Effects of Treatment Combining 9300 nm Carbon Dioxide Lasers and Fluoride on Prevention of Enamel Caries: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Focused Question
2.2. Search Strategy
2.3. Study Selection and Data Extraction
2.3.1. Inclusion Criteria
2.3.2. Exclusion Criteria
- Studies on other lasers or CO2 lasers wavelengths other than 9300 nm;
- Studies on dental erosion;
- Literature reviews, case reports, conference papers, and book sections.
2.4. Data Extraction
2.5. Assessment of Risk of Bias
2.6. Data Analysis
3. Results
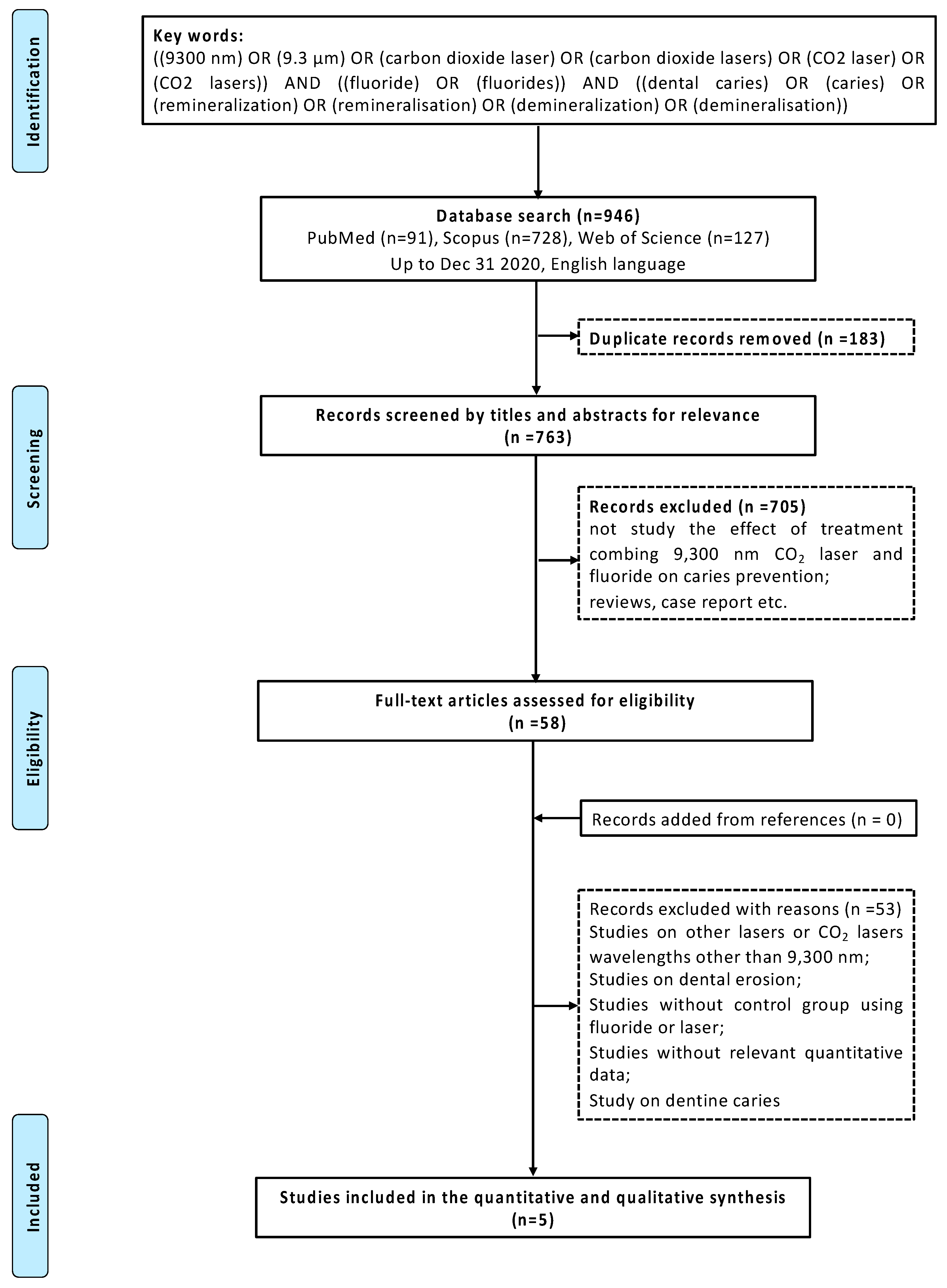
3.1. Search and Selection
3.2. Descriptive Analysis
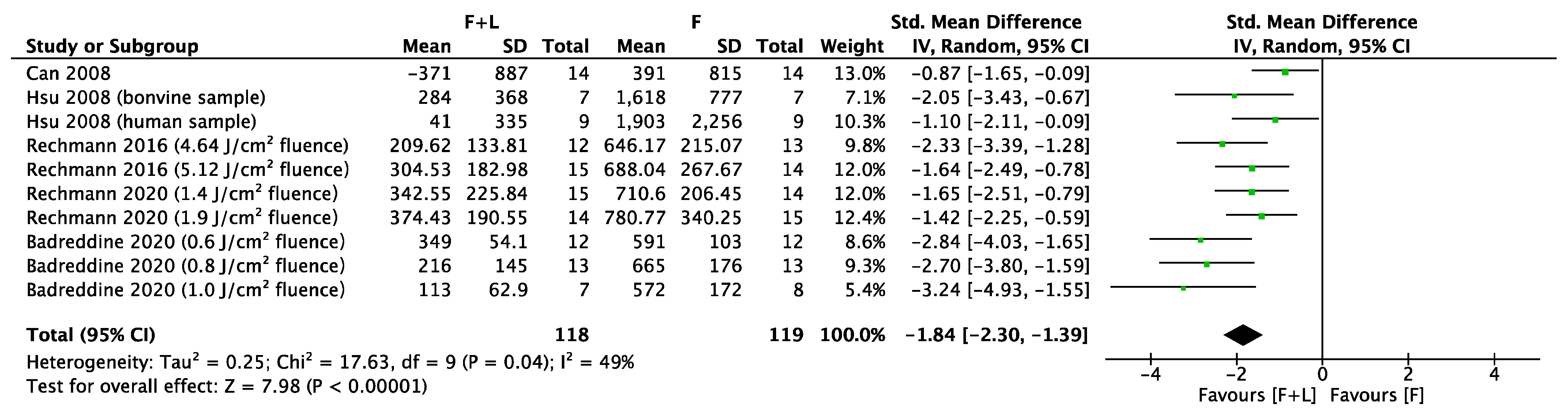
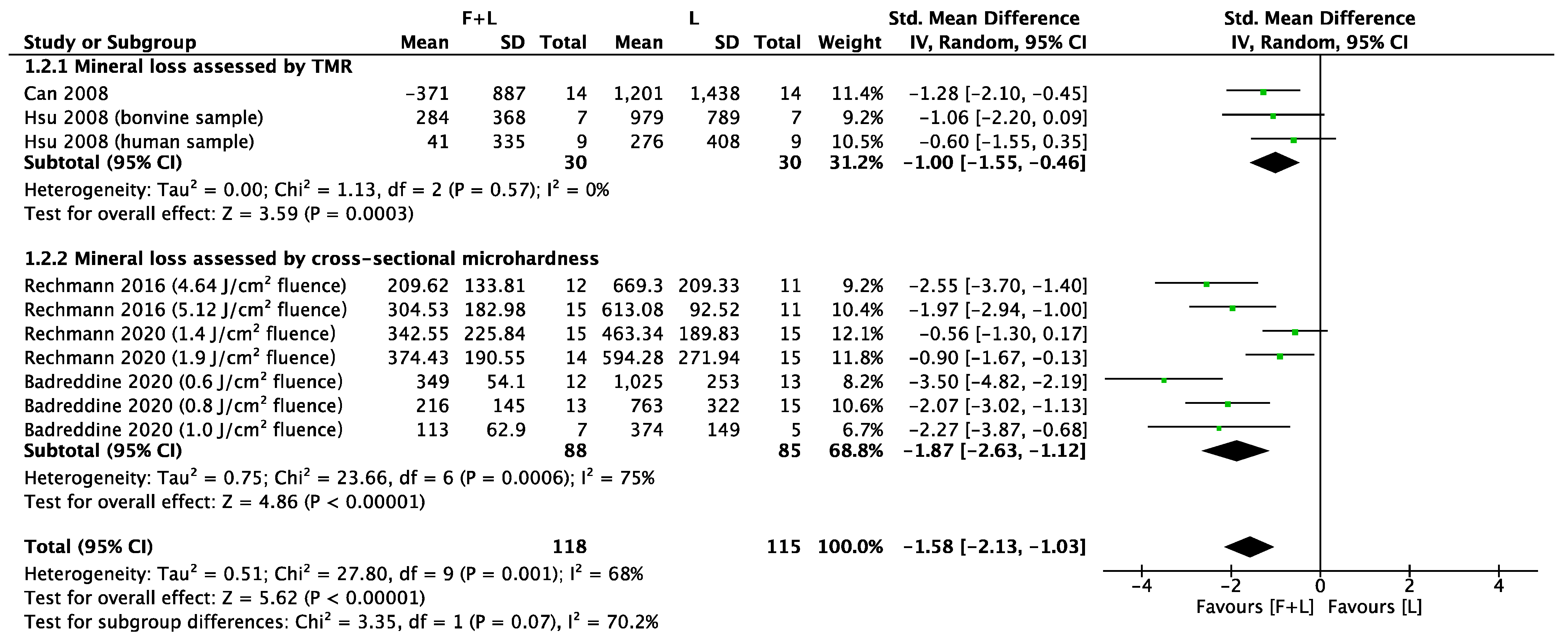
3.3. Meta-Analyses
3.4. Risk of Bias
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Petersen, P.E.; Lennon, M.A. Effective use of fluorides for the prevention of dental caries in the 21st century: The WHO approach. Coasmmunity Dent. Oral Epidemio. 2004, 32, 319–321. [Google Scholar] [CrossRef] [PubMed]
- Featherstone, J. Dental caries: A dynamic disease process. Aust. Dent. J. 2008, 53, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Shah, N. Dental Caries: The Disease and Its Clinical Management, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2009; Volume 206, p. 498. [Google Scholar]
- American Dental Association Council on Scientific Affairs. Professionally applied topical fluoride: Evidence-based clinical recommendations. J. Am. Dent. Assoc. 2006, 137, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.S.; Zhang, S.; Mei, M.L.; Lo, E.C.M.; Chu, C.H. Caries remineralisation and arresting effect in children by professionally applied fluoride treatment–a systematic review. BMC Oral Health. 2016, 16, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rosin-Grget, K.; Lincir, I. Current concept on the anticaries fluoride mechanism of the action. Coll. Antropol. 2001, 25, 703–712. [Google Scholar] [PubMed]
- Li, X.; Wang, J.; Joiner, A.; Chang, J. The remineralisation of enamel: A review of the literature. J. Dent. 2014, 42, S12–S20. [Google Scholar] [CrossRef]
- Mei, M.L.; Li, Q.; Chu, C.H.; Lo, E.C.M.; Samaranayake, L.P. Antibacterial effects of silver diamine fluoride on multi-species cariogenic biofilm on caries. Ann. Clin. Microbiol. Antimicrob. 2013, 12, 4. [Google Scholar] [CrossRef]
- Balzar Ekenbäck, S.; Linder, L.E.; Sund, M.L.; Lönnies, H. Effect of fluoride on glucose incorporation and metabolism in biofilm cells of Streptococcus mutans. Eur. J. Oral Sci. 2001, 109, 182–186. [Google Scholar] [CrossRef]
- Mei, M.L.; Li, Q.; Chu, C.H.; Yiu, C.K.; Lo, E.C.M. The inhibitory effects of silver diamine fluoride at different concentrations on matrix metalloproteinases. Dent. Mater. 2012, 28, 903–908. [Google Scholar] [CrossRef]
- Al-Maliky, M.A.; Frentzen, M.; Meister, J. Laser-assisted prevention of enamel caries: A 10-year review of the literature. Lasers Med. Sci. 2020, 35, 13–30. [Google Scholar] [CrossRef]
- Chun, K.; Choi, H.; Lee, J. Comparison of mechanical property and role between enamel and dentin in the human teeth. J. Dent. Biomech. 2014, 5, 1758736014520809. [Google Scholar] [CrossRef] [PubMed]
- Luk, K.; Zhao, I.S.; Gutknecht, N.; Chu, C.H. Use of carbon dioxide lasers in dentistry. Laser Dent. Sci. 2019, 3, 1–9. [Google Scholar] [CrossRef]
- Pagano, S.; Lombardo, G.; Orso, M.; Abraha, I.; Capobianco, B.; Cianetti, S. Lasers to prevent dental caries: A systematic review. BMJ Open. 2020, 10, e038638. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, L.K.; Nobre dos Santos, M.; Pereira, D.; Assaf, A.V.; Pardi, V. Carbon dioxide laser in dental caries prevention. J. Dent. 2004, 32, 531–540. [Google Scholar] [CrossRef]
- Fantarella, D.; Kotlow, L. The 9.3-µm CO2 dental laser: Technical development and early clinical experiences. J. Laser Dent. 2014, 22, 1. [Google Scholar]
- Rechmann, P.; Rechmann, B.M.; Groves, W.H., Jr.; Le, C.Q.; Rapozo-Hilo, M.L.; Kinsel, R.; Featherstone, J.D. Caries inhibition with a CO2 9.3 μm laser: An in vitro study. Lasers Surg. Med. 2016, 48, 546–554. [Google Scholar] [CrossRef] [PubMed]
- McCormack, S.M.; Fried, D.; Featherstone, J.D.; Glena, R.E.; Seka, W. Scanning electron microscope observations of CO2 laser effects on dental enamel. J. Dent. Res. 1995, 74, 1702–1708. [Google Scholar] [CrossRef]
- Fried, D.; Featherstone, J.D.; Le, C.Q.; Fan, K. Dissolution studies of bovine dental enamel surfaces modified by high-speed scanning ablation with a lambda = 9.3-microm TEA CO2 laser. Lasers Surg. Med. 2006, 38, 837–845. [Google Scholar] [CrossRef]
- Chokhachi Zadeh Moghadam, N.; Seraj, B.; Chiniforush, N.; Ghadimi, S. Effects of Laser and Fluoride on the Prevention of Enamel Demineralization: An In Vitro Study. J. Lasers Med. Sci. 2018, 9, 177–182. [Google Scholar] [CrossRef]
- Lee, R.; Chan, K.H.; Jew, J.; Simon, J.C.; Fried, D. Synergistic effect of fluoride and laser irradiation for the inhibition of the demineralization of dental enamel. Proc. SPIE Int. Soc. Opt. Eng. 2017, 10044. [Google Scholar] [CrossRef]
- Chang, N.N.; Jew, J.M.; Simon, J.C.; Chen, K.H.; Lee, R.C.; Fried, W.A.; Cho, J.; Darling, C.L.; Fried, D. Influence of multi-wavelength laser irradiation of enamel and dentin surfaces at 0.355, 2.94, and 9.4 μm on surface morphology, permeability, and acid resistance. Lasers Surg. Med. 2017, 49, 913–927. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, L.K.A.; Nobre Dos Santos, M.; Featherstone, J.D.B. In situ mineral loss inhibition by CO2 laser and fluoride. J. Dent. Res. 2006, 85, 617–621. [Google Scholar] [CrossRef]
- Gao, X.L.; Hsu, C.Y. Synergistic effects of fluoride and CO2 laser on root demineralization. J. Dent. Res. 2003, 82, 650. [Google Scholar]
- Anaraki, S.N.; Serajzadeh, M.; Fekrazad, R. Effects of laser-assisted fluoride therapy with a CO2 laser and Er, Cr:YSGG laser on enamel demineralization. Pediatr. Dent. 2012, 34, e92–e96. [Google Scholar] [PubMed]
- Mirhashemi, A.H.; Hakimi, S.; Ahmad Akhoundi, M.S.; Chiniforush, N. Prevention of enamel adjacent to bracket demineralization following carbon dioxide laser radiation and titanium tetra fluoride solution treatment: An In vitro Study. J Lasers Med. Sci. 2016, 7, 192–196. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Belcheva, A.; El Feghali, R.; Nihtianova, T.; Parker, S. Effect of the carbon dioxide 10,600-nm laser and topical fluoride gel application on enamel microstructure and microhardness after acid challenge: An in vitro study. Lasers Med. Sci. 2018, 33, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Bahrololoomi, Z.; Ardakani, F.F.; Sorouri, M. In vitro comparison of the effects of diode laser and CO2 Laser on topical fluoride uptake in Primary Teeth. J. Dent. 2015, 12, 585–591. [Google Scholar]
- Vlacic, J.; Meyers, I.; Walsh, L. Photonic conversion of hydroxyapapite to fluorapatite: A possible mechanism for laser-activated fluoride therapy. J. Oral Laser Appl. 2008, 8, 95–102. [Google Scholar]
- Rechmann, P.; Charland, D.A.; Rechmann, B.M.; Le, C.Q.; Featherstone, J.D. In-vivo occlusal caries prevention by pulsed CO2 -laser and fluoride varnish treatment--a clinical pilot study. Lasers Surg. Med. 2013, 45, 302–310. [Google Scholar] [CrossRef]
- Fekrazad, R.; Najafi, A.; Mahfar, R.; Namdari, M.; Azarsina, M. Comparison of enamel remineralization potential after. application of titanium tetra fluoride and carbon dioxide laser. Laser Ther. 2017, 26, 113–119. [Google Scholar] [CrossRef]
- Moher, D. Corrigendum to: Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Green, S. Cochrane handbook for Systematic Reviews of Interventions. Version 6.2.0 [updated April 2021]. The Cochrane Collaboration. 2021. Available online: www.cochrane-handbook.org (accessed on 27 April 2021).
- Martins, F.V.; Vasques, W.F.; Fonseca, E.M. Evaluation of the efficiency of fluoride-releasing adhesives for preventing secondary caries in vitro: A systematic review and meta-analysis. Eur. J. Paediatr. Dent. 2018, 20, 1–8. [Google Scholar] [CrossRef]
- Hbibi, A.; Sikkou, K.; Khedid, K.; El Hamzaoui, S.; Bouziane, A.; Benazza, D. Antimicrobial activity of honey in periodontal disease: A systematic review. J. Antimicrob. Chemother. 2020, 75, 807–826. [Google Scholar] [CrossRef] [PubMed]
- Soares, F.Z.; Follak, A.; da Rosa, L.S.; Montagner, A.F.; Lenzi, T.L.; Rocha, R.O. Bovine tooth is a substitute for human tooth on bond strength studies: A systematic review and meta-analysis of in vitro studies. Dent. Mater. 2016, 32, 1385–1393. [Google Scholar] [CrossRef]
- Can, A.M.; Darling, C.L.; Ho, C.; Fried, D. Non-destructive assessment of inhibition of demineralization in dental enamel irradiated by a lambda=9.3-microm CO2 laser at ablative irradiation intensities with PS-OCT. Lasers Surg. Med. 2008, 40, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Hsu, D.J.; Darling, C.L.; Lachica, M.M.; Fried, D. Nondestructive assessment of the inhibition of enamel demineralization by CO2 laser treatment using polarization sensitive optical coherence tomography. J. Biomed. Opt. 2008, 13. [Google Scholar] [CrossRef]
- Rechmann, P.; Le, C.Q.; Kinsel, R.; Kerbage, C.; Rechmann, B.M.T. In vitro CO2 9.3-μm short-pulsed laser caries prevention-effects of a newly developed laser irradiation pattern. Lasers Med. Sci. 2020, 35, 979–989. [Google Scholar] [CrossRef] [PubMed]
- Badreddine, A.H.; Couitt, S.; Donovan, J.; Cantor-Balan, R.; Kerbage, C.; Rechmann, P. Demineralization inhibition by high-speed scanning of 9.3 µm CO2 single laser pulses over enamel. Lasers Surg. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Churchley, D.; Lippert, F.; Lynch, R.; Alton, J.; Gonzalez-Cabezas, C.; Eder, J. A comparison of terahertz-pulsed imaging with transverse microradiography and microhardness to measure mineral changes in enamel after treatment with fluoride dentifrices. In Lasers in Dentistry XV; Proc SPIE XV; International Society for Optics and Photonics: Washington, DC, USA, 2009; p. 716203. [Google Scholar]
- Manesh, S.K.; Darling, C.L.; Fried, D. Nondestructive Assessment of Dentin Demineralization Using Polarization-Sensitive Optical Coherence Tomography After Exposure to Fluoride and Laser Irradiation. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 90B, 802–812. [Google Scholar] [CrossRef]
- Robinson, C.; Shore, R.C.; Brookes, S.J.; Strafford, S.; Wood, S.R.; Kirkham, J. The chemistry of enamel caries. Crit. Rev. Oral. Biol. Med. 2000, 11, 481–495. [Google Scholar] [CrossRef]
- Yu, O.Y.; Zhao, I.S.; Mei, M.L.; Lo, E.C.M.; Chu, C.H. Caries-arresting effects of silver diamine fluoride and sodium fluoride on dentine caries lesions. J. Dent. 2018, 78, 65–71. [Google Scholar] [CrossRef]
- Yassen, G.H.; Platt, J.A.; Hara, A.T. Bovine teeth as substitute for human teeth in dental research: A review of literature. J. Oral Sci. 2011, 53, 273–282. [Google Scholar] [CrossRef]
- Featherstone, J.D.; Mellberg, J.R. Relative rates of progress of artificial carious lesions in bovine, ovine and human enamel. Caries Res. 1981, 15, 109–114. [Google Scholar] [CrossRef]
- Song, F.; Koo, H.; Ren, D. Effects of material properties on bacterial adhesion and biofilm formation. J. Dent. Res. 2015, 94, 1027–1034. [Google Scholar] [CrossRef]
- Hu, X.L.; Ho, B.; Lim, C.T.; Hsu, C.S. Thermal treatments modulate bacterial adhesion to dental enamel. J. Dent. Res. 2011, 90, 1451–1456. [Google Scholar] [CrossRef]
- Cohen, J.; Featherstone, J.D.B.; Le, C.Q.; Steinberg, D.; Feuerstein, O. Effects of CO2 laser irradiation on tooth enamel coated. with biofilm. Lasers Surg. Med. 2014, 46, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.J.; Oh, H.W.; Lee, D.W.; Kim, C.H.; Ahn, J.Y.; Kim, Y.; Shin, H.B.; Kim, C.Y.; Park, S.H.; Jeon, J.G. chronologic trends in studies on fluoride mechanisms of action. J. Dent. Res. 2017, 96, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Esteves-Oliveira, M.; El-Sayed, K.F.; Dörfer, C.; Schwendicke, F. Impact of combined CO2 laser irradiation and fluoride on enamel and dentin biofilm-induced mineral loss. Clin. Oral Investig. 2017, 21, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Xuelian, H.; Qiang, G.; Biao, R.; Yuqing, L.; Xuedong, Z. Models in Caries Research, Dental Caries; Springer: Berlin, Germany, 2016; pp. 157–173. [Google Scholar]
- Yu, O.Y.; Zhao, I.S.; Mei, M.L.; Lo, E.C.M.; Chu, C.H. A review of the common models used in mechanistic studies on demineralization-remineralization for cariology research. Dent. J. 2017, 5, 20. [Google Scholar] [CrossRef] [PubMed]
- Skucha-Nowak, M.; Gibas, M.; Tanasiewicz, M.; Twardawa, H.; Szklarski, T. Natural and controlled demineralization for study purposes in minimally invasive dentistry. Adv. Clin. Exp. Med. 2015, 24, 891–898. [Google Scholar] [CrossRef]
- Staninec, M.; Darling, C.L.; Goodis, H.E.; Pierre, D.; Cox, D.P.; Fan, K.; Larson, M.; Parisi, R.; Hsu, D.; Manesh, S.K. Pulpal effects of enamel ablation with a microsecond pulsed λ = 9.3-µm CO2 laser. Lasers Surg. Med. 2009, 41, 256–263. [Google Scholar] [CrossRef]
- Oliveira, M.R.C.; Oliveira, P.H.C.; Oliveira, L.H.C.; Sfalcin, R.A.; Prates, R.A.; Navarro, R.S.; Cesar, P.F.; Deana, A.M.; Chavantes, M.C.; Bussadori, S.K.; et al. Influence of Ultrapulsed CO2 Laser, before Application of Different Types of Fluoride, on the Increase of Microhardness of Enamel In Vitro. Biomed. Res. Int. 2018, 5852948. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, M.N.; Featherstone, J.D.B.; Fried, D. Effect of a new carbon dioxide laser and fluoride on sound and demineralized enamel. Proc. SPIE. 2001, 4249, 169–174. [Google Scholar] [CrossRef]
- Esteves-Oliveira, M.; Pasaporti, C.; Heussen, N.; Eduardo, C.P.; Lampert, F.; Apel, C. Rehardening of acid-softened enamel and prevention of enamel softening through CO2 laser irradiation. J. Dent. 2011, 39, 414–421. [Google Scholar] [CrossRef] [PubMed]
| Authors, Year [Reference] | Laser Machine; Manufacturer | Cooling | Fluence | Pulse Duration | Beam Diameter | Frequency | Spot/ Motion | Irradiation Time |
|---|---|---|---|---|---|---|---|---|
| J/cm2 | µs | μm | Hz | mm/s | s | |||
| Can et al., 2008 [37] | Impact 2500; GSI Lumonics | Air and water | 20 | 15 | 320 | 100 | Motion 1.5 | - |
| Hsu et al., 2008 [38] | Impact 2500; GSI Lumonics | No | 1 | 15 | 100 | 200 | Motion 3.0 | - |
| Rechmann et al., 2016 [17] | Impact 2500; GSI Lumonics | No | 4.6 | 5 | 250 | 43 | Spot | 120 |
| No | 5.12 | 6 | 250 | 43 | Spot | 120 | ||
| Rechmann et al., 2020 [39] | Solea, Convergent Dental | Air Air | 1.4 1.9 | 11.4 14.6 | 630 630 | 100 100 | Spot Spot | 25 25 |
| Badreddine et al., 2020 [40] | Solea, Convergent Dental | Air | 0.6 0.8 1.0 | 17 22 27 | 1000 1000 1000 | 750 | Spot | 0.3 |
| Authors Year [Reference] | Specimen | Study Design | Cariogenic Challenge | Fluoride, Concentration | Fluoride Application | Assessment |
|---|---|---|---|---|---|---|
| Can et al., 2008 [37] | Bovine enamel | In vitro, chemical model | pH 4.9, 9 days | Acidulated phosphate fluoride, 12,300 ppm | After laser irradiation | PS-OCT, PLM, TMR |
| Hsu et al., 2008 [38] | Bovine and human enamel | In vitro, chemical model | pH 4.9, 7 days | Acidulated phosphate fluoride, 12,300 ppm | After laser irradiation | PS-OCT, PLM, TMR, LIF |
| Rechmann et al., 2016 [17] | Human enamel | In vitro, chemical model | pH-cycling pH 4.4/7.0, 9 days | Sodium fluoride, 825 ppm | After laser irradiation | MHT |
| Rechmann et al., 2020 [39] | Human enamel | In vitro, chemical model | pH-cycling pH 4.4/7.0, 9 days | Sodium fluoride, 825 ppm | After laser irradiation | MHT |
| Badreddine et al., 2020 [40] | Human enamel | In vitro, chemical model | pH-cycling pH 4.4/7.0, 9 days | Sodium fluoride, 825 ppm | After laser irradiation | MHT |
| Authors, Year [Reference] | Can et al., 2008 [37] | Hsu et al., 2008 [38] | Rechmann et al., 2016 [17] | Rechmann et al., 2020 [39] | Badreddine et al., 2020 [40] |
|---|---|---|---|---|---|
| Quality check of samples | - | - | - | - | Yes |
| Randomization of samples | - | - | - | - | - |
| Sample-size calculation | - | - | Yes | Yes | Yes |
| Homogeneity of samples | Yes | Yes | - | - | - |
| Details of laser parameters used | Yes | Yes | Yes | Yes | Yes |
| Fluoride application protocol | - | Yes | Yes | Yes | Yes |
| Operator training | - | - | - | - | - |
| Blinding of operator | - | - | - | Yes | - |
| Risk of bias | High | High | High | Medium | Medium |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, V.W.; Zhao, I.S.; Yin, I.X.; Niu, J.Y.; Lo, E.C.M.; Chu, C.H. Effects of Treatment Combining 9300 nm Carbon Dioxide Lasers and Fluoride on Prevention of Enamel Caries: A Systematic Review and Meta-Analysis. Appl. Sci. 2021, 11, 3996. https://doi.org/10.3390/app11093996
Xue VW, Zhao IS, Yin IX, Niu JY, Lo ECM, Chu CH. Effects of Treatment Combining 9300 nm Carbon Dioxide Lasers and Fluoride on Prevention of Enamel Caries: A Systematic Review and Meta-Analysis. Applied Sciences. 2021; 11(9):3996. https://doi.org/10.3390/app11093996
Chicago/Turabian StyleXue, Vicky Wenqing, Irene Shuping Zhao, Iris Xiaoxue Yin, John Yun Niu, Edward Chin Man Lo, and Chun Hung Chu. 2021. "Effects of Treatment Combining 9300 nm Carbon Dioxide Lasers and Fluoride on Prevention of Enamel Caries: A Systematic Review and Meta-Analysis" Applied Sciences 11, no. 9: 3996. https://doi.org/10.3390/app11093996
APA StyleXue, V. W., Zhao, I. S., Yin, I. X., Niu, J. Y., Lo, E. C. M., & Chu, C. H. (2021). Effects of Treatment Combining 9300 nm Carbon Dioxide Lasers and Fluoride on Prevention of Enamel Caries: A Systematic Review and Meta-Analysis. Applied Sciences, 11(9), 3996. https://doi.org/10.3390/app11093996








