Governing Functionality of Silver Ion-Exchanged Photo-Thermo-Refractive Glass Matrix by Small Additives
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
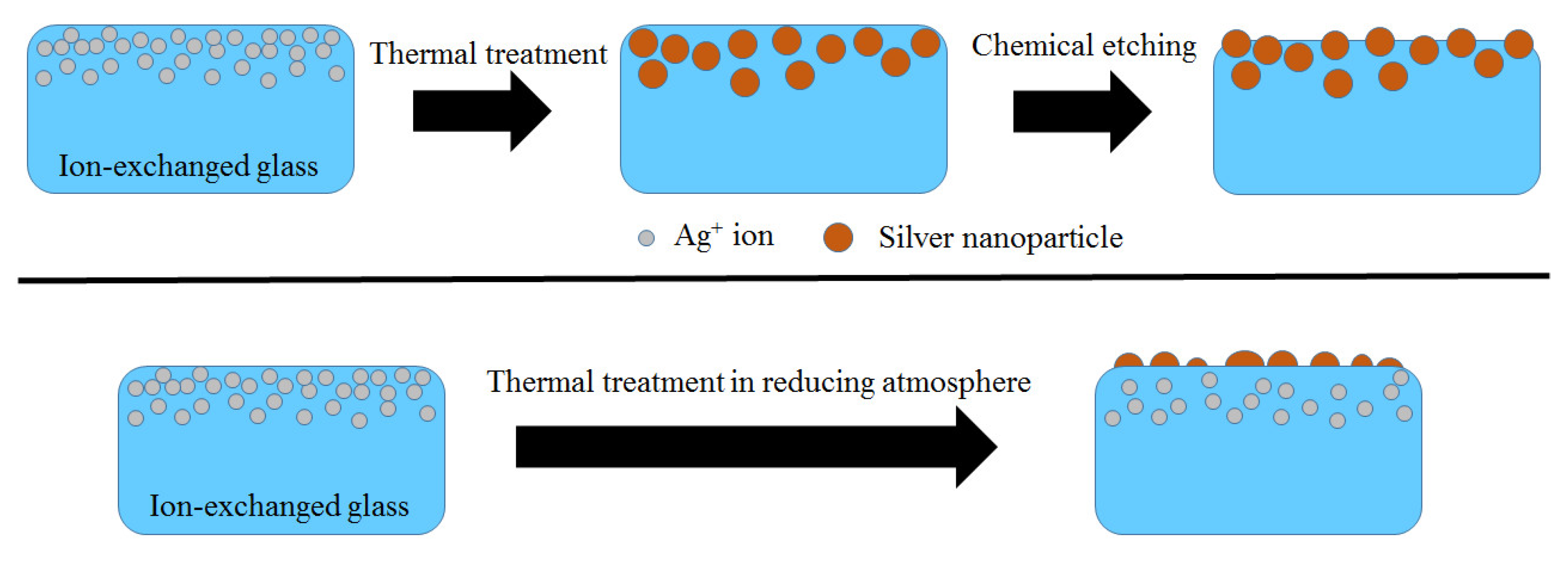
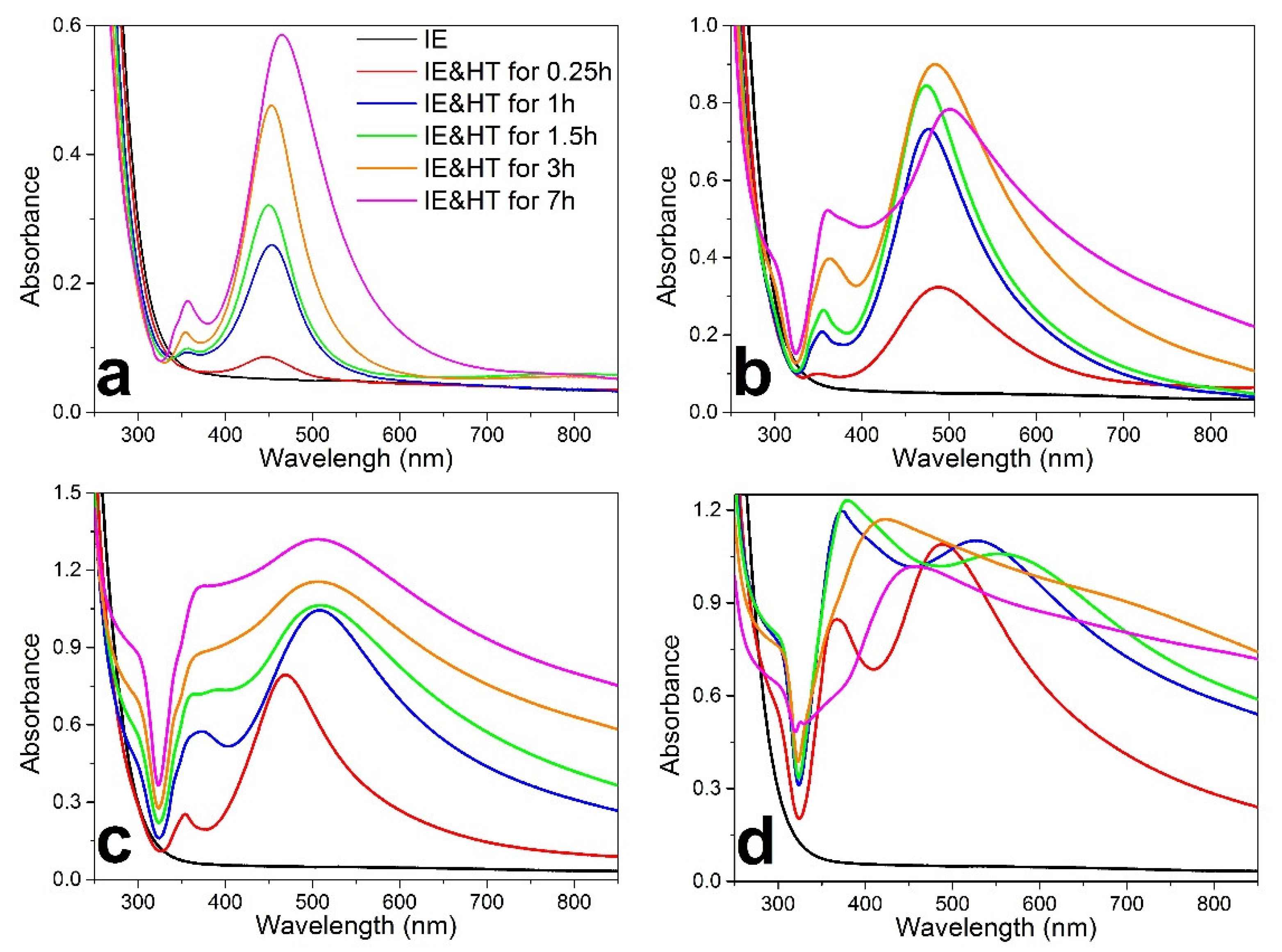
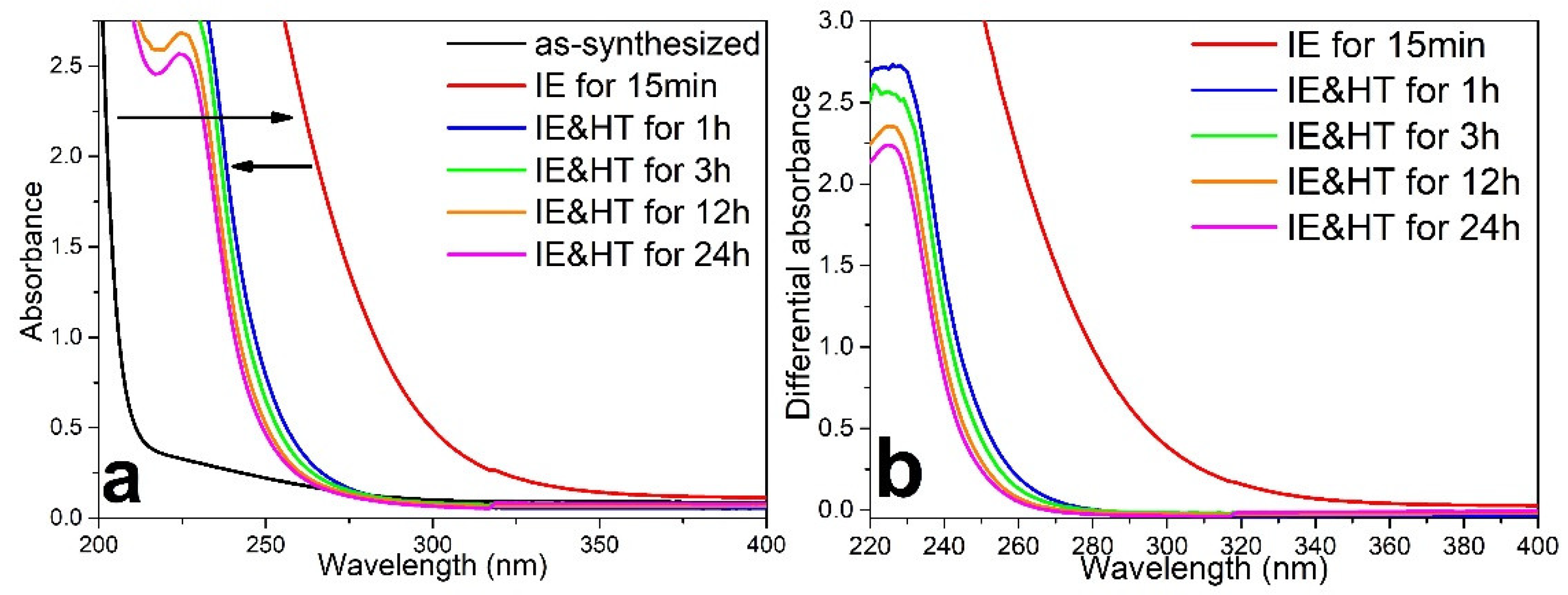
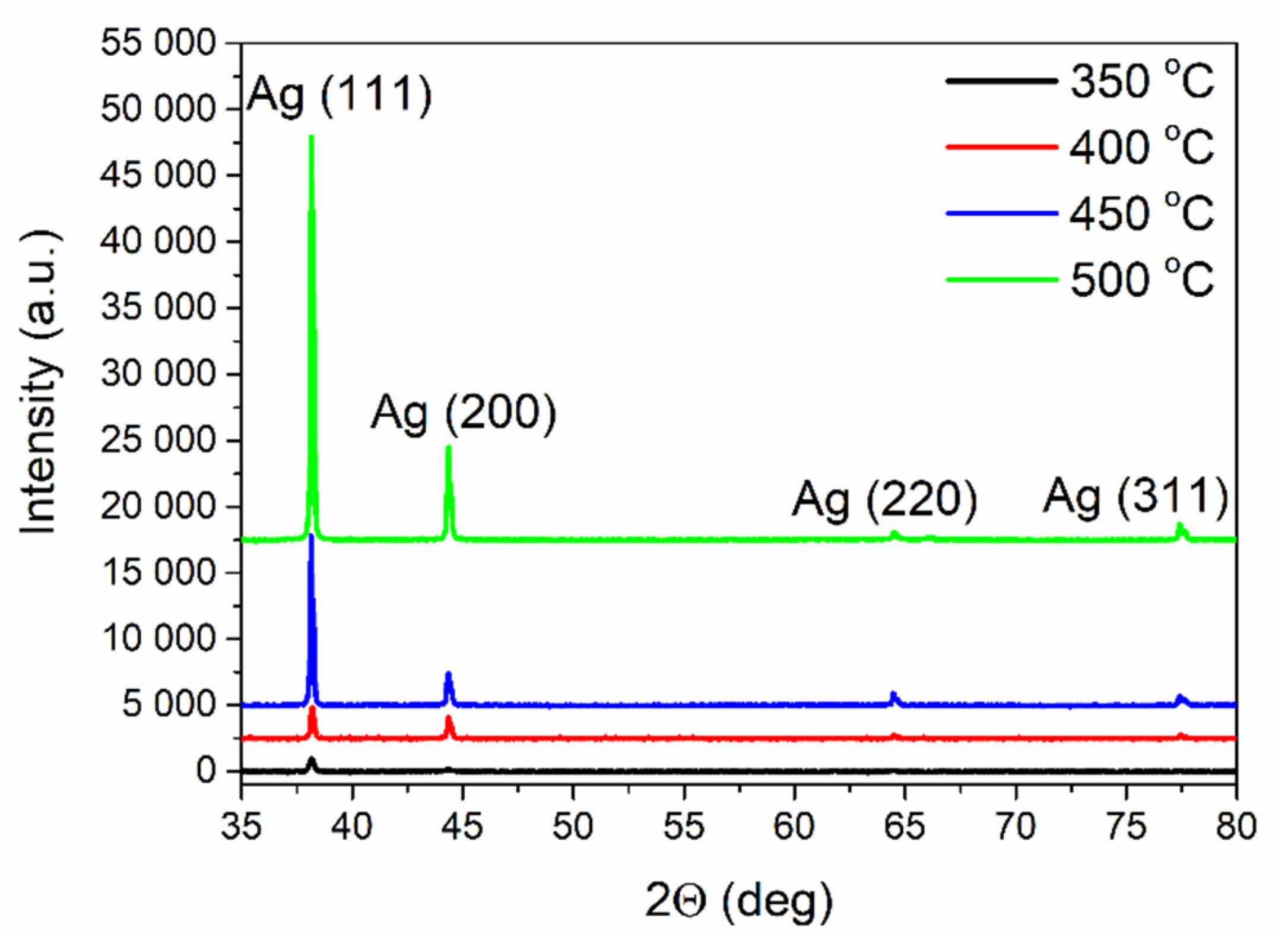
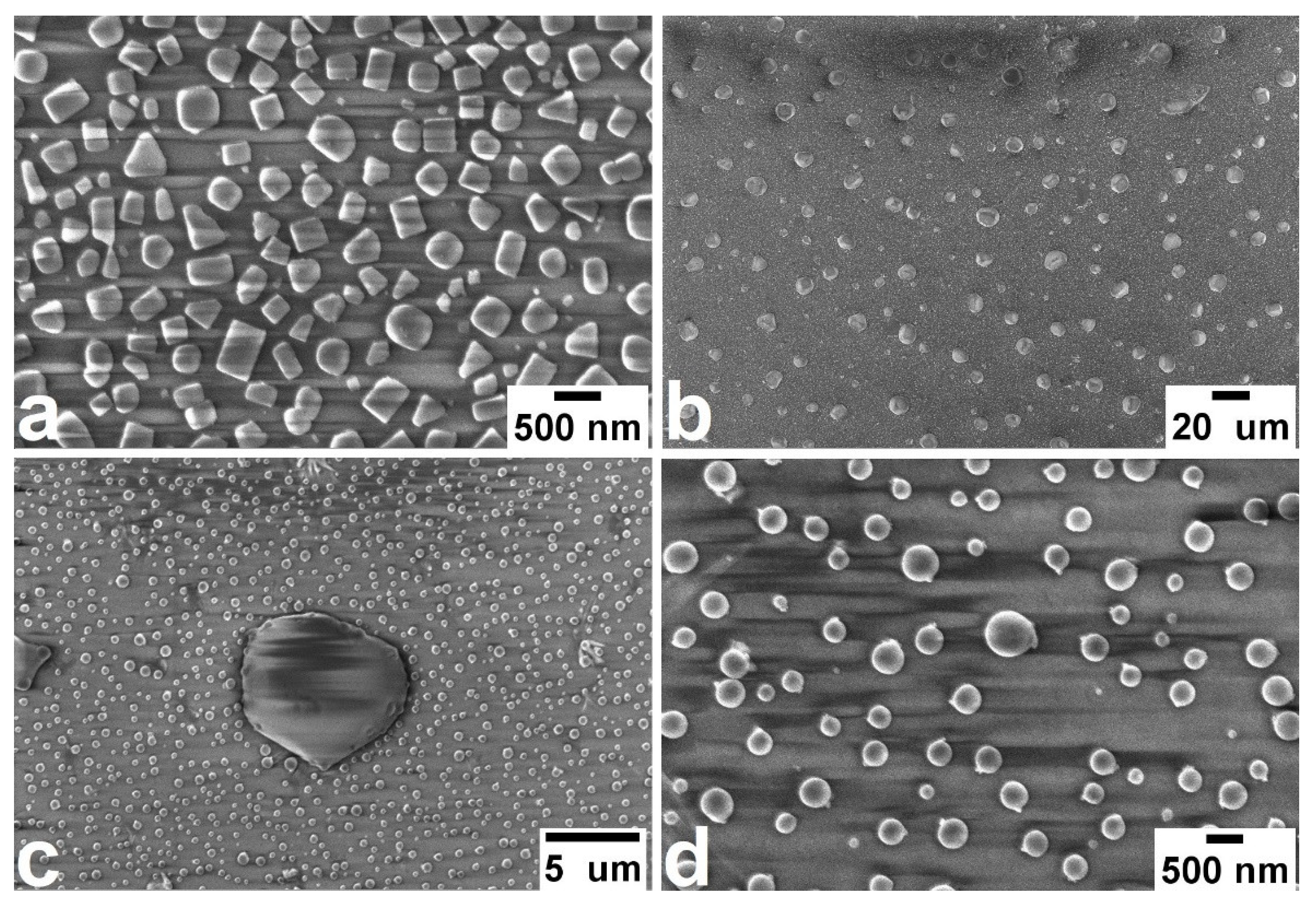
3.1. Spontaneous Growth of Silver Nanoparticles on the Surface of Ion-Exchanged Photo-Thermo-Refractive Glass Heat-Treated in Air Atmosphere
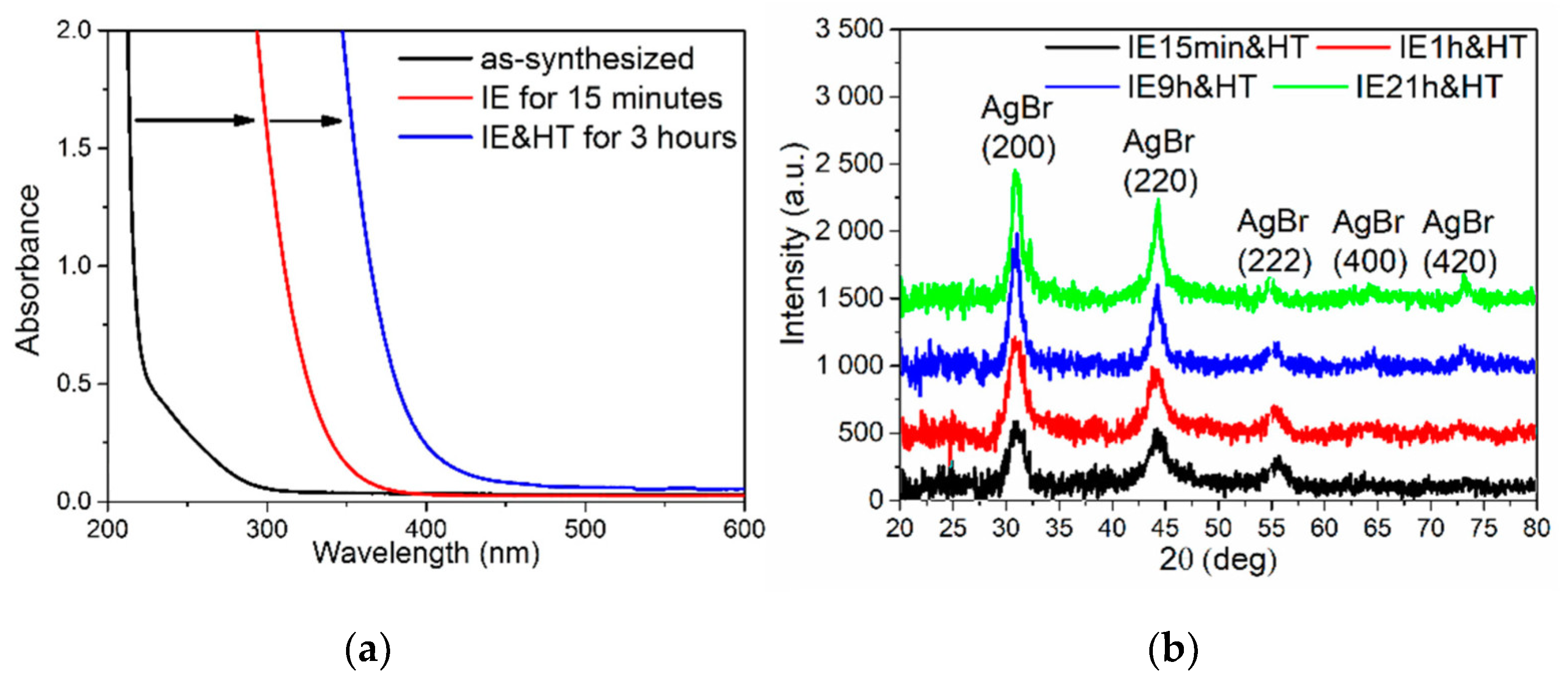
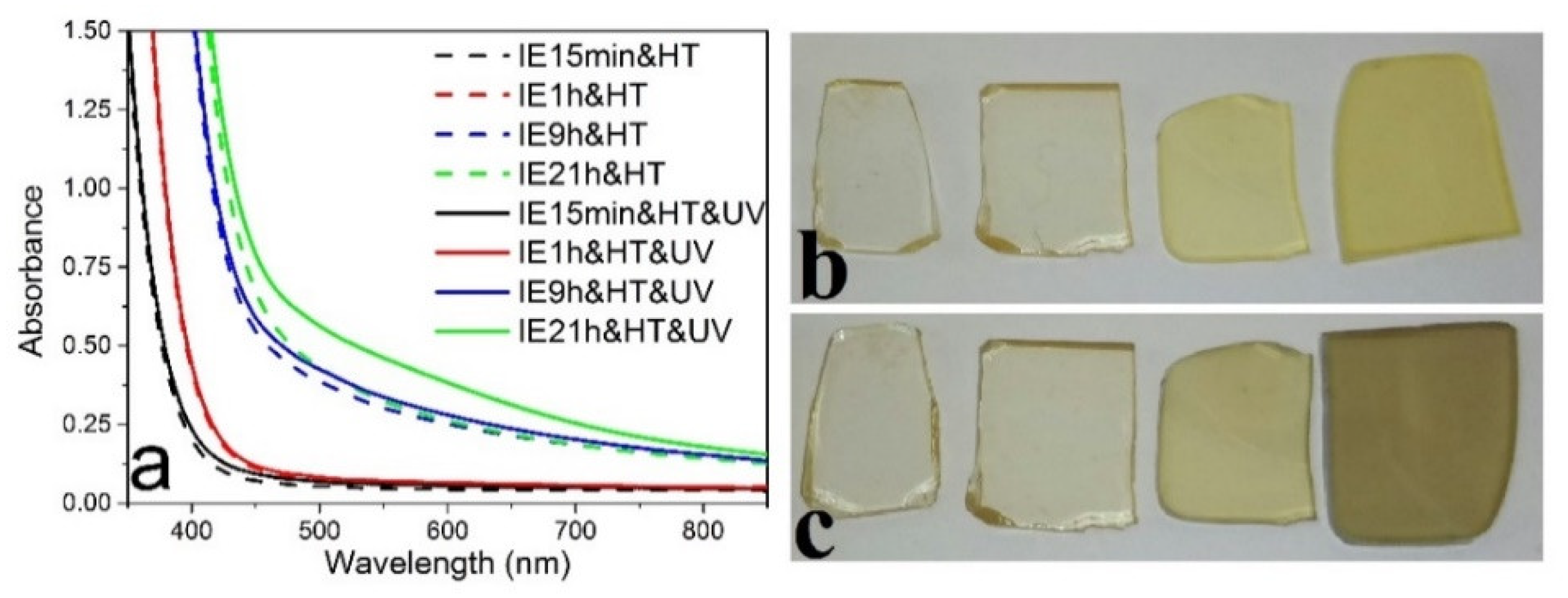
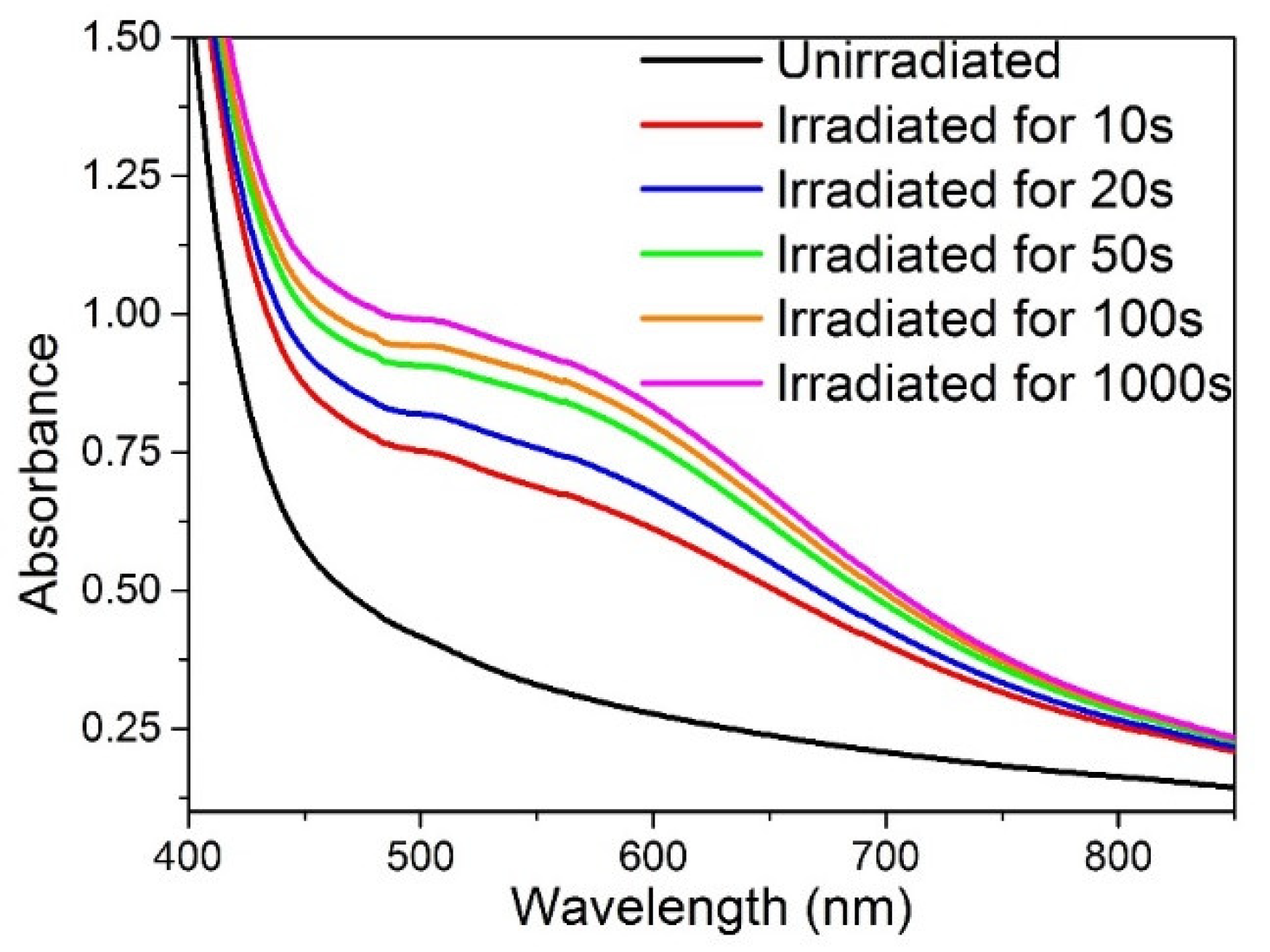
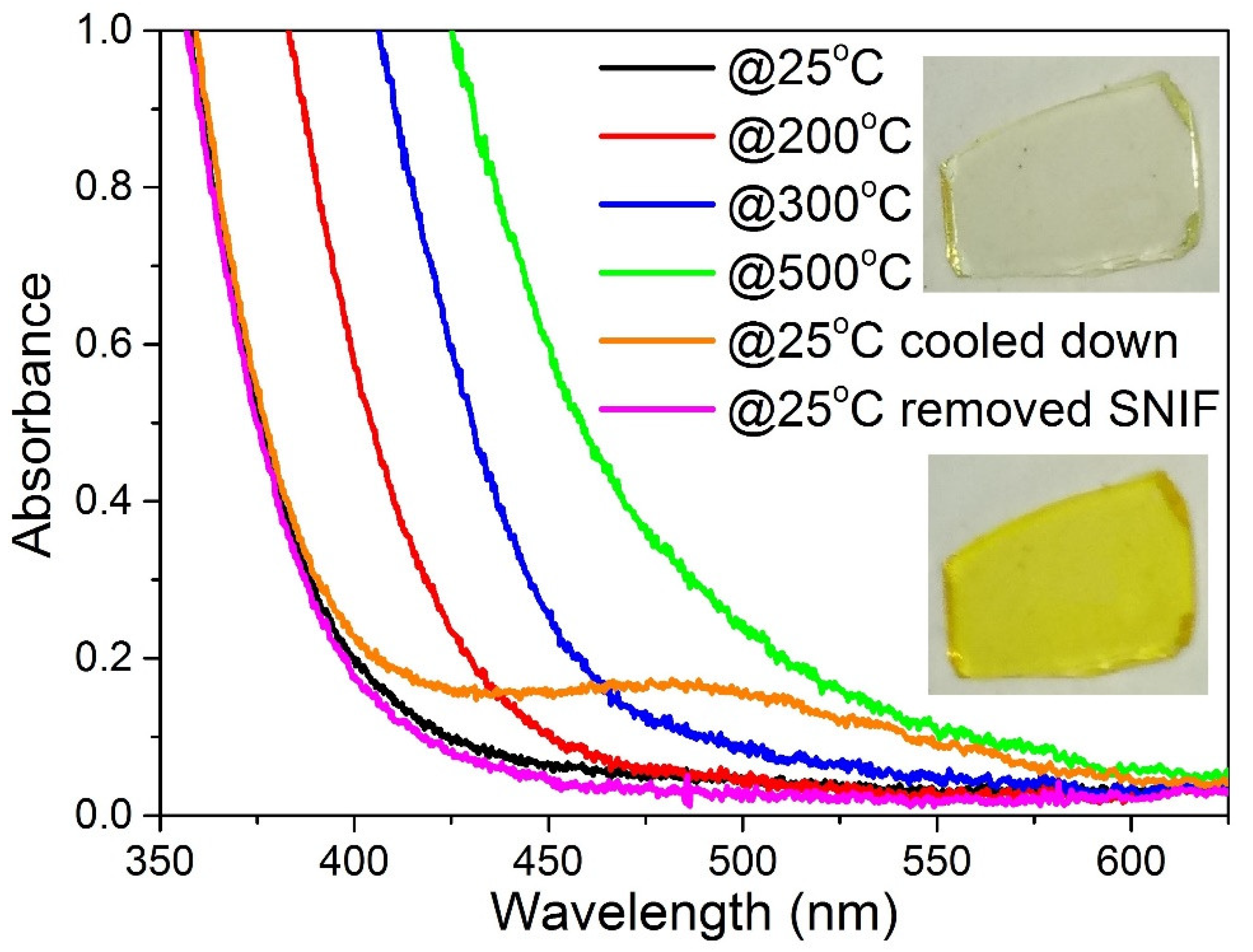
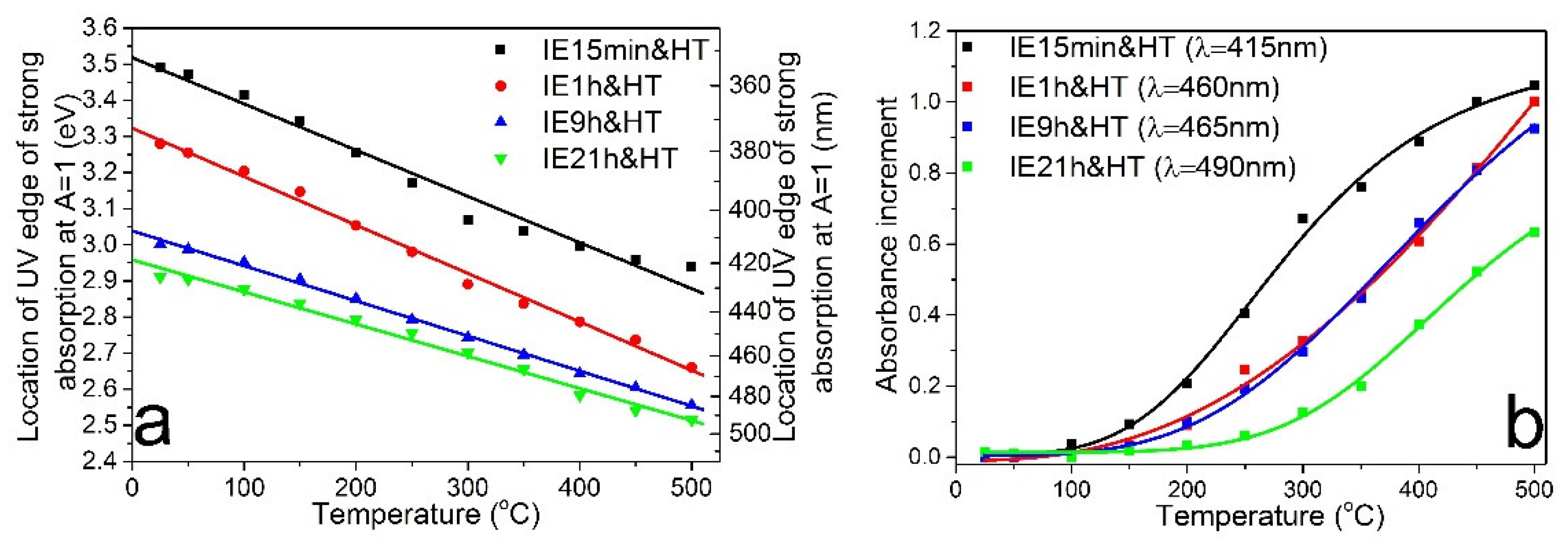
3.2. Photo- and Thermochromic Behavior of Na+–Ag+ Ion-Exchanged Br-Doped Glasses
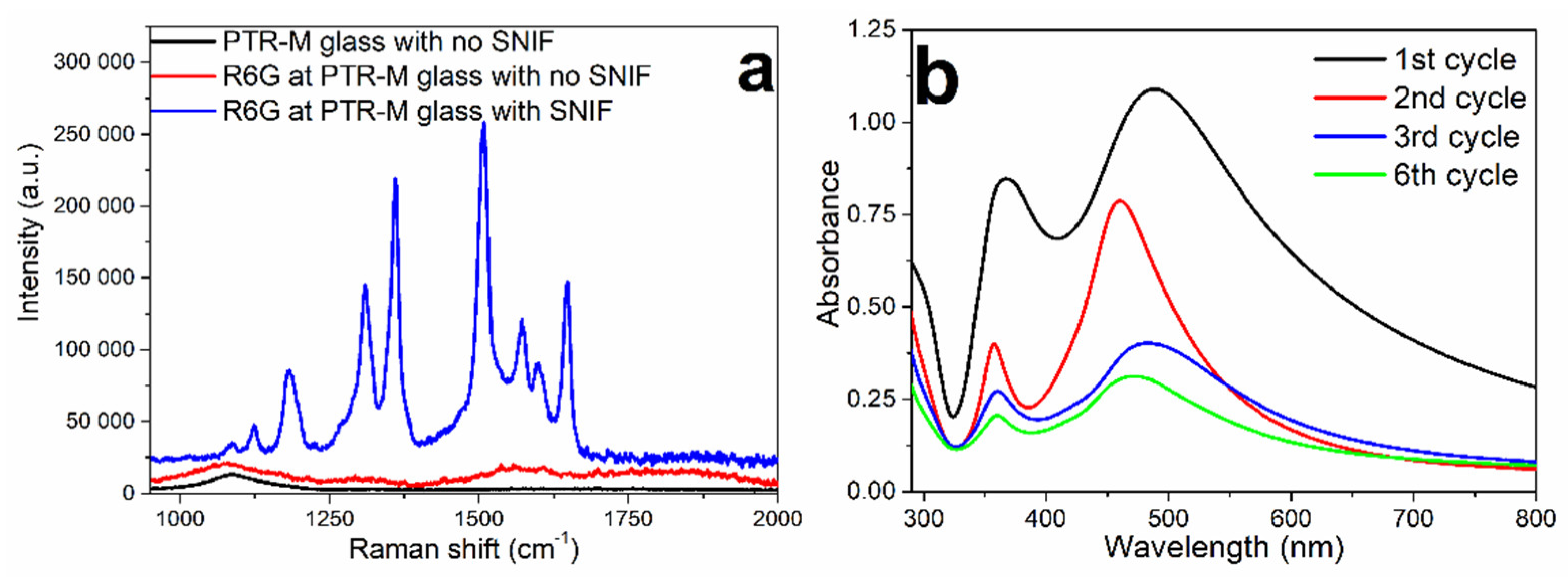
3.3. Luminescent Properties of Silver Molecular Clusters Formed in Ce- and Sb-Doped Glasses
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nikonorov, N.; Aseev, V.; Dubrovin, V.; Ignatiev, A.; Ivanov, S.; Sgibnev, Y.; Sidorov, A. Photonic, Plasmonic, Fluidic, and Luminescent Devices Based on New Polyfunctional Photo-Thermo-Refractive Glass. In Optics, Photonics and Laser Technology; Ribeiro, P., Raposo, M., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; p. 246. ISBN 978-3-319-98547-3. [Google Scholar]
- Nikonorov, N.; Ivanov, S.; Dubrovin, V.; Ignatiev, A. New Photo-Thermo-Refractive Glasses for Holographic Optical Elements: Properties and Applications. In Holographic Materials and Optical Systems; Naydenova, I., Ed.; IntechOpen: London, UK, 2017; p. 503. [Google Scholar]
- Lumeau, J.; Zanotto, E.D. A review of the photo-thermal mechanism and crystallization of photo-thermo-refractive (PTR) glass. Int. Mater. Rev. 2017, 62, 348–366. [Google Scholar] [CrossRef]
- Ivanov, S.; Dubrovin, V.; Nikonorov, N.; Stolyarchuk, M.; Ignatiev, A. Origin of refractive index change in photo-thermo-refractive glass. J. Non. Cryst. Solids 2019, 521, 119496. [Google Scholar] [CrossRef]
- Sato, Y.; Taira, T.; Smirnov, V.; Glebova, L.; Glebov, L. Continuous-wave diode-pumped laser action of Nd3+-doped photo-thermo-refractive glass. Opt. Lett. 2011, 36, 2257–2259. [Google Scholar] [CrossRef]
- Ivanov, S.A.; Lebedev, V.F.; Ignat’ev, A.I.; Nikonorov, N.V. Laser action on neodymium heavily doped photo-thermo-refractive glass. In Proceedings of the 1st International Symposium on Advanced Photonic Materials, St. Petersburg, Russia, 27 June–1 July; ITMO University: St. Petersburg, Russia, 2016; pp. 29–31. [Google Scholar]
- Sgibnev, Y.; Nikonorov, N.; Ignatiev, A.; Vasilyev, V.; Sorokina, M. Photostructurable photo-thermo-refractive glass. Opt. Express 2016, 24, 4563. [Google Scholar] [CrossRef]
- Sgibnev, Y.M.; Nikonorov, N.V.; Vasilev, V.N.; Ignatiev, A.I. Optical Gradient Waveguides in Photo-Thermo-Refractive Glass Formed by Ion Exchange Method. J. Light. Technol. 2015, 33, 3730–3735. [Google Scholar] [CrossRef]
- Stepanov, A.L.; Hole, D.E.; Townsend, P.D. Formation of silver nanoparticles in soda-lime silicate glass by ion implantation near room temperature. J. Non. Cryst. Solids 1999, 260, 65–74. [Google Scholar] [CrossRef]
- Arnold, G.W.; Borders, J.A. Aggregation and migration of ion-implanted silver in lithia-alumina-silica glass. J. Appl. Phys. 1977, 48, 1488–1496. [Google Scholar] [CrossRef]
- Onbaşlı, M.C.; Tandia, A.; Mauro, J.C. Mechanical and compositional design of high-strength Corning Gorilla® Glass. In Handbook of Materials Modeling: Applications: Current and Emerging Materials; Springer International Publishing: NewYork, NY, USA, 2020; pp. 1997–2019. [Google Scholar]
- Findakly, T. Glass waveguides by ion exchange: A review. Opt. Eng. 1985, 25, 244–250. [Google Scholar] [CrossRef]
- Tervonen, A.; West, B.R.; Honkanen, S. Ion-exchanged glass waveguide technology: A review. Opt. Eng. 2011, 50, 71107. [Google Scholar] [CrossRef]
- Karlsson, S.; Jonson, B.; Stålhandske, C. The technology of chemical glass strengthening—A review. Glas. Technol. Eur. J. Glas. Sci. Technol. Part A 2010, 51, 41–54. [Google Scholar]
- Haes, A.J.; Van Duyne, R.P. A nanoscale optical biosensor: Sensitivity and selectivity of an approach based on the localized surface plasmon resonance spectroscopy of triangular silver nanoparticles. J. Am. Chem. Soc. 2002, 124, 10596–10604. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Brolo, A.G. Silver Nanoparticles Self Assembly as SERS Substrates with Near Single Molecule Detection Limit. Phys. Chem. Chem. Phys. 2009, 11, 7381–7389. [Google Scholar] [CrossRef]
- Enrichi, F.; Quandt, A.; Righini, G.C. Plasmonic enhanced solar cells: Summary of possible strategies and recent results. Renew. Sustain. Energy Rev. 2018, 82, 2433–2439. [Google Scholar] [CrossRef]
- Ivanova, N.; Gugleva, V.; Dobreva, M.; Pehlivanov, I.; Stefanov, S.; Andonova, V. Silver Nanoparticles as Multi-Functional Drug Delivery Systems. In Nanomedicines; IntechOpen: London, UK, 2018. [Google Scholar]
- Guo, H.; Feng, M.; Song, F.; Li, H.; Ren, A.; Wei, X.; Li, Y.; Xu, X. Q-switched Erbium-doped Fiber Laser Based on Silver Nanoparticles as a Saturable Absorber. IEEE Photonics Technol. Lett. 2015, 28, 135–138. [Google Scholar] [CrossRef]
- Sarina, S.; Waclawik, E.R.; Zhu, H. Photocatalysis on Supported Gold and Silver Nanoparticles under Ultraviolet and Visible Light Irradiation. Green Chem. 2013, 17, 1814–1833. [Google Scholar] [CrossRef]
- Fleischmann, M.; Hendra, P.J.; McQuillan, A.J. Raman spectra of pyridine adsorbed at a silver electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Doering, W.E.; Nie, S. Single-Molecule and Single-Nanoparticle SERS: Examining the Roles of Surface Active Sites and Chemical Enhancement. J. Phys. Chem. B 2002, 106, 311–317. [Google Scholar] [CrossRef]
- Kneipp, J.; Kneipp, K. SERS—A single-molecule and nanoscale tool for bioanalytics. Chem. Soc. Rev. 2008, 37, 1052–1060. [Google Scholar] [CrossRef]
- Chen, Y.; Karvonen, L.; Säynätjoki, A.; Ye, C.; Tervonen, A.; Honkanen, S. Ag nanoparticles embedded in glass by two-step ion exchange and their SERS application. Opt. Mater. Express 2011, 1, 164. [Google Scholar] [CrossRef]
- Chen, Y.; Jaakola, J.J.; Säynätjoki, A.; Tervonen, A.; Honkanen, S. Glass-embedded silver nanoparticle patterns by masked ion-exchange process for surface-enhanced Raman scattering. J. Raman Spectrosc. 2011, 42, 936–940. [Google Scholar] [CrossRef]
- Zhurikhina, V.V.; Brunkov, P.N.; Melehin, V.G.; Kaplas, T.; Svirko, Y.; Rutckaia, V.V.; Lipovskii, A.A. Self-assembled silver nanoislands formed on glass surface via out-diffusion for multiple usages in SERS applications. Nanoscale Res. Lett. 2012, 7, 676. [Google Scholar] [CrossRef]
- Chervinskii, S.; Sevriuk, V.; Reduto, I.; Lipovskii, A. Formation and 2D-patterning of silver nanoisland film using thermal poling and out-diffusion from glass. J. Appl. Phys. 2013, 114, 224301. [Google Scholar] [CrossRef]
- Heisler, F.; Babich, E.; Scherbak, S.; Chervinskii, S.; Hasan, M.; Samusev, A.; Lipovskii, A.A. Resonant Optical Properties of Single Out-Diffused Silver Nanoislands. J. Phys. Chem. C 2015, 119, 26692–26697. [Google Scholar] [CrossRef]
- Babich, E.S.; Redkov, A.V.; Reduto, I.V.; Scherbak, S.A.; Kamenskii, A.N.; Lipovskii, A.A. Raman enhancement by individual silver hemispheroids. Appl. Surf. Sci. 2017, 397, 119–124. [Google Scholar] [CrossRef]
- Cattaruzza, E.; Mardegan, M.; Trave, E.; Battaglin, G.; Calvelli, P.; Enrichi, F.; Gonella, F. Modifications in silver-doped silicate glasses induced by ns laser beams. Appl. Surf. Sci. 2011, 257, 5434–5438. [Google Scholar] [CrossRef]
- Ajami, A.; Husinsky, W.; Svecova, B.; Vytykacova, S.; Nekvindova, P. Saturable absorption of silver nanoparticles in glass for femtosecond laser pulses at 400 nm. J. Non. Cryst. Solids 2015, 426, 159–163. [Google Scholar] [CrossRef]
- Sgibnev, E.M.; Ignatiev, A.I.; Nikonorov, N.V.; Efimov, A.M.; Postnikov, E.S. Effects of silver ion exchange and subsequent treatments on the UV-VIS spectra of silicate glasses. I. Undoped, CeO2-doped, and (CeO 2 + Sb2O3)-codoped photo-thermo-refractive matrix glasses. J. Non. Cryst. Solids 2013, 378, 213–226. [Google Scholar] [CrossRef]
- Kinnan, M.K.; Chumanov, G. Plasmon Coupling in Two-Dimensional Arrays of Silver Nanoparticles: II. Effect of the Particle Size and Interparticle Distance. J. Phys. Chem. C 2010, 114, 7496–7501. [Google Scholar] [CrossRef]
- Spierings, G. Optical absorption of Ag+ ions in 11(Na, Ag)2O·11B2O3·78SiO2 glass. J. Non. Cryst. Solids 1987, 94, 407–411. [Google Scholar] [CrossRef]
- Efimov, A.M.; Ignatiev, A.I.; Nikonorov, N.V.; Postnikov, E.S. Ultraviolet-VIS spectroscopic manifestations of silver in photo-thermo-refractive glass matrices. Eur. J. Glas. Sci. Technol. Part A 2013, 54, 155–164. [Google Scholar]
- Sgibnev, Y.M.; Nikonorov, N.V.; Ignatiev, A.I. High efficient luminescence of silver clusters in ion-exchanged antimony-doped photo-thermo-refractive glasses: Influence of antimony content and heat treatment parameters. J. Lumin. 2017, 188, 172–179. [Google Scholar] [CrossRef]
- Sgibnev, Y.; Asamoah, B.; Nikonorov, N.; Honkanen, S. Tunable photoluminescence of silver molecular clusters formed in Na+-Ag+ ion-exchanged antimony-doped photo-thermo-refractive glass matrix. J. Lumin. 2020, 226, 117411. [Google Scholar] [CrossRef]
- Luo, W.; Hu, W.; Xiao, S. Size effect on the thermodynamic properties of silver nanoparticles. J. Phys. Chem. C 2008, 112, 2359–2369. [Google Scholar] [CrossRef]
- Asoro, M.A.; Damiano, J.; Ferreira, P.J. Size effects on the melting temperature of silver nanoparticles: In-situ TEM observations. Microsc. Microanal. 2009, 15, 706–707. [Google Scholar] [CrossRef]
- Zhao, S.J.; Wang, S.Q.; Cheng, D.Y.; Ye, H.Q. Three distinctive melting mechanisms in isolated nanoparticles. J. Phys. Chem. B 2001, 105, 12857–12860. [Google Scholar] [CrossRef]
- Kumbhar, A.S.; Kinnan, M.K.; Chumanov, G. Multipole plasmon resonances of submicron silver particles. J. Am. Chem. Soc. 2005, 127, 12444–12445. [Google Scholar] [CrossRef]
- Kaganovskii, Y.; Mogilko, E.; Lipovskii, A.A.; Rosenbluh, M. Formation of nanoclusters in silver-doped glasses in wet atmosphere. J. Phys. Conf. Ser. 2007, 61, 508–512. [Google Scholar] [CrossRef]
- Araujo, R. Colorless glasses containing ion-exchanged silver. Appl. Opt. 1992, 31, 5221. [Google Scholar] [CrossRef]
- Tutihasi, S. Optical absorption by silver halides. Phys. Rev. 1954, 105, 882–884. [Google Scholar] [CrossRef]
- Dotsenko, L.B.; Glebov, V.A.T. Physics and Chemistry of Photochromic Glasses; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar]
- Garfinkel, H.M. Photochromic glass by silver ion exchange. Appl. Opt. 1968, 7, 789–794. [Google Scholar] [CrossRef]
- Araujo, R.J. Photochromic Glass. Treatise Mater. Sci. Technol. 1977, 12, 91–122. [Google Scholar] [CrossRef]
- Madelung, O. Semiconductors: Data Handbook; Springer: Berlin/Heidelberg, Germany, 2004; ISBN 9783662036488. [Google Scholar]
- Kuznetsov, A.S.; Tikhomirov, V.K.; Moshchalkov, V.V. UV-driven efficient white light generation by Ag nanoclusters dispersed in glass host. Mater. Lett. 2013, 92, 4–6. [Google Scholar] [CrossRef]
- Dubrovin, V.D.; Ignatiev, A.I.; Nikonorov, N.V.; Sidorov, A.I.; Shakhverdov, T.A.; Agafonova, D.S. Luminescence of silver molecular clusters in photo-thermo-refractive glasses. Opt. Mater. Amst. 2014, 36, 753–759. [Google Scholar] [CrossRef]
- Cattaruzza, E.; Caselli, V.M.; Mardegan, M.; Gonella, F.; Bottaro, G.; Quaranta, A.; Valotto, G.; Enrichi, F. Ag+↔Na+ ion exchanged silicate glasses for solar cells covering: Down-shifting properties. Ceram. Int. 2015, 41, 7221–7226. [Google Scholar] [CrossRef]
- Sgibnev, Y.; Cattaruzza, E.; Dubrovin, V.; Vasilyev, V. Photo-Thermo-Refractive Glasses Doped with Silver Molecular Clusters as Luminescence Downshifting Material for Photovoltaic Applications. Part. Part. Syst. Charact. 2018, 35, 1800141. [Google Scholar] [CrossRef]
- Bourhis, K.; Royon, A.; Papon, G.; Bellec, M.; Petit, Y.; Canioni, L.; Dussauze, M.; Rodriguez, V.; Binet, L.; Caurant, D.; et al. Formation and thermo-assisted stabilization of luminescent silver clusters in photosensitive glasses. Mater. Res. Bull. 2013, 48, 1637–1644. [Google Scholar] [CrossRef]
- Efimov, A.M.; Ignatiev, A.I.; Nikonorov, N.V.; Postnikov, E.S. Quantitative UV–VIS spectroscopic studies of photo-thermo-refractive glasses. II. Manifestations of Ce3+ and Ce(IV) valence states in the UV absorption spectrum of cerium-doped photo-thermo-refractive matrix glasses. J. Non. Cryst. Solids 2013, 361, 26–37. [Google Scholar] [CrossRef]
- Ehrt, D. Photoluminescence in the UV-VIS region of polyvalent ions in glasses. J. Non. Cryst. Solids 2004, 348, 22–29. [Google Scholar] [CrossRef]
- Efimov, A.M.; Ignatiev, A.I.; Nikonorov, N.V.; Postnikov, E.S. Photo-Thermo-Refractive Glasses: Effects of Dopants on Their Ultraviolet Absorption Spectra. Int. J. Appl. Glas. Sci. 2015, 6, 109–127. [Google Scholar] [CrossRef]
- Zhurikhina, V.V.; Petrov, M.I.; Sokolov, K.S.; Shustova, O.V. Ion-exchange characteristics of sodium-calcium-silicate glass: Calculation from mode spectra. Tech. Phys. 2010, 55, 1447–1452. [Google Scholar] [CrossRef]






| Parameter | Ion Exchange | Ion Implantation |
|---|---|---|
| Simplicity/complication | Very simple and flexible technology | Requires sophisticated equipment |
| Applications of modified glass | Optical waveguides; glass strengthening; nanostructuring | Optical waveguides; glass hardening; nanostructuring |
| Variety of metal nanostructures | Limited to copper and silver nanostructures due to possibility of ions to effectively exchange with alkali ions from a glass substrate | Depends on ion source: Ag, Cu, Ni, Co, Ti, Cr, and Zn nanoparticles were obtained and studied in glasses |
| Requirements for glass substrate | High chemical durability to salt melt; limited to alkali-containing glasses | No requirements |
| Thickness of the glass modified region | The range is very wide and depends on particular application, typically in the range from few to tens of microns | Typically, below 2 µm |
| Temperature during modification | Should be at least above melting temperature of salt melt; in most cases, temperature is in the range 250–450 °C. | Low-temperature processing |
| Reproducibility | Excellent | Excellent |
| Allows overcoming solubility of dopant | Yes | Yes |
| High-scale production capability | Already used for mass production | Already used for mass production |
| Allows overcoming solubility of dopant in glass matrix | Yes | Yes |
| Glass | Sb2O5, mol.% | Br, mol.% |
|---|---|---|
| PTR-M | 0 | 0 |
| PTR-Br | 0 | 1.4 |
| PTR-Sb2 | 0.002 | 0 |
| PTR-Sb4 | 0.004 | 0 |
| PTR-Sb10 | 0.01 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sgibnev, Y.; Nikonorov, N.; Ignatiev, A. Governing Functionality of Silver Ion-Exchanged Photo-Thermo-Refractive Glass Matrix by Small Additives. Appl. Sci. 2021, 11, 3891. https://doi.org/10.3390/app11093891
Sgibnev Y, Nikonorov N, Ignatiev A. Governing Functionality of Silver Ion-Exchanged Photo-Thermo-Refractive Glass Matrix by Small Additives. Applied Sciences. 2021; 11(9):3891. https://doi.org/10.3390/app11093891
Chicago/Turabian StyleSgibnev, Yevgeniy, Nikolay Nikonorov, and Alexander Ignatiev. 2021. "Governing Functionality of Silver Ion-Exchanged Photo-Thermo-Refractive Glass Matrix by Small Additives" Applied Sciences 11, no. 9: 3891. https://doi.org/10.3390/app11093891
APA StyleSgibnev, Y., Nikonorov, N., & Ignatiev, A. (2021). Governing Functionality of Silver Ion-Exchanged Photo-Thermo-Refractive Glass Matrix by Small Additives. Applied Sciences, 11(9), 3891. https://doi.org/10.3390/app11093891






