1. The Healing of Acute and Chronic Wounds
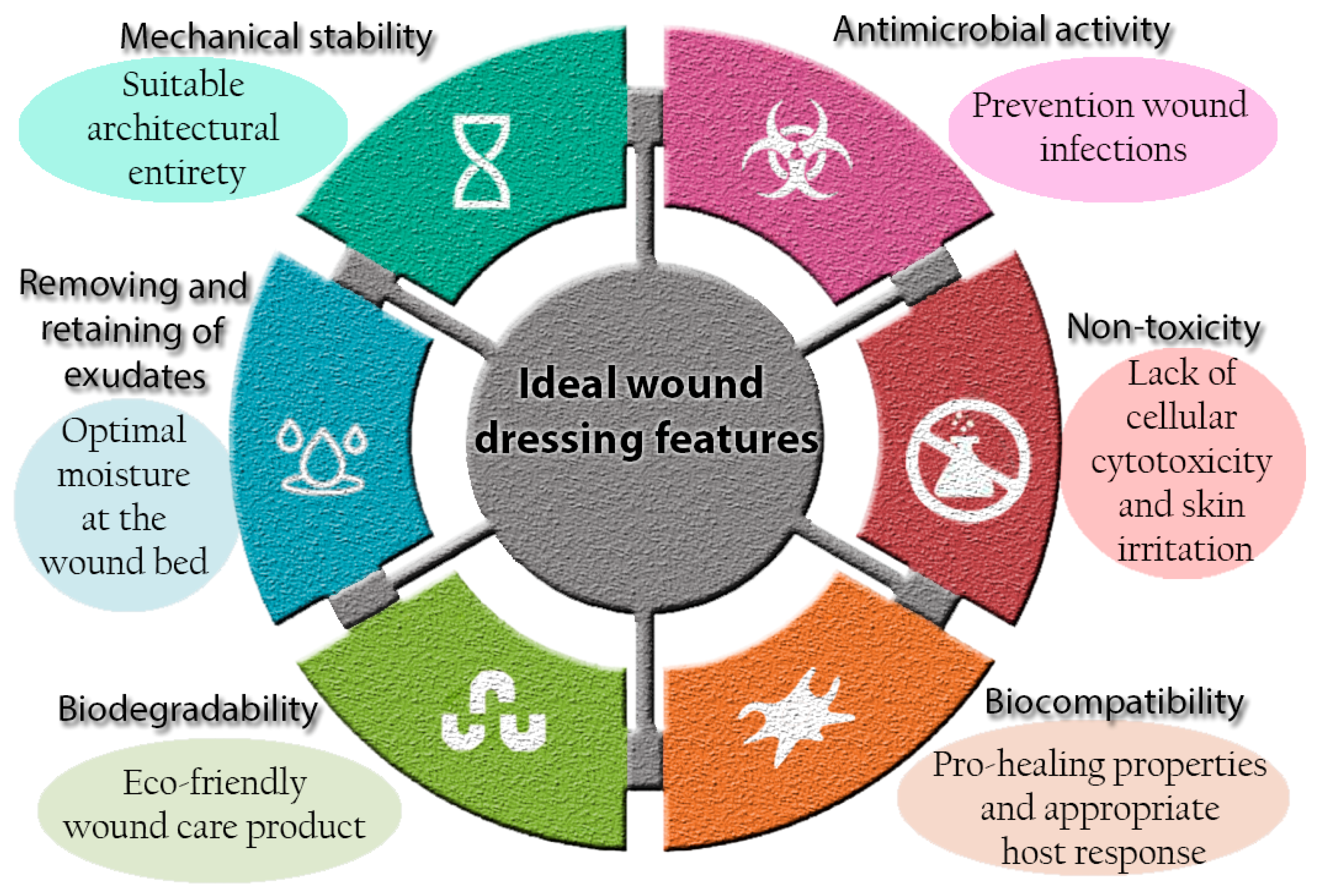
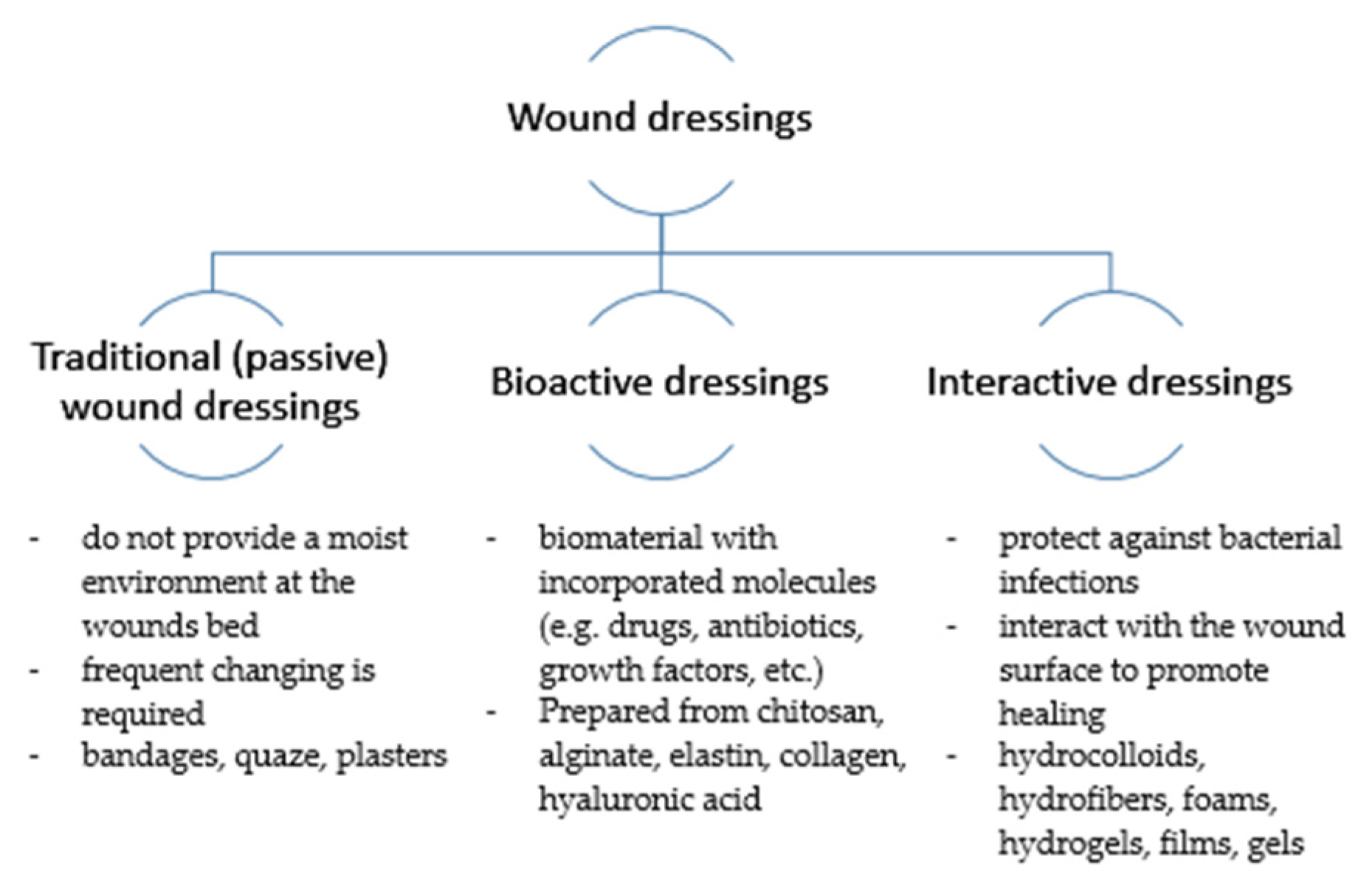
2. Current Concepts in Wound Dressings
3. Pro-Healing Wound Dressings
3.1. The Effect of Natural Compounds on Skin Regeneration
3.1.1. Curcumin-Loaded Biomaterials
3.1.2. Essential-Oil-Loaded Biomaterials
3.2. The Effect of Vitamins on Skin Regeneration
Bioactive Dressings Enriched with Vitamins
4. Patented and Commercial Bioactive Dressings
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound dressings—A review. Biomed. 2015, 5, 24–28. [Google Scholar] [CrossRef]
- Awadhiya, A.; Tyeb, S.; Rathore, K.; Verma, V. Agarose bioplastic-based drug delivery system for surgical and wound dressings. Eng. Life Sci. 2017, 17, 204–214. [Google Scholar] [CrossRef]
- Deng, Z.; Wang, T.; Chen, X.; Liu, Y. Applications of chitosan-based biomaterials: A focus on dependent antimicrobial properties. Mar. Life Sci. Technol. 2020, 2, 398–413. [Google Scholar] [CrossRef]
- Moeini, A.; Pedram, P.; Makvandi, P.; Malinconico, M.; Gomez d’Ayala, G. Wound healing and antimicrobial effect of active secondary metabolites in chitosan-based wound dressings: A review. Carbohydr. Polym. 2020, 233, 115839. [Google Scholar] [CrossRef]
- Cañedo-Dorantes, L.; Cañedo-Ayala, M. Skin Acute Wound Healing: A Comprehensive Review. Int. J. Inflam. 2019, 2019, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Armato, U.; Wu, J. Targeting Tunable Physical Properties of Materials for Chronic Wound Care. Front. Bioeng. Biotechnol. 2020, 8, 1–14. [Google Scholar] [CrossRef]
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahsan, S.M.; Thomas, M.; Reddy, K.K.; Sooraparaju, S.G.; Asthana, A.; Bhatnagar, I. Chitosan as biomaterial in drug delivery and tissue engineering. Int. J. Biol. Macromol. 2018, 110, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Przekora, A. A concise review on tissue engineered artificial skin grafts for chronic wound treatment: Can we reconstruct functional skin tissue in vitro? Cells 2020, 9, 1622. [Google Scholar] [CrossRef] [PubMed]
- Opt Veld, R.C.; Walboomers, X.F.; Jansen, J.A.; Wagener, F.A.D.T.G. Design Considerations for Hydrogel Wound Dressings: Strategic and Molecular Advances. Tissue Eng. Part B Rev. 2020, 26, 230–248. [Google Scholar] [CrossRef] [PubMed]
- Portela, R.; Leal, C.R.; Almeida, P.L.; Sobral, R.G. Bacterial cellulose: A versatile biopolymer for wound dressing applications. Microb. Biotechnol. 2019, 12, 586–610. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kumar, S.; Udupa, E.P.; Kumar, U.; Rao, P.; Honnegowda, T. Role of angiogenesis and angiogenic factors in acute and chronic wound healing. Plast. Aesthetic Res. 2015, 2, 243. [Google Scholar] [CrossRef] [Green Version]
- Lemraski, E.G.; Jahangirian, H.; Dashti, M.; Khajehali, E.; Sharafinia, S.; Moghaddam, R.R.; Webster, T.J. Antimicrobial double-layer wound dressing based on chitosan/polyvinyl alcohol/copper: In vitro and in vivo assessment. Int. J. Nanomedicine 2021, 16, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Kruse, C.R.; Singh, M.; Targosinski, S.; Sinha, I.; Sørensen, J.A.; Eriksson, E.; Nuutila, K. The effect of pH on cell viability, cell migration, cell proliferation, wound closure, and wound reepithelialization: In vitro and in vivo study. Wound Repair Regen. 2017, 25, 260–269. [Google Scholar] [CrossRef]
- Borda, L.J.; Macquhae, F.E.; Kirsner, R.S. Wound Dressings: A Comprehensive Review. Curr. Dermatol. Rep. 2016, 5, 287–297. [Google Scholar] [CrossRef]
- Rezvani Ghomi, E.; Khalili, S.; Nouri Khorasani, S.; Esmaeely Neisiany, R.; Ramakrishna, S. Wound dressings: Current advances and future directions. J. Appl. Polym. Sci. 2019, 136, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Wang, C.; Li, C.; Qin, Y.; Wang, Z.; Yang, F.; Li, Z.; Wang, J. A functional chitosan-based hydrogel as a wound dressing and drug delivery system in the treatment of wound healing. RSC Adv. 2018, 8, 7533–7549. [Google Scholar] [CrossRef] [Green Version]
- Khorasani, M.T.; Joorabloo, A.; Moghaddam, A.; Shamsi, H.; MansooriMoghadam, Z. Incorporation of ZnO nanoparticles into heparinised polyvinyl alcohol/chitosan hydrogels for wound dressing application. Int. J. Biol. Macromol. 2018, 114, 1203–1215. [Google Scholar] [CrossRef]
- Zaman, H.U.; Islam, J.M.M.; Khan, M.A.; Khan, R.A. Physico-mechanical properties of wound dressing material and its biomedical application. J. Mech. Behav. Biomed. Mater. 2011, 4, 1369–1375. [Google Scholar] [CrossRef]
- Aljghami, M.E.; Saboor, S.; Amini-Nik, S. Emerging innovative wound dressings. Ann. Biomed. Eng. 2019, 47, 659–675. [Google Scholar] [CrossRef]
- Peppas, N.A.; Hoffman, A.S. Hydrogels. In Biomaterials Science; Elsevier: Amsterdam, The Netherlands, 2020; pp. 153–166. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Khademhosseini, A. Advances in engineering hydrogels. Science 2017, 356. [Google Scholar] [CrossRef] [PubMed]
- Nuutila, K.; Eriksson, E. Moist Wound Healing with Commonly Available Dressings. Adv. Wound Care 2020, 1, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Moura, L.I.F.; Dias, A.M.A.; Carvalho, E.; De Sousa, H.C. Recent advances on the development of wound dressings for diabetic foot ulcer treatment—A review. Acta Biomater. 2013, 9, 7093–7114. [Google Scholar] [CrossRef] [Green Version]
- Bharambe, S.V.; Darekar, A.B.; Saudagar, R.B. Wound healing dressings and drug delivery systems: A review. Int. J. Pharm. Technol. 2013, 5, 2764–2786. [Google Scholar] [CrossRef]
- Dabiri, G.; Damstetter, E.; Phillips, T. Choosing a wound dressing based on common wound characteristics. Adv. Wound Care 2016, 5, 32–41. [Google Scholar] [CrossRef] [Green Version]
- Aderibigbe, B.A.; Buyana, B. Alginate in wound dressings. Pharmaceutics 2018, 10, 42. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [Green Version]
- Topuz, F.; Henke, A.; Richtering, W.; Groll, J. Magnesium ions and alginate do form hydrogels: A rheological study. Soft Matter 2012, 8, 4877–4881. [Google Scholar] [CrossRef]
- Zhao, W.Y.; Fang, Q.Q.; Wang, X.F.; Wang, X.W.; Zhang, T.; Shi, B.H.; Zheng, B.; Zhang, D.D.; Hu, Y.Y.; Ma, L.; et al. Chitosan-calcium alginate dressing promotes wound healing: A preliminary study. Wound Repair Regen. 2020, 28, 326–337. [Google Scholar] [CrossRef]
- Lei, J.; Sun, L.; Li, P.; Zhu, C.; Lin, Z. The Wound Dressings and Their Applications in Wound Healing and Management. Heal. Sci. J. 2019, 13, 1–8. [Google Scholar]
- Roducts, P.; Matica, A.; Ostafe, V. Biodegradability of chitosan based. New Front. Chem. 2017, 26, 75–86. [Google Scholar]
- Pang, Y.; Qin, A.; Lin, X.; Yang, L.; Wang, Q.; Wang, Z.; Shan, Z.; Li, S.; Wang, J.; Fan, S.; et al. Biodegradable and biocompatible high elastic chitosan scaffold is cell-friendly both in vitro and in vivo. Oncotarget 2017, 8, 35583–35591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laokuldilok, T.; Potivas, T.; Kanha, N.; Surawang, S.; Seesuriyachan, P.; Wangtueai, S.; Phimolsiripol, Y.; Regenstein, J.M. Physicochemical, antioxidant, and antimicrobial properties of chitooligosaccharides produced using three different enzyme treatments. Food Biosci. 2017, 18, 28–33. [Google Scholar] [CrossRef]
- Limem, K.; Majdoub, H.; Rouatbi, S. Antioxidant and antimicrobial proprieties of chitin and chitosan extracted from Parapenaeus Longirostris shrimp shell waste. Ann. Pharm. Fr. 2016, 74, 27–33. [Google Scholar] [CrossRef]
- Mata, M.A.L.; Ruiz, S.; José, C.; Ornelas, D.J.; Lizette, C.; Toro, D.; Enrique, S.; Ríos, M.; Silva, N.P.; Luis, B.; et al. Mechanical, Barrier and Antioxidant Properties of Chitosan Films Incorporating Cinnamaldehyde. J. Polym. Environ. 2018, 26, 452–461. [Google Scholar] [CrossRef]
- Wu, T.; Wu, C.; Fu, S.; Wang, L.; Yuan, C.; Chen, S.; Hu, Y. Integration of lysozyme into chitosan nanoparticles for improving antibacterial activity. Carbohydr. Polym. 2017, 155, 192–200. [Google Scholar] [CrossRef]
- Yadav, P.; Chaudhary, S.; Saxena, R.K.; Talwar, S.; Yadav, S. Evaluation of Antimicrobial and Antifungal efficacy of Chitosan as endodontic irrigant against Enterococcus Faecalis and Candida Albicans Biofilm formed on tooth substrate. J. Clin. Exp. Dent. 2017, 9. [Google Scholar] [CrossRef] [Green Version]
- Lu, Z.; Gao, J.; He, Q.; Wu, J.; Liang, D.; Yang, H.; Chen, R. Enhanced antibacterial and wound healing activities of microporous chitosan-Ag / ZnO composite dressing. Carbohydr. Polym. 2017, 156, 460–469. [Google Scholar] [CrossRef]
- Matica, A.; Ostafe, V. Toxicity of chitosan based products. New Front. Chem. 2017, 26, 65–74. [Google Scholar]
- Yadav, P.; Bandyopadhyay, A.; Chakraborty, A.; Sarkar, K. Enhancement of anticancer activity and drug delivery of chitosan-curcumin nanoparticle via molecular docking and simulation analysis. Carbohydr. Polym. 2018, 182, 188–198. [Google Scholar] [CrossRef]
- Quagliariello, V.; Masarone, M.; Giudice, A.; Barbarisi, M.; Caraglia, M.; Barbarisi, A.; Persico, M. Chitosan-coated liposomes loaded with butyric acid demonstrate anticancer and anti-inflammatory activity in human hepatoma HepG2 cells. Oncol. Rep. 2019, 1476–1486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matica, M.A.; Aachmann, F.L.; Tøndervik, A.; Sletta, H.; Ostafe, V. Chitosan as a wound dressing starting material: Antimicrobial properties and mode of action. Int. J. Mol. Sci. 2019, 20, 5889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davison-kotler, E.; Marshall, W.S.; Garc, E. Sources of Collagen for Biomaterials in Skin Wound Healing. Bioengineering 2019, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J. Cellulose and its derivatives: Towards biomedical applications. Cellulose 2021, 0123456789. [Google Scholar] [CrossRef]
- Mandla, S.; Davenport Huyer, L.; Radisic, M. Review: Multimodal bioactive material approaches for wound healing. APL Bioeng. 2018, 2. [Google Scholar] [CrossRef] [PubMed]
- Weller, C.D.; Team, V.; Sussman, G. First-line interactive wound dressing update: A comprehensive review of the evidence. Front. Pharmacol. 2020, 11, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Schoukens, G. Bioactive Dressings to Promote Wound Healing, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Liang, J.; Cui, L.; Li, J.; Guan, S.; Zhang, K.; Li, J. Aloe vera: A Medicinal Plant Used in Skin Wound Healing. Tissue Eng. Part B Rev. 2020, 1–67. [Google Scholar] [CrossRef]
- Pérez-Recalde, M.; Ruiz Arias, I.E.; Hermida, É.B. Could essential oils enhance biopolymers performance for wound healing? A systematic review. Phytomedicine 2018, 38, 57–65. [Google Scholar] [CrossRef]
- Gaspar-Pintiliescu, A.; Stanciuc, A.-M.; Craciunescu, O. Natural composite dressings based on collagen, gelatin and plant bioactive compounds for wound healing: A review. Int. J. Biol. Macromol. 2019, 138, 854–865. [Google Scholar] [CrossRef]
- Abbas, M.; Hussain, T.; Arshad, M.; Ansari, A.R.; Irshad, A.; Nisar, J.; Hussain, F.; Masood, N.; Nazir, A.; Iqbal, M. Wound healing potential of curcumin cross-linked chitosan/polyvinyl alcohol. Int. J. Biol. Macromol. 2019, 140, 871–876. [Google Scholar] [CrossRef]
- Sadeghianmaryan, A.; Yazdanpanah, Z.; Soltani, Y.A.; Sardroud, H.A.; Nasirtabrizi, M.H.; Chen, X. Curcumin-loaded electrospun polycaprolactone/montmorillonite nanocomposite: Wound dressing application with anti-bacterial and low cell toxicity properties. J. Biomater. Sci. Polym. Ed. 2020, 31, 169–187. [Google Scholar] [CrossRef] [PubMed]
- Alven, S.; Nqoro, X.; Aderibigbe, B.A. Polymer-based materials loaded with curcumin for wound healing applications. Polymers 2020, 12, 2286. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Keddie, D.J.; Kannappan, V.; Gibson, H.; Khalil, I.R.; Kowalczuk, M.; Martin, C.; Shuai, X.; Radecka, I. Production and characterisation of bacterial cellulose hydrogels loaded with curcumin encapsulated in cyclodextrins as wound dressings. Eur. Polym. J. 2019, 118, 437–450. [Google Scholar] [CrossRef]
- Mutlu, G.; Calamak, S.; Ulubayram, K.; Guven, E. Curcumin-loaded electrospun PHBV nanofibers as potential wound-dressing material. J. Drug Deliv. Sci. Technol. 2018, 43, 185–193. [Google Scholar] [CrossRef]
- Pankongadisak, P.; Sangklin, S.; Chuysinuan, P.; Suwantong, O.; Supaphol, P. The use of electrospun curcumin-loaded poly(L-lactic acid) fiber mats as wound dressing materials. J. Drug Deliv. Sci. Technol. 2019, 53, 101121. [Google Scholar] [CrossRef]
- Saeed, S.M.; Mirzadeh, H.; Zandi, M.; Barzin, J. Designing and fabrication of curcumin loaded PCL/PVA multi-layer nanofibrous electrospun structures as active wound dressing. Prog. Biomater. 2017, 6, 39–48. [Google Scholar] [CrossRef] [Green Version]
- Kaolaor, A.; Phunpee, S.; Ruktanonchai, U.R.; Suwantong, O. Effects of β-cyclodextrin complexation of curcumin and quaternization of chitosan on the properties of the blend films for use as wound dressings. J. Polym. Res. 2019, 26. [Google Scholar] [CrossRef]
- Shefa, A.A.; Sultana, T.; Park, M.K.; Lee, S.Y.; Gwon, J.G.; Lee, B.T. Curcumin incorporation into an oxidized cellulose nanofiber-polyvinyl alcohol hydrogel system promotes wound healing. Mater. Des. 2020, 186, 108313. [Google Scholar] [CrossRef]
- Venkatasubbu, G.D.; Anusuya, T. Investigation on Curcumin nanocomposite for wound dressing. Int. J. Biol. Macromol. 2017, 98, 366–378. [Google Scholar] [CrossRef]
- Huang, B.; Liu, M.; Zhou, C. Cellulose–halloysite nanotube composite hydrogels for curcumin delivery. Cellulose 2017, 24, 2861–2875. [Google Scholar] [CrossRef]
- Rosa, J.M.; Bonato, L.B.; Mancuso, C.B.; Martinelli, L.; Okura, M.H.; Malpass, G.R.P.; Granato, A.C. Antimicrobial wound dressing films containing essential oils and oleoresins of pepper encapsulated in sodium alginate films. Ciência Rural 2018, 48, 1–5. [Google Scholar] [CrossRef]
- Pereira dos Santos, E.; Nicácio, P.H.M.; Coêlho Barbosa, F.; Nunes da Silva, H.; Andrade, A.L.S.; Lia Fook, M.V.; Farias Leite, I. Chitosan/Essential Oils Formulations for Potential Use as Wound Dressing: Physical and Antimicrobial Properties. Materials 2019, 12, 2223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiji, S.; Udhayakumar, S.; Rose, C.; Muralidharan, C.; Kadirvelu, K. Thymol enriched bacterial cellulose hydrogel as effective material for third degree burn wound repair. Int. J. Biol. Macromol. 2019, 122, 452–460. [Google Scholar] [CrossRef]
- García-Salinas, S.; Evangelopoulos, M.; Gámez-Herrera, E.; Arruebo, M.; Irusta, S.; Taraballi, F.; Mendoza, G.; Tasciotti, E. Electrospun anti-inflammatory patch loaded with essential oils for wound healing. Int. J. Pharm. 2020, 577, 119067. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Qiu, Y.; Chen, W.; Wei, Q. Electrospun thymol-loaded porous cellulose acetate fibers with potential biomedical applications. Mater. Sci. Eng. C 2020, 109, 110536. [Google Scholar] [CrossRef]
- Ardekani, N.T.; Khorram, M.; Zomorodian, K.; Yazdanpanah, S.; Veisi, H.; Veisi, H. Evaluation of electrospun poly (vinyl alcohol)-based nanofiber mats incorporated with Zataria multiflora essential oil as potential wound dressing. Int. J. Biol. Macromol. 2019, 125, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Lee, S. Electrospun Nanofibrous Membranes with Essential Oils for Wound Dressing Applications. Fibers Polym. 2020, 21, 999–1012. [Google Scholar] [CrossRef]
- Altaf, F.; Niazi, M.B.K.; Jahan, Z.; Ahmad, T.; Akram, M.A.; Safdar, A.; Butt, M.S.; Noor, T.; Sher, F. Synthesis and Characterization of PVA/Starch Hydrogel Membranes Incorporating Essential Oils Aimed to be Used in Wound Dressing Applications. J. Polym. Environ. 2021, 29, 156–174. [Google Scholar] [CrossRef]
- Kuznetsova, T.A.; Andryukov, B.G.; Besednova, N.N.; Zaporozhets, T.S.; Kalinin, A.V. Marine algae polysaccharides as basis for wound dressings, drug delivery, and tissue engineering: A review. J. Mar. Sci. Eng. 2020, 8, 481. [Google Scholar] [CrossRef]
- Krishnan K, A.; Thomas, S. Recent advances on herb-derived constituents-incorporated wound-dressing materials: A review. Polym. Adv. Technol. 2019, 30, 823–838. [Google Scholar] [CrossRef]
- Vivcharenko, V.; Wojcik, M.; Palka, K.; Przekora, A. Highly Porous and Superabsorbent Biomaterial Made of Marine-Derived Polysaccharides and Ascorbic Acid as an Optimal Dressing for Exuding Wound Management. Materials 2021, 14, 1211. [Google Scholar] [CrossRef] [PubMed]
- Farzanfar, S.; Kouzekonan, G.S.; Mirjani, R.; Shekarchi, B. Vitamin B12-loaded polycaprolacton/gelatin nanofibrous scaffold as potential wound care material. Biomed. Eng. Lett. 2020, 10, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; DiPietro, L.A. Critical review in oral biology & medicine: Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Carr, A.C.; Maggini, S. Vitamin C and immune function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammed, B.M.; Fisher, B.J.; Kraskauskas, D.; Ward, S.; Wayne, J.S.; Brophy, D.F.; Fowler, A.A.; Yager, D.R.; Natarajan, R. Vitamin C promotes wound healing through novel pleiotropic mechanisms. Int. Wound J. 2016, 13, 572–584. [Google Scholar] [CrossRef]
- Pullar, J.M.; Carr, A.C.; Vissers, M.C.M. The roles of vitamin C in skin health. Nutrients 2017, 9, 866. [Google Scholar] [CrossRef] [Green Version]
- Müller, W.E.G.; Tolba, E.; Dorweiler, B.; Schröder, H.C.; Diehl-Seifert, B.; Wang, X. Electrospun bioactive mats enriched with Ca-polyphosphate/retinol nanospheres as potential wound dressing. Biochem. Biophys. Rep. 2015, 3, 150–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehterami, A.; Salehi, M.; Farzamfar, S.; Samadian, H.; Vaez, A.; Ghorbani, S.; Ai, J.; Sahrapeyma, H. Chitosan/alginate hydrogels containing Alpha-tocopherol for wound healing in rat model. J. Drug Deliv. Sci. Technol. 2019, 51, 204–213. [Google Scholar] [CrossRef]
- Prakoeswa, C.R.S.; Natallya, F.R.; Harnindya, D.; Thohiroh, A.; Oktaviyanti, R.N.; Pratiwi, K.D.; Rubianti, M.A.; Yogatri, B.; Primasari, P.I.; Herwanto, N.; et al. The efficacy of topical human amniotic membrane-mesenchymal stem cell-conditioned medium (hAMMSC-CM) and a mixture of topical hAMMSC-CM + vitamin C and hAMMSC-CM + vitamin E on chronic plantar ulcers in leprosy:a randomized control trial. J. Dermatolog. Treat. 2018, 29, 835–840. [Google Scholar] [CrossRef] [Green Version]
- Voss, G.T.; Gularte, M.S.; Vogt, A.G.; Giongo, J.L.; Vaucher, R.A.; Echenique, J.V.Z.; Soares, M.P.; Luchese, C.; Wilhelm, E.A.; Fajardo, A.R. Polysaccharide-based film loaded with vitamin C and propolis: A promising device to accelerate diabetic wound healing. Int. J. Pharm. 2018, 552, 340–351. [Google Scholar] [CrossRef]
- Madni, A.; Khan, R.; Ikram, M.; Naz, S.S.; Khan, T.; Wahid, F. Fabrication and characterization of chitosan–Vitamin C–lactic acid composite membrane for potential skin tissue engineering. Int. J. Polym. Sci. 2019, 2019. [Google Scholar] [CrossRef]
- Ehterami, A.; Salehi, M.; Farzamfar, S.; Samadian, H.; Vaez, A.; Sahrapeyma, H.; Ghorbani, S. A promising wound dressing based on alginate hydrogels containing vitamin D3 cross-linked by calcium carbonate/d-glucono-δ-lactone. Biomed. Eng. Lett. 2020, 10, 309–319. [Google Scholar] [CrossRef]
- Cerchiara, T.; Giordani, B.; Melgoza, L.M.; Prata, C.; Parolin, C.; Dalena, F.; Abruzzo, A.; Bigucci, F.; Luppi, B.; Vitali, B. New Spanish Broom dressings based on Vitamin E and Lactobacillus plantarum for superficial skin wounds. J. Drug Deliv. Sci. Technol. 2020, 56, 101499. [Google Scholar] [CrossRef]
- Li, H.; Wang, M.; Williams, G.R.; Wu, J.; Sun, X.; Lv, Y.; Zhu, L.-M. Electrospun gelatin nanofibers loaded with vitamins A and E as antibacterial wound dressing materials. RSC Adv. 2016, 6, 50267–50277. [Google Scholar] [CrossRef]
- Kheradvar, S.A.; Nourmohammadi, J.; Tabesh, H.; Bagheri, B. Starch nanoparticle as a vitamin E-TPGS carrier loaded in silk fibroin-poly(vinyl alcohol)-Aloe vera nanofibrous dressing. Colloids Surf. B Biointerfaces 2018, 166, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Ousey, K. HydroClean ® plus: A new and debridement. Wounds UK 2016, 12, 94–104. [Google Scholar]
- Rafter, L.; Reynolds, T.; Collier, M.; Rafter, M.; West, M. A clinical evaluation of Algivon® Plus manuka honey dressings for chronic wounds. Wounds UK 2017, 13, 132–140. [Google Scholar]
- Davies, P.; McCarty, S.; Hamberg, K. Silver-containing foam dressings with Safetac: A review of the scientific and clinical data. J. Wound Care 2017, 26 (Suppl. 6a), S1–S32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebert, M.; Assadian, O.; Hübner, N.O.; Koburger, T.; Kramer, A. Antimicrobial efficacy of the silver wound dressing Biatain Ag in a disc carrier test simulating wound secretion. Skin Pharmacol. Physiol. 2011, 24, 337–341. [Google Scholar] [CrossRef]
- Varela, P.; Marlinghaus, L.; Sartori, S.; Viebahn, R.; Salber, J.; Ciardelli, G. Response of Human Macrophages to Clinically Applied Wound Dressings Loaded With Silver. Front. Bioeng. Biotechnol. 2020, 8, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khampieng, T.; Wongkittithavorn, S.; Chaiarwut, S.; Ekabutr, P.; Pavasant, P.; Supaphol, P. Silver nanoparticles-based hydrogel: Characterization of material parameters for pressure ulcer dressing applications. J. Drug Deliv. Sci. Technol. 2018, 44, 91–100. [Google Scholar] [CrossRef]
| Type of Biomaterial | Composition of the Biomaterial | Experimental Model | Biological Properties and Advantages | Limitations | Ref. |
|---|---|---|---|---|---|
| Hydrogel | Curcumin, bacterial cellulose | In vitro (A549—human lung adenocarcinoma, MSTO—human mesothelioma, PANC1—human pancreatic ductal adenocarcinoma, U251MG—human glioblastoma, horse blood cells) | Non-cytotoxicity, antibacterial (S. aureus) and antioxidant properties | Not provided | [55] |
| Nanofiber | Curcumin, poly(3-hydroxybutyric acid-co-3-hydroxyvaleric acid) (PHBV) | In vitro (L929—mouse fibroblast cell line) | Enhanced cell adhesion and proliferation | Low mechanical properties related to high curcumin concentration | [56] |
| Fiber mat | Curcumin, pure poly-L-lactic acid (PLLA) | In vitro (HDFa—human adult dermal fibroblasts) | Enhanced cell adhesion and proliferation, antioxidant properties | Not provided | [57] |
| Membrane | Curcumin, chitosan, polyvinyl alcohol (PVA) | In vivo (rabbit model) | Biodegradability, low production cost, antibacterial (P. multocida, S. aureus, E. coli, B. subtilis) and antioxidant properties | Dedicated mainly to burn wounds | [52] |
| Nanofiber | Curcumin, polylactic acid (PLA), polycaprolactone (PCL) | In vitro (L929—mouse fibroblast cell line) | Antibacterial (E.coli, S. aureus) activity, hydrophobic behavior | Slight toxicity | [58] |
| Film | Curcumin, chitosan, β-cyclodextrin | In vitro (NHDF—normal human dermal fibroblast cell line, NCTC clone 929 cells—mouse subcutaneous fibroblast cell line | Enhanced mechanical properties, antioxidant activity | Slight reduction in water swelling | [59] |
| Hydrogel | Curcumin, PVA, TEMPO-oxidized cellulose nanofiber (TOCN) | In vitro (L929—mouse fibroblast cell line), In vivo (rat model) | Enhanced collagen organization, supported wound contraction | Not provided | [60] |
| Nanocomposite (gauze) | Curcumin, cotton | Not provided | Enhanced water absorption and drying time | Not provided | [61] |
| Hydrogel | Curcumin, cellulose–halloysite nanotube | In vitro (MC3T3-E1—mouse calvarial preosteoblasts, MCF-7—human breast cancer cell line) | Anticancer properties | Reduced cell proliferation | [62] |
| Nanocomposite | Curcumin, PCL, quaternary ammonium salt-modified montmorillonite (MMT) | In vitro (L929—mouse fibroblast cell line) | Enhanced antibacterial activity (E.coli, S. aureus) | Initial burst release of curcumin | [53] |
| Type of Biomaterial | Composition of the Biomaterial | Experimental Model | Biological Properties and Advantages | Limitations | Ref. |
|---|---|---|---|---|---|
| Hydrogel | Thymol, bacterial cellulose | In vitro (NIH 3T3—mouse fibroblast cell line)In vivo (rat model) | Enhanced antibacterial activity (E. coli, S. aureus, P. aeruginosa, K. pneumoniae) and wound closure speed | Decreased water vapor transmission rate | [65] |
| Nanofiber | Thymol, tyrosol, PCL | In vitro (J774A.1—macrophage cell line) | Anti-inflammatory activity | Not provided | [66] |
| Fibrous membrane | Thymol, cellulose | In vitro (L929—mouse fibroblast cell line) | Enhanced antibacterial activity (E. coli, S. aureus) | Decreased wettability | [67] |
| Nanofiber mat | Zataria multiflora essential oil, chitosan, PVA, gelatin | In vitro (L929—mouse fibroblast cell line) | Enhanced antimicrobial activity (C. albicans, S. aureus, P. aeruginosa) | Decreased swelling degree | [68] |
| Film | Eugenia caryophyllata essential oil, Melaleuca alternifolia essential oil, chitosan | Not provided | Enhanced biomaterial elasticity and flexibility | Decreased mechanical strength | [64] |
| Nanofiber membrane | Cymbopogon martini essential oil, Chamaecyparis obtusa essential oil, PVA | Not provided | Enhanced antimicrobial activity (S. aureus, C. albicans) and aqueous stability | Not provided | [69] |
| Hydrogel | Clove essential oil, tea tree essential oil, oregano essential oil, PVA, starch | Not provided | Enhanced antibacterial activity (E.coli, S. aureus) | Decreased mechanical strength | [70] |
| Type of Biomaterial | Composition of the Biomaterial | Experimental Model | Biological Properties and Advantages | Limitations | Ref. |
|---|---|---|---|---|---|
| Foam-like, hydrocolloid type | Vitamin C, agarose, chitosan | In vitro (BJ—normal human skin fibroblast cell line) | Enhanced fibroblasts viability and proliferation, supported platelet-derived growth factor (PDGF-BB) synthesis | Initial burst release of vitamin C | [73] |
| Film | Vitamin C, Brazilian propolis, cellulose, PVA | In vivo (mouse model) | Enhanced absorptive capacity, accelerated wound closure rate | Not provided | [82] |
| Membrane | Vitamin C, chitosan, polyethylene glycol (PEG), glycerol | In vitro (NIH 3T3—mouse fibroblast cell line) | Enhanced biocompatibility | Increased fragility | [83] |
| Nanofibrous scaffold | Vitamin B12, PCL, gelatin (type A) | In vitro (L929—mouse fibroblast cell line), In vivo (rat model) | Enhanced wound closure rate and cell viability, increased epithelial thickness | Not provided | [74] |
| Hydrogel | Vitamin D3, alginate | In vitro (L929—mouse fibroblast cell line), In vivo (rat model) | Promoted cells proliferation, accelerated wound healing | Swelling percentage decreased with time | [84] |
| Hydrogel | Vitamin E, chitosan, alginate | In vitro (L929—mouse fibroblast cell line), In vivo (rat model) | Enhanced wound closure and re-epithelialization | Not provided | [80] |
| Gauzes/fibers | Vitamin E, Lactobacillus plantarum, Spanish Broom fibers, cotton | In vitro (BJ—normal human skin fibroblast cell line) | Enhanced antioxidant properties | Initial burst release of the vitamin E | [85] |
| Nanofibers mats | Vitamin E, silk fibroin, PVA, Aloe vera | In vitro (L929—mouse fibroblast cell line) | Enhanced cell-matrix interactions and cellular viability, antioxidant activity | Initial burst release of vitamin E | [87] |
| Nanofibers | Vitamin A and E, gelatin | In vitro (L929—mouse fibroblast cell line), In vivo (rat model) | Enhanced antibacterial activity (E. coli, S. aureus) and L929 fibroblast cells growth | Decreased fiber diameter | [86] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vivcharenko, V.; Przekora, A. Modifications of Wound Dressings with Bioactive Agents to Achieve Improved Pro-Healing Properties. Appl. Sci. 2021, 11, 4114. https://doi.org/10.3390/app11094114
Vivcharenko V, Przekora A. Modifications of Wound Dressings with Bioactive Agents to Achieve Improved Pro-Healing Properties. Applied Sciences. 2021; 11(9):4114. https://doi.org/10.3390/app11094114
Chicago/Turabian StyleVivcharenko, Vladyslav, and Agata Przekora. 2021. "Modifications of Wound Dressings with Bioactive Agents to Achieve Improved Pro-Healing Properties" Applied Sciences 11, no. 9: 4114. https://doi.org/10.3390/app11094114
APA StyleVivcharenko, V., & Przekora, A. (2021). Modifications of Wound Dressings with Bioactive Agents to Achieve Improved Pro-Healing Properties. Applied Sciences, 11(9), 4114. https://doi.org/10.3390/app11094114







