Green and Efficient Processing of Wood with Supercritical CO2: A Review
Abstract
1. Introduction
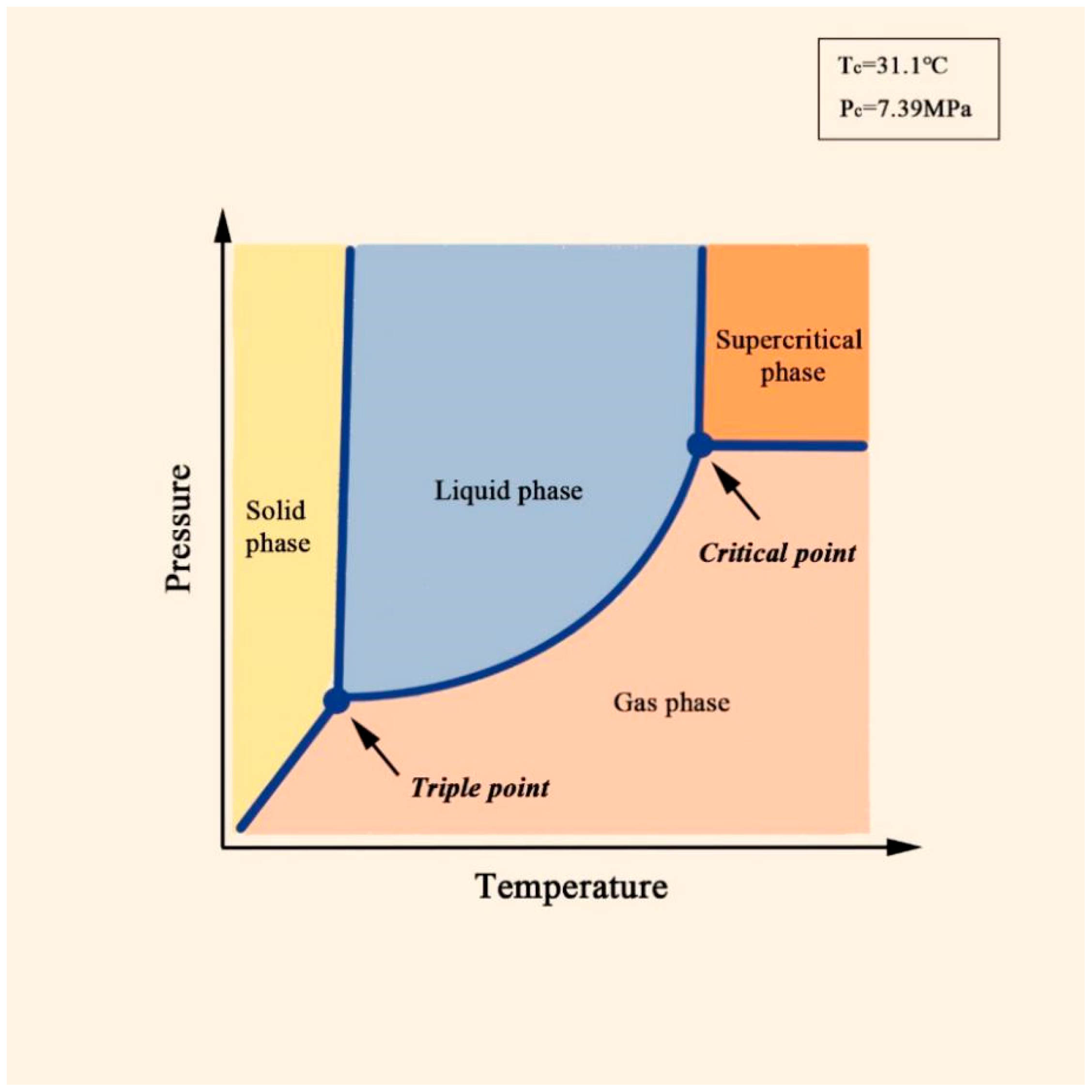
2. Characteristics of Supercritical CO2
3. Wood Impregnation
3.1. Wood Preservation with Biocides
3.2. Flame Retardancy of Wood
3.3. Wood Dyeing
3.4. Wood Acetylation
4. Wood Drying
5. Wood Thermochemical Conversion
5.1. Wood Gasification
5.2. Wood Liquidation
5.3. Bio-Oil Purification
6. Wood Extraction
6.1. Natural Component of Wood
6.2. CCA Wood Detoxification
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, X.Y.; Lv, M.Q.; Liu, M.; Lv, J.F. Repeated humidity cycling’s effect on physical properties of three kinds of wood based panels. Bioresources 2019, 14, 9444–9453. [Google Scholar] [CrossRef]
- Liu, X.Y.; Tu, X.W.; Liu, M. Effects of light thermal treatments on the color, hygroscopity and dimensional stability of wood. Wood Res. 2021, 66, 95–104. [Google Scholar] [CrossRef]
- Zhou, L.; Fu, Y. Flame-retardant wood composites based on immobilizing with chitosan/sodium phytate/nano-TiO2-ZnO coatings via layer-by-layer self-assembly. Coatings 2020, 10, 296. [Google Scholar] [CrossRef]
- Santos, A.; Carvalho, A.; Barbosa-Póvoa, A.P.; Marques, A.; Amorim, P. Assessment and optimization of sustainable forest wood supply chains–A systematic literature review. For. Policy Econ. 2019, 105, 112–135. [Google Scholar] [CrossRef]
- Zhao, Z.; Sakai, S.; Wu, D.; Chen, Z.; Zhu, N.; Huang, C.; Sun, S.; Zhang, M.; Umemura, K.; Yong, Q. Further exploration of sucrose–citric acid adhesive: Investigation of optimal hot-pressing conditions for plywood and curing behavior. Polymers 2019, 11, 1996. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.Q.; Ma, Q.R.; Yuan, Y.Y.; Wu, Z.H.; Zhang, M. Current situation and key manufacturing considerations of green furniture in China: A review. J. Clean. Prod. 2020, 267, 121957. [Google Scholar] [CrossRef]
- Lu, X.R.; Teng, Q.C.; Li, Z.R.; Zhang, X.L.; Wang, X.M.; Komatsu, K.H.; Que, Z.L. Study on shear property of spruce glulam and steel plate connected with inclined screw. J. For. Eng. 2020, 5, 48–53. [Google Scholar] [CrossRef]
- Huang, C.X.; He, J.; Liang, C.; Tang, S.; Yong, Q. Progress in applications of high value-added lignin materials. J. For. Eng. 2019, 4, 17–26. [Google Scholar] [CrossRef]
- Tchinda, J.B.S.; Ndikontar, M.K.; Belinga, A.D.F.; Mounguengui, S.; Njankouo, J.M.; Durmaçay, S.; Gerardin, P. Inhibition of fungi with wood extractives and natural durability of five Cameroonian wood species. Ind. Crops Prod. 2018, 123, 183–191. [Google Scholar] [CrossRef]
- Susilawati, D.; Kanowski, P. Cleaner production in the Indonesian pulp and paper sector: Improving sustainability and legality compliance in the value chain. J. Clean. Prod. 2020, 248, 119259. [Google Scholar] [CrossRef]
- Jebrane, M.; Sèbe, G. A novel simple route to wood acetylation by transesterification with vinyl acetate. Holzforschung 2007, 61, 143–147. [Google Scholar] [CrossRef]
- Bijaisoradat, O.; Yue, L.; Manas-Zloczower, I.; Manuspiya, H. Wood flour-high density polyethylene composites: Influence of silanization and esterification on mechanical properties. J. Appl. Polym. Sci. 2021, 138, e50197. [Google Scholar] [CrossRef]
- Nypelö, T.; Laine, C.; Aoki, M.; Tammelin, T.; Henniges, U. Etherification of wood-Based hemicelluloses for interfacial activity. Biomacromolecules 2016, 17, 1894–1901. [Google Scholar] [CrossRef]
- Sun, B.; Wang, X.; Liu, J. Changes in dimensional stability and mechanical properties of Eucalyptus pellita by melamine–urea–formaldehyde resin impregnation and heat treatment. Eur. J. Wood Prod. 2013, 71, 557–562. [Google Scholar] [CrossRef]
- Deka, M.; Saikia, C.N. Chemical modification of wood with thermosetting resin: Effect on dimensional stability and strength property. Bioresour. Technol. 2000, 73, 179–181. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, W.; Hughes, M.; Wu, M.; Zhang, S.; Li, J. Various polymeric monomers derived from renewable rosin for the modification of fast-growing poplar wood. Compos. Part B 2019, 174, 106902. [Google Scholar] [CrossRef]
- Fawaz, M.; Lautenberger, C.; Bond, T.C. Prediction of organic aerosol precursor emission from the pyrolysis of thermally thick wood. Fuel 2020, 269, 117333. [Google Scholar] [CrossRef]
- Chu, D.; Zhang, X.; Mu, J.; Avramidis, S.; Xue, L.; Li, Y. A greener approach to byproducts from the production of heat-treated poplar wood: Analysis of volatile organic compound emissions and antimicrobial activities of its condensate. J. Clean. Prod. 2019, 213, 521–527. [Google Scholar] [CrossRef]
- Moreira, R.; Mendes, C.V.T.; Banaco, M.B.F.; Carvalho, M.G.V.S.; Portugal, A. New insights in the fractionation of Pinus pinaster wood: Sequential autohydrolysis, soda ethanol organosolv and acidic precipitation. Ind. Crops Prod. 2020, 152, 112499. [Google Scholar] [CrossRef]
- Machmudah, S.; Wicaksono, D.T.; Happy, M.; Winardi, S.; Wahyudiono, K.H.; Goto, M. Water removal from wood biomass by liquefied dimethyl ether for enhancing heating value. Energy Rep. 2020, 6, 824–831. [Google Scholar] [CrossRef]
- Borges, J.C.M.; Haddi, K.; Oliveira, E.E.; Andrade, B.S.; Nascimento, V.L.; Melo, T.S.; Didonet, J.; Carvalho, J.C.T.; Cangussu, A.S.; Soares, I.M.; et al. Mosquiticidal and repellent potential of formulations containing wood residue extracts of a Neotropical plant, Tabebuia heptaphylla. Ind. Crops Prod. 2019, 129, 424–433. [Google Scholar] [CrossRef]
- Haq, M.; Chun, B.S. Characterization of phospholipids extracted from Atlantic salmon by-product using supercritical CO2 with ethanol as co-solvent. J. Clean. Prod. 2018, 178, 186–195. [Google Scholar] [CrossRef]
- Dawson, B.S.W.; Pearson, H. Effect of supercritical CO2 dewatering followed by oven-drying of softwood and hardwood timbers. Wood Sci. Technol. 2017, 51, 771–784. [Google Scholar] [CrossRef]
- Náthia-Neves, G.; Vardanega, R.; Urango, A.C.M.; Meireles, M.A.A. Supercritical CO2 extraction of α-bisabolol from different parts of candeia wood (Eremanthus erythropappus). J. Supercrit. Fluids 2020, 166, 105026. [Google Scholar] [CrossRef]
- Jaxel, J.; Amer, H.; Bacher, M.; Roller, A.; Guggenberger, M.; Zwirchmayr, N.S.; Hansmann, C.; Liebner, F. Facile synthesis of 1-butylamino- and 1,4-bis(butylamino)-2-alkyl-9,10-anthraquinone dyes for improved supercritical carbon dioxide dyeing. Dye. Pigment. 2020, 173, 107991. [Google Scholar] [CrossRef]
- Todd, R.; Baroutian, S. A techno-economic comparison of subcritical water, supercritical CO2 and organic solvent extraction of bioactives from grape marc. J. Clean. Prod. 2017, 158, 349–358. [Google Scholar] [CrossRef]
- Ferrentino, G.; Morozova, K.; Mosibo, O.K.; Ramezani, M.; Scampicchio, M. Biorecovery of antioxidants from apple pomace by supercritical fluid extraction. J. Clean. Prod. 2018, 186, 253–261. [Google Scholar] [CrossRef]
- Freire, A.L.; Moura-Nickel, C.D.; Scaratti, G.; De Rossi, A.; Araújo, M.H.; De Noni Júnior, A.; Rodrigues, A.E.; Castellón, E.R.; de Fátima Peralta Muniz Moreira, R. Geopolymers produced with fly ash and rice husk ash applied to CO2 capture. J. Clean. Prod. 2020, 273, 122917. [Google Scholar] [CrossRef]
- Belbute, J.M.; Pereira, A.M. Reference forecasts for CO2 emissions from fossil-fuel combustion and cement production in Portugal. Energy Policy 2020, 144, 111642. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, Y.; Xu, J.; Jia, G. Carbon element flow analysis and CO2 emission reduction in iron and steel works. J. Clean. Prod. 2018, 172, 709–723. [Google Scholar] [CrossRef]
- Ehsan, M.M.; Guan, Z.; Klimenko, A.Y. A comprehensive review on heat transfer and pressure drop characteristics and correlations with supercritical CO2 under heating and cooling applications. Renew. Sustain. Energy Rev. 2018, 92, 658–675. [Google Scholar] [CrossRef]
- Cabeza, L.F.; de Gracia, A.; Fernández, A.I.; Farid, M.M. Supercritical CO2 as heat transfer fluid: A review. Appl. Therm. Eng. 2017, 125, 799–810. [Google Scholar] [CrossRef]
- Tabernero, A.; Martín Del Valle, E.M.; Galán, M.A. Supercritical fluids for pharmaceutical particle engineering: Methods, basic fundamentals and modelling. Chem. Eng. Process. Process Intensif. 2012, 60, 9–25. [Google Scholar] [CrossRef]
- Zhang, X.; Heinonen, S.; Levänen, E. Applications of supercritical carbon dioxide in materials processing and synthesis. RSC Adv. 2014, 4, 61137–61152. [Google Scholar] [CrossRef]
- Tabernero, A.; Cardea, S. Supercritical carbon dioxide techniques for processing microbial exopolysaccharides used in biomedical applications. Mater. Sci. Eng. C 2020, 112, 110940. [Google Scholar] [CrossRef]
- Luo, X.; Ren, X.; Wang, S. Supercritical CO2-water-shale Interactions under Supercritical CO2 Stimulation Conditions. Energy Procedia 2018, 144, 182–185. [Google Scholar] [CrossRef]
- Alexandre, A.M.R.C.; Serra, A.T.; Matias, A.A.; Duarte, C.M.M.; Bronze, M.R. Supercritical fluid extraction of Arbutus unedo distillate residues–Impact of process conditions on antiproliferative response of extracts. J. CO2 Util. 2020, 37, 29–38. [Google Scholar] [CrossRef]
- Pishnamazi, M.; Zabihi, S.; Jamshidian, S.; Hezaveh, H.Z.; Hezave, A.Z.; Shirazian, S. Measuring solubility of a chemotherapy-anti cancer drug (busulfan) in supercritical carbon dioxide. J. Mol. Liq. 2020, 317, 113954. [Google Scholar] [CrossRef]
- Kanimozhi, B.; Mahalingam, S.; Pranesh, V.; Kesavakumar, R.; Senthil, S.; Ravikumar, S.; Pradeep, S.; Senthil, S.; Murugan, R. Colloidal release in high temperature porous media with oversaturated fines during supercritical CO2 transport. J. Pet. Sci. Eng. 2020, 192, 107345. [Google Scholar] [CrossRef]
- Sahena, F.; Zaidul, I.S.M.; Jinap, S.; Karim, A.A.; Abbas, K.A.; Norulaini, N.A.N.; Omar, A.K.M. Application of supercritical CO2 in lipid extraction—A review. J. Food Eng. 2009, 95, 240–253. [Google Scholar] [CrossRef]
- Smith, R.M. Supercritical fluids in separation science-The dreams, the reality and the future. J. Chromatogr. A. 1999, 856, 83–115. [Google Scholar] [CrossRef]
- Machado, B.A.S.; Pereira, C.G.; Nunes, S.B.; Padilha, F.F.; Umsza-Guez, M.A. Supercritical fluid extraction using CO2: Main applications and future perspectives. Sep. Sci. Technol. 2013, 48, 2741–2760. [Google Scholar] [CrossRef]
- Saltanov, E.; Pioro, I.; Mann, D.; Gupta, S.; Mokry, S.; Harvel, G. Study on Specifics of Forced-Convective Heat Transfer in Supercritical Carbon Dioxide. J. Nucl. Eng. Radiat. Sci. 2015, 1, 11008. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, X.R. Flow and heat transfer characteristics of supercritical CO2 in a natural circulation loop. Int. J. Therm. Sci. 2012, 58, 52–60. [Google Scholar] [CrossRef]
- Zhao, D.; Yin, J. Overview of supercritical fluid extraction and its application (In Chinese). J. Anhui Agric. Sci. 2014, 42, 4772–4780. [Google Scholar] [CrossRef]
- Pinto, D.; de la Luz Cádiz-Gurrea, M.; Sut, S.; Ferreira, A.S.; Leyva-Jimenez, F.J.; Dall’Acqua, S.; Segura-Carretero, A.; Delerue-Matos, C.; Rodrigues, F. Valorisation of underexploited Castanea sativa shells bioactive compounds recovered by supercritical fluid extraction with CO2: A response surface methodology approach. J. CO2 Util. 2020, 40, 101194. [Google Scholar] [CrossRef]
- Bermejo, D.V.; Ibáñez, E.; Reglero, G.; Fornari, T. Effect of cosolvents (ethyl lactate, ethyl acetate and ethanol) on the supercritical CO2 extraction of caffeine from green tea. J. Supercrit. Fluids 2016, 107, 507–512. [Google Scholar] [CrossRef]
- García-Pérez, J.S.; Cuéllar-Bermúdez, S.P.; de la Cruz-Quiroz, R.; Arévalo-Gallegos, A.; Esquivel-Hernandez, D.A.; Rodríguez-Rodríguez, J.; García-García, R.; Iqbal, H.M.N.; Parra-Saldivar, R. Supercritical CO2-based tailor made valorization of Origanum vulgare L extracts: A green approach to extract high-value compounds with applied perspectives. J. Environ. Manag. 2019, 232, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Wu, Y.; Xu, W.; Zhan, X.X.; Zhang, J.L. Preparation and characterization of heating floor impregnated by graphene/phenol-formaldehyde resin. J. For. Eng. 2019, 4, 167–173. [Google Scholar] [CrossRef]
- Cao, J.Z. A review on wood protectant dispersion systems and their liquid penetration. J. For. Eng. 2019, 4, 1–9. [Google Scholar] [CrossRef]
- Fernandes, J.; Kjellow, A.W.; Henriksen, O. Modeling and optimization of the supercritical wood impregnation process-Focus on pressure and temperature. J. Supercrit. Fluids 2012, 66, 307–314. [Google Scholar] [CrossRef]
- Comin, L.M.; Temelli, F.; Saldaña, M.D.A. Barley β-glucan aerogels as a carrier for flax oil via supercritical CO2. J. Food Eng. 2012, 111, 625–631. [Google Scholar] [CrossRef]
- Goñi, M.L.; Gañán, N.A.; Strumia, M.C.; Martini, R.E. Eugenol-loaded LLDPE films with antioxidant activity by supercritical carbon dioxide impregnation. J. Supercrit. Fluids 2016, 111, 28–35. [Google Scholar] [CrossRef]
- Siau, J.F. Permeability. In Transport Processes in Wood; Springer Series in Wood Science; Springer: Berlin/Heidelberg, Germany, 1984. [Google Scholar]
- Demessie, E.S.; Hassan, A.; Levien, K.L.; Kumar, S.; Morrell, J.J. Supercritical CO2 treatment: Effect on permeability of douglas-fir heartwood. Wood Fiber Sci. 1995, 27, 296–300. [Google Scholar]
- Matsunaga, M.; Matsunaga, H.; Kataoka, Y.; Matsui, H. Improved water permeability of sugi heartwood by pretreatment with supercritical carbon dioxide. J. Wood Sci. 2005, 51, 195–197. [Google Scholar] [CrossRef]
- Cookson, L.J.; Qader, A.; Creffield, J.W.; Scown, D.K. Treatment of timber with permethrin in supercritical carbon dioxide to control termites. J. Supercrit. Fluids 2009, 49, 203–208. [Google Scholar] [CrossRef]
- Muin, M.; Tsunoda, K. Preservative treatment of wood-based composites with 3-iodo-2-propynyl butylcarbamate using supercritical carbon dioxide impregnation. J. Wood Sci. 2003, 49, 430–436. [Google Scholar] [CrossRef]
- Kang, S.M.; Cho, M.W.; Kim, K.M.; Kang, D.; Koo, W.M.; Kim, K.H.; Park, J.Y.; Lee, S.S. Cyproconazole impregnation into wood using sub- and supercritical carbon dioxide. Wood Sci. Technol. 2012, 46, 643–656. [Google Scholar] [CrossRef]
- Eastman, S.A.; Lesser, A.J.; McCarthy, T.J. Supercritical CO2-assisted, silicone-modified wood for enhanced fire resistance. J. Mater. Sci. 2009, 44, 1275–1282. [Google Scholar] [CrossRef]
- Jaxel, J.; Fontaine, L.; Krenke, T.; Hansmann, C.; Liebner, F. Bio-inspired conformational lipophilization of wood for scCO2-assisted colouring with disperse dyes. J. Supercrit. Fluids 2019, 147, 116–125. [Google Scholar] [CrossRef]
- Matsunaga, M.; Kataoka, Y.; Matsunaga, H.; Matsui, H. A novel method of acetylation of wood using supercritical carbon dioxide. J. Wood Sci. 2010, 56, 293–298. [Google Scholar] [CrossRef]
- Duke, S.O.; Powles, S.B. Glyphosate: A once-in-a-century herbicide. Pest. Manag. Sci. 2008, 63, 1100–1106. [Google Scholar] [CrossRef]
- Brocco, V.F.; Paes, J.B.; Da Costa, L.G.; Brazolin, S.; Arantes, M.D.C. Potential of teak heartwood extracts as a natural wood preservative. J. Cleaner Prod. 2017, 142, 2093–2099. [Google Scholar] [CrossRef]
- Reinprecht, L. Chemical Protection of Wood. In Wood Deterioration, Protection and Maintenance, 1st ed.; Wiley & Sons, Ltd.: Chichester, UK, 2016; pp. 145–217. [Google Scholar] [CrossRef]
- Pant, H.; Tripathi, S. Fungal decay resistance of wood fumigated with chlorpyrifos. Int. Biodeterior. Biodegrad. 2010, 64, 665–669. [Google Scholar] [CrossRef]
- Lucas, S.; González, E.; Calvo, M.P.; Palencia, C.; Alonso, E.; Cocero, M.J. Supercritical CO2 impregnation of Radiata pine with organic fungicides: Effect of operating conditions and two-parameters modeling. J. Supercrit. Fluids 2007, 40, 462–469. [Google Scholar] [CrossRef]
- Muin, M.; Adachi, A.; Inoue, M.; Yoshimura, T.; Tsunoda, K. Feasibility of supercritical carbon dioxide as a carrier solvent for preservative treatment of wood-based composites. J. Wood Sci. 2003, 49, 65–72. [Google Scholar] [CrossRef]
- Acda, M.N.; Morrell, J.J.; Levien, K.L. Supercritical fluid impregnation of selected wood species with tebuconazole. Wood Sci. Technol. 2001, 35, 127–136. [Google Scholar] [CrossRef]
- Kang, S.M.; Ra, J.B.; Levien, K.L.; Morrell, J.J. Developing diffusion coefficients for SCF impregnation of Douglas Fir heartwood with cyproconazole. J. Wood Chem. Technol. 2006, 26, 111–124. [Google Scholar] [CrossRef]
- Acda, M.N. Supercritical Fluid Impregnation of Wood-Based Composites. Ph.D. Thesis, Oregon State University, Corvallis, OR, USA, 1995. [Google Scholar]
- Sun, S.; Zhao, Z.; Umemura, K. Further exploration of sucrose-citric acid adhesive: Synthesis and application on plywood. Polymers 2019, 11, 1875. [Google Scholar] [CrossRef]
- Lu, J.; Jiang, P.; Chen, Z.; Li, L.; Huang, Y. Flame retardancy, thermal stability, and hygroscopicity of wood materials modified with melamine and amino trimethylene phosphonic acid. Constr. Build. Mater. 2021, 267, 121042. [Google Scholar] [CrossRef]
- Wang, S.Y.; Yang, T.H.; Lin, L.T.; Lin, C.J.; Tsai, M.J. Fire-retardant-treated low-formaldehyde-emission particleboard made from recycled wood-waste. Bioresour. Technol. 2008, 99, 2072–2077. [Google Scholar] [CrossRef] [PubMed]
- Tsioptsias, C.; Panayiotou, C. Thermal stability and hydrophobicity enhancement of wood through impregnation with aqueous solutions and supercritical carbon dioxide. J. Mater. Sci. 2011, 46, 5406–5411. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, J. Investigation of polystyrene-based microspheres from different copolymers and their structural color coatings on wood surface. Coatings 2021, 11, 14. [Google Scholar] [CrossRef]
- Liu, Y. Self-assembly of poly (styrene-methyl methacrylate-acrylic acid) (P(St-MMA-AA)) colloidal microspheres on wood surface by thermal-assisted gravity deposition. Wood Sci. Technol. 2021, 55, 403–417. [Google Scholar] [CrossRef]
- Jebrane, M.; Pichavant, F.; Sèbe, G. A comparative study on the acetylation of wood by reaction with vinyl acetate and acetic anhydride. Carbohydr. Polym. 2011, 83, 339–345. [Google Scholar] [CrossRef]
- Matsunaga, M.; Hewage, D.C.; Kataoka, Y.; Ishikawa, A.; Kobayashi, M.; Kiguchi, M. Acetylation of wood using supercritical carbon dioxide. J. Trop. For Sci. 2016, 28, 132–138. Available online: https://www.jstor.org/stable/43799216 (accessed on 25 April 2021).
- Khouya, A. Performance assessment of a heat pump and a concentrated photovoltaic thermal system during the wood drying process. Appl. Therm. Eng. 2020, 180, 115923. [Google Scholar] [CrossRef]
- Franich, R.A.; Gallagher, S.; Kroese, H. Dewatering green sapwood using carbon dioxide cycled between supercritical fluid and gas phase. J. Supercrit. Fluids 2014, 89, 113–118. [Google Scholar] [CrossRef]
- Yang, L.; Liu, H. A review of Eucalyptus wood collapse and its control during drying. BioResources 2018, 13, 2171–2181. [Google Scholar] [CrossRef]
- Dawson, B.S.W.; Pearson, H.; Kroese, H.W.; Sargent, R. Effect of specimen dimension and pre-heating temperature on supercritical CO2 dewatering of radiata pine sapwood. Holzforschung 2015, 69, 421–430. [Google Scholar] [CrossRef]
- Gabitov, R.F.; Khairutdinov, V.F.; Gumerov, F.M.; Gabitov, F.R.; Zaripov, Z.I.; Gaifullina, R.; Farakhov, M.I. Drying and impregnation of wood with propiconazole using supercritical carbon dioxide. Russ. J. Phys. Chem. B 2017, 11, 1223–1230. [Google Scholar] [CrossRef]
- Dawson, B.S.W.; Pearson, H.; Kimberley, M.O.; Davy, B.; Dickson, A.R. Effect of supercritical CO2 treatment and kiln drying on collapse in Eucalyptus nitens wood. Eur. J. Wood Wood Prod. 2020, 78, 209–217. [Google Scholar] [CrossRef]
- Behr, V.C.; Hill, S.J.; Meder, R.; Sandquist, D.; Hindmarsh, J.P.; Franich, R.A.; Newman, R.H. Carbon-13 NMR chemical-shift imaging study of dewatering of green sapwood by cycling carbon dioxide between the supercritical fluid and gas phases. J. Supercrit. Fluids 2014, 95, 535–540. [Google Scholar] [CrossRef]
- Meder, R.; Franich, R.A.; Callaghan, P.T.; Behr, V.C. A comparative study of dewatering of Pinus radiata sapwood using supercritical CO2 and conventional forced air-drying via in situ magnetic resonance microimaging (MRI). Holzforschung 2015, 69, 1137–1142. [Google Scholar] [CrossRef]
- Newman, R.H.; Franich, R.A.; Meder, R.; Hill, S.J.; Kroese, H.; Sandquist, D.; Hindmarsh, J.P.; Schmid, M.W.; Fuchs, J.; Behr, V.C. Proton magnetic resonance imaging used to investigate dewatering of green sapwood by cycling carbon dioxide between supercritical fluid and gas phase. J. Supercrit. Fluids 2016, 111, 36–42. [Google Scholar] [CrossRef]
- Franich, R.A.; Meder, R.; Falge, M.; Fuchs, J.; Behr, V.C. Uncovering supercritical CO2 wood dewatering via interleaved 1H-imaging and 13C-spectroscopy with real-time reconstruction. J. Supercrit. Fluids 2019, 144, 56–62. [Google Scholar] [CrossRef]
- Gollakota, A.R.K.; Kishore, N.; Gu, S. A review on hydrothermal liquefaction of biomass. Renew. Sustain. Energy Rev. 2018, 81, 1378–1392. [Google Scholar] [CrossRef]
- Parvez, A.M.; Afzal, M.T.; Hebb, T.G.V.; Schmid, M. Utilization of CO2 in thermochemical conversion of biomass for enhanced product properties: A review. J. CO2 Util. 2020, 40, 101217. [Google Scholar] [CrossRef]
- Kumar, A.; Jones, D.D.; Hanna, M.A. Thermochemical biomass gasification: A review of the current status of the technology. Energies 2009, 2, 556–581. [Google Scholar] [CrossRef]
- Billaud, J.; Valin, S.; Peyrot, M.; Salvador, S. Influence of H2O, CO2 and O2 addition on biomass gasification in entrained flow reactor conditions: Experiments and modelling. Fuel 2016, 166, 166–178. [Google Scholar] [CrossRef]
- Dhyani, V.; Bhaskar, T. A comprehensive review on the pyrolysis of lignocellulosic biomass. Renew. Energy 2018, 129, 695–716. [Google Scholar] [CrossRef]
- Cui, X.X.; He, L.H.; An, Z.; Zhang, M.H.; Han, W. The research of rheological properties of salix polyol liquefied products. J. For. Eng. 2020, 5, 90–96. [Google Scholar] [CrossRef]
- Guizani, C.; Louisnard, O.; Sanz, F.J.E.; Salvador, S. Gasification of woody biomass under high heating rate conditions in pure CO2: Experiments and modelling. Biomass Bioenergy 2015, 83, 169–182. [Google Scholar] [CrossRef]
- Butterman, H.C.; Castaldi, M.J. CO2 as a carbon neutral fuel source via enhanced biomass gasification. Environ. Sci. Technol. 2009, 43, 9030–9037. [Google Scholar] [CrossRef]
- Pohořelý, M.; Jeremiáš, M.; Svoboda, K.; Kameníková, P.; Skoblia, S.; Beňo, Z. CO2 as moderator for biomass gasification. Fuel 2014, 117, 198–205. [Google Scholar] [CrossRef]
- Chaiwatanodom, P.; Vivanpatarakij, S.; Assabumrungrat, S. Thermodynamic analysis of biomass gasification with CO2 recycle for synthesis gas production. Appl. Energy 2014, 114, 10–17. [Google Scholar] [CrossRef]
- Wang, Q.; Li, K.; Guo, Z.; Fang, M.; Luo, Z.; Cen, K. Effects of CO2 atmosphere on slow pyrolysis of high-ash lignite. Carbon Resour. Convers. 2018, 1, 94–103. [Google Scholar] [CrossRef]
- Ding, N.; Azargohar, R.; Dalai, A.K.; Kozinski, J.A. Catalytic gasification of cellulose and pinewood to H2 in supercritical water. Fuel 2014, 118, 416–425. [Google Scholar] [CrossRef]
- Cengiz, N.Ü.; Eren, S.; Sağlam, M.; Yüksel, M.; Ballice, L. Influence of temperature and pressure on hydrogen and methane production in the hydrothermal gasification of wood residues. J. Supercrit. Fluids 2016, 107, 243–249. [Google Scholar] [CrossRef]
- Knez, Ž.; Hrnčič, M.K.; Čolnik, M.; Škerget, M. Chemicals and value added compounds from biomass using sub- and supercritical water. J. Supercrit. Fluids 2017, 133, 591–602. [Google Scholar] [CrossRef]
- Gökkaya, D.S.; Çokkuvvetli, T.; Sağlam, M.; Yüksel, M.; Ballice, L. Hydrothermal gasification of poplar wood chips with alkali, mineral, and metal impregnated activated carbon catalysts. J. Supercrit. Fluids 2019, 152, 104542. [Google Scholar] [CrossRef]
- Chan, Y.H.; Quitain, A.T.; Yusup, S.; Uemura, Y.; Sasaki, M.; Kida, T. Optimization of hydrothermal liquefaction of palm kernel shell and consideration of supercritical carbon dioxide mediation effect. J. Supercrit. Fluids 2018, 133, 640–646. [Google Scholar] [CrossRef]
- García-Serna, J.; García-Merino, E.; Cocero, M.J. Gasification of charcoal using supercritical CO2 at high pressures. J. Supercrit. Fluids 2007, 43, 228–235. [Google Scholar] [CrossRef]
- Baloch, H.A.; Nizamuddin, S.; Siddiqui, M.T.H.; Riaz, S.; Jatoi, A.S.; Dumbre, D.K.; Mubarak, N.M.; Srinivasan, M.P.; Griffin, G.J. Recent advances in production and upgrading of bio-oil from biomass: A critical overview. J. Environ. Chem. Eng. 2018, 6, 5101–5118. [Google Scholar] [CrossRef]
- Peterson, A.A.; Vogel, F.; Lachance, R.P.; Fröling, M.; Jr Antal, M.J.; Tester, J.W. Thermochemical biofuel production in hydrothermal media: A review of sub- and supercritical water technologies. Energy Environ. Sci. 2008, 1, 32–65. [Google Scholar] [CrossRef]
- Morais, A.R.C.; da Costa Lopes, A.M.; Bogel-Łukasik, R. Carbon dioxide in biomass processing: Contributions to the green biorefinery concept. Chem. Rev. 2015, 115, 3–27. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Lin, H.; Zheng, Y.; Zhao, J.; Pelletier, A.; Li, K. Effects of solvents and catalysts in liquefaction of pinewood sawdust for the production of bio-oils. Biomass Bioenergy 2013, 59, 158–167. [Google Scholar] [CrossRef]
- De Caprariis, B.; Bavasso, I.; Bracciale, M.P.; Damizia, M.; De Filippis, P.; Scarsella, M. Enhanced bio-crude yield and quality by reductive hydrothermal liquefaction of oak wood biomass: Effect of iron addition. J. Anal. Appl. Pyrolysis 2019, 139, 123–130. [Google Scholar] [CrossRef]
- Yu, J.; Biller, P.; Mamahkel, A.; Klemmer, M.; Becker, J.; Glasius, M.; Iversen, B.B. Catalytic hydrotreatment of bio-crude produced from the hydrothermal liquefaction of aspen wood: A catalyst screening and parameter optimization study. Sustain. Energy Fuels 2017, 1, 832–841. [Google Scholar] [CrossRef]
- Jogi, R.; Mäki-Arvela, P.; Virtanen, P.; Kumar, N.; Hemming, J.; Russo, V.; Samikannu, A.; Lestander, T.A.; Mikkola, J.P. Understanding the formation of phenolic monomers during fractionation of birch wood under supercritical ethanol over iron based catalysts. J. Energy Inst. 2020, 93, 2055–2062. [Google Scholar] [CrossRef]
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of Transportation Fuels from Biomass: Chemistry, Catalysts, and Engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef]
- Elliott, D.C. Historical Developments in Hydroprocessing Bio-oils. Energy Fuels 2007, 21, 1792–1815. [Google Scholar] [CrossRef]
- Feng, Y.; Meier, D. Extraction of value-added chemicals from pyrolysis liquids with supercritical carbon dioxide. J. Anal. Appl. Pyrolysis 2015, 113, 174–185. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, R.; Yin, R.; Mei, Y. Upgrading of bio-oil from biomass fast pyrolysis in China: A review. Renewable Sustainable Energy Rev. 2013, 24, 66–72. [Google Scholar] [CrossRef]
- Naik, S.; Goud, V.V.; Rout, P.R.; Dalai, A.K. Supercritical CO2 fractionation of bio-oil produced from wheat–hemlock biomass. Bioresour. Technol. 2010, 101, 7605–7613. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Meier, D. Supercritical carbon dioxide extraction of fast pyrolysis oil from softwood. J. Supercrit. Fluids 2017, 128, 6–17. [Google Scholar] [CrossRef]
- Rout, P.K.; Naik, M.K.; Naik, S.N.; Goud, V.V.; Das, L.M.; Dalai, A.K. Supercritical CO2 fractionation of bio-oil produced from mixed biomass of wheat and wood sawdust. Energy Fuels 2009, 23, 6181–6188. [Google Scholar] [CrossRef]
- Danh, L.T.; Mammucari, R.; Truong, P.; Foster, N. Response surface method applied to supercritical carbon dioxide extraction of Vetiveria zizanioides essential oil. Chem. Eng. J. 2009, 155, 617–626. [Google Scholar] [CrossRef]
- Dos Santos, N.O.; Mariane, B.; Lago, J.H.G.; Sartorelli, P.; Rosa, W.; Soares, M.G.; Da Silva, A.M.; Lorenzi, H.; Vallim, M.A.; Pascon, R.C. Assessing the chemical composition and antimicrobial activity of essential oils from Brazilian plants-Eremanthus erythropappus (Asteraceae), Plectrantuns barbatus, and P. amboinicus (Lamiaceae). Molecules 2015, 20, 8440–8452. [Google Scholar] [CrossRef]
- Santos, K.A.; Klein, E.J.; Gazim, Z.C.; Gonçalves, J.E.; Cardozo-Filho, L.; Corazza, M.L.; da Silva, E.A. Wood and industrial residue of candeia (Eremanthus erythropappus): Supercritical CO2 oil extraction, composition, antioxidant activity and mathematical modeling. J. Supercrit. Fluids 2016, 114, 1–8. [Google Scholar] [CrossRef]
- Queiroz, A.; Cajaib, J. A sustainable process for (−)-α-bisabolol extraction from Eremanthus erythropappus using supercritical CO2 and ethanol as co-solvent. J. Supercrit. Fluids 2016, 110, 39–46. [Google Scholar] [CrossRef]
- De Souza, A.T.; Benazzi, T.L.; Grings, M.B.; Cabral, V.; Antônio da Silva, E.; Cardozo-Filho, L.; Ceva Antunes, O.A. Supercritical extraction process and phase equilibrium of Candeia (Eremanthus erythropappus) oil using supercritical carbon dioxide. J. Supercrit. Fluids 2008, 47, 182–187. [Google Scholar] [CrossRef]
- Yesil-Celiktas, O.; Otto, F.; Gruener, S.; Parlar, H. Determination of extractability of pine bark using supercritical CO2 extraction and different solvents: Optimization and prediction. J. Agric. Food Chem. 2009, 57, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Sarikaki, V.; Rallis, M.; Tanojo, H.; Panteri, I.; Dotsikas, Y.; Loukas, Y.L.; Papaioannou, G.; Demetzos, C.; Weber, S.; Moini, H.; et al. In Vitro Percutaneous Absorption of pine bark extract (Pycnogenol) in human skin. J. Toxicol. Cutan Ocul. Toxicol. 2005, 23, 149–158. [Google Scholar] [CrossRef]
- Conde, E.; Hemming, J.; Smeds, A.; Reinoso, B.D.; Moure, A.; Willför, S.; Domínguez, H.; Parajó, J.C. Extraction of low-molar-mass phenolics and lipophilic compounds from Pinus pinaster wood with compressed CO2. J. Supercrit. Fluids 2013, 81, 193–199. [Google Scholar] [CrossRef]
- Braga, M.E.M.; Santos, R.M.S.; Seabra, I.J.; Facanali, R.; Marques, M.O.M.; De Sousa, H.C. Fractioned SFE of antioxidants from maritime pine bark. J. Supercrit. Fluids 2008, 47, 37–48. [Google Scholar] [CrossRef]
- Ramezani, H.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Antifungal activity of the volatile oil of Eucalyptus citriodora. Fitoterapia 2002, 73, 261–262. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, D. Supercritical CO2 extraction of Eucalyptus leaves oil and comparison with Soxhlet extraction and hydro-distillation methods. Sep. Purif. Technol. 2014, 133, 443–451. [Google Scholar] [CrossRef]
- Zhou, L.J.; Huang, L.J.; Yang, Z.R.; Bai, L.H. Optimization of supercritical CO2 extraction conditions for essential oil from Eucalyptus·grandis × Eucalyptus·urophylla using box-behnken design-response suface methodology (In Chinese). J. Sichuan Univ. (Nat. Sci. Ed.) 2014, 51, 1319–1324. [Google Scholar] [CrossRef]
- Santos, S.A.O.; Villaverde, J.J.; Silva, C.M.; Neto, C.P.; Silvestre, A.J.D. Supercritical fluid extraction of phenolic compounds from Eucalyptus globulus Labill bark. J. Supercrit. Fluids 2012, 71, 71–79. [Google Scholar] [CrossRef]
- Patinha, D.J.S.; Domingues, R.M.A.; Villaverde, J.J.; Silva, A.M.S.; Silva, C.M.; Freire, C.S.R.; Neto, C.P.; Silvestre, A.J.D. Lipophilic extractives from the bark of Eucalyptus grandis × globulus, a rich source of methyl morolate: Selective extraction with supercritical CO2. Ind. Crops Prod. 2013, 43, 340–348. [Google Scholar] [CrossRef]
- Eller, F.J.; Kirker, G.T.; Mankowski, M.E.; Hay, W.T.; Palmquist, D.E. Effect of burgundy solid extracted from Eastern Red Cedar heartwood on subterranean termites and Wood-decay fungi. Ind. Crops Prod. 2020, 144, 112023. [Google Scholar] [CrossRef]
- Gaspar, M.C.; De Sousa, H.C.; Seabra, I.J.; Braga, M.E.M. Environmentally-safe scCO2 P. pinaster branches extracts: Composition and properties. J. CO2 Util. 2020, 37, 74–84. [Google Scholar] [CrossRef]
- Gwee, Y.L.; Yusup, S.; Tan, R.R.; Yiin, C.L. Techno-economic and life-cycle assessment of volatile oil extracted from Aquilaria sinensis using supercritical carbon dioxide. J. CO2 Util. 2020, 38, 158–167. [Google Scholar] [CrossRef]
- Ramsey, E.; Sun, Q.; Zhang, Z.; Zhang, C.; Gou, W. Mini-Review: Green sustainable processes using supercritical fluid carbon dioxide. J. Environ. Sci. 2009, 21, 720–726. [Google Scholar] [CrossRef]
- El-Fatah, S.A.; Goto, M.; Kodama, A.; Hirose, T. Supercritical fluid extraction of hazardous metals from CCA wood. J. Supercrit. Fluids 2004, 28, 21–27. [Google Scholar] [CrossRef]
- Wang, J.S.; Chiu, K. Extraction of chromated copper arsenate from wood wastes using green solvent supercritical carbon dioxide. J. Hazard. Mater. 2008, 158, 384–391. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Liquid | Gas | Supercritical Fluid |
|---|---|---|---|
| Density/g·cm−3 | 0.6~1.6 | 0.006~0.02 | 0.2~0.5 |
| Viscosity/10−4 g·cm−3·s−1 | 20~300 | 1~3 | 1~3 |
| Diffusivity/ cm2·s−1 | (0.2~2) × 10−3 | 0.1~0.4 | 0.7 × 10−3 |
| Application | Environment Benefits with scCO2 | Efficiency with scCO2 | References |
|---|---|---|---|
| Wood preservation with biocide | No VOCs emission No metal components Non-toxic solvent | The higher insecticide retention value Better protection Fast treatment | [50,57,58,59] |
| Flame retardancy of wood | Fire-resistant additives (non-polar compound) have appreciable solubility in scCO2 | [60] | |
| Wood dyeing | Facilitating dye uptake at moderate temperatures Improving recycling rates of both CO2 and dyes | [25,61] | |
| Wood acetylation | Acetylation reagent penetrate into the core of wood | [62] |
| References | Wood Species | Gasification Agent | Catalyst | Conditions | Syngas | Conversion |
|---|---|---|---|---|---|---|
| [21] | Eucalyptus grandis | Supercritical water | — | 400 °C–450 °C | Gas mixture of H2 and CH4 | 64–73% |
| NiFe2O4 | 83–95.5% | |||||
| [100] | Wood chips oak and beech (supplied by J. Rettenmaier & Sohne GmbH) | CO2 | dolomitic limestone | 850 °C | Gas mixture of CO and CH4 | 89% |
| [101] | The wood residues of the pine tree | Supercritical water | — | 500–600 °C; 19.8 MPa–43 MPa | Gas mixture of H2, CH4 and CO2 | 46.9–73.2% |
| K2CO3 | 59.1–80.9% | |||||
| [104] | Populus alba L. | Supercritical water | — | 400–600 °C | Gas mixture of H2 and CH4 | 45.2–70.4% |
| K2CO3 | 47.6–81.0% | |||||
| [106] | The wood residues of the fir tree | ScCO2 | — | 650 °C–800 °C; 30 MPa | CO | 43.2–65.8% |
| K2CO3 | 56.7–77.5% |
| References | Wood Species | Liquefaction Media | Catalyst | Conditions | Bio-Oil Yield |
|---|---|---|---|---|---|
| [109] | Palm kernel (Elaeis guineensis Jacq.) shell | H2O | — | 300 °C; 30 MPa | 3.00~6.59 wt% |
| ScCO2 | 300 °C; 25 MPa | 12.03 wt% | |||
| [110] | White pine (Pinus strobus L.) sawdust | ScCO2 | — | 8 wt% | |
| K2CO3 | 300 °C; 11 MPa | 29.3 wt% | |||
| Acetone | K2CO3 | 300 °C; 4.5 MPa | 27.9 wt% | ||
| Ethanol | K2CO3 | 300 °C; 7.3 MPa | 30.8 wt% | ||
| H2O | K2CO3 | 300 °C; 7.9 MPa | 17.3 wt% | ||
| [111] | Oak wood (Quercus pubescens) | H2O | K2CO3 | 320 °C | 27 wt% |
| Fe | 32 wt% | ||||
| [112] | Aspen wood (Populus tremula L.) | H2O | — | 350 °C; 15 MPa | 17 wt% |
| NiMo | 70.3 wt% | ||||
| [113] | Silver birch (Betula sp.) | Supercritical ethanol | — | 234 °C | 19 wt% |
| 5 wt% iron modified beta zeolite | 25 wt% |
| References | Wood | Effective Constituent |
|---|---|---|
| [121] | Candeia (Eremanthus erythropappus) | α-bisabolol |
| [122] | Candeia (Eremanthus erythropappus) | (−)-α-bisabolol |
| [123] | Candeia (Eremanthus erythropappus) | α-bisabolol |
| [125] | Pinus brutia | (-)-catechin, (-)-epicatechin, (-)-catechin gallate |
| [126] | Maritime pine (Pinus pinaster) | phenolic compounds |
| [127] | Maritime pine (Pinus pinaster) | Catechin, epicatechin |
| [130] | Eucalyptus globulus | phenolics |
| [131] | Eucalyptus. grandis × Eucalyptus. urophylla | Eucalyptol, α-pinene |
| [132] | Eucalyptus grandis × Eucalyptus globulus | Lipophilic extractives |
| [133] | Aquilaria sinensis | Volatile oil |
| [134] | Pinus pinaster | α-pinene, β-pinene, β-myrcene, β-caryophyllene |
| [137] | Eastern red cedar (Juniperus virginiana L.) | Oil |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Yang, L.; Liu, H. Green and Efficient Processing of Wood with Supercritical CO2: A Review. Appl. Sci. 2021, 11, 3929. https://doi.org/10.3390/app11093929
Zhang J, Yang L, Liu H. Green and Efficient Processing of Wood with Supercritical CO2: A Review. Applied Sciences. 2021; 11(9):3929. https://doi.org/10.3390/app11093929
Chicago/Turabian StyleZhang, Jingwen, Lin Yang, and Honghai Liu. 2021. "Green and Efficient Processing of Wood with Supercritical CO2: A Review" Applied Sciences 11, no. 9: 3929. https://doi.org/10.3390/app11093929
APA StyleZhang, J., Yang, L., & Liu, H. (2021). Green and Efficient Processing of Wood with Supercritical CO2: A Review. Applied Sciences, 11(9), 3929. https://doi.org/10.3390/app11093929






