Performance Study of Nano/SiO2 Films and the Antimicrobial Application on Cantaloupe Fruit Shelf-Life
Abstract
1. Introduction
2. Materials and Methods
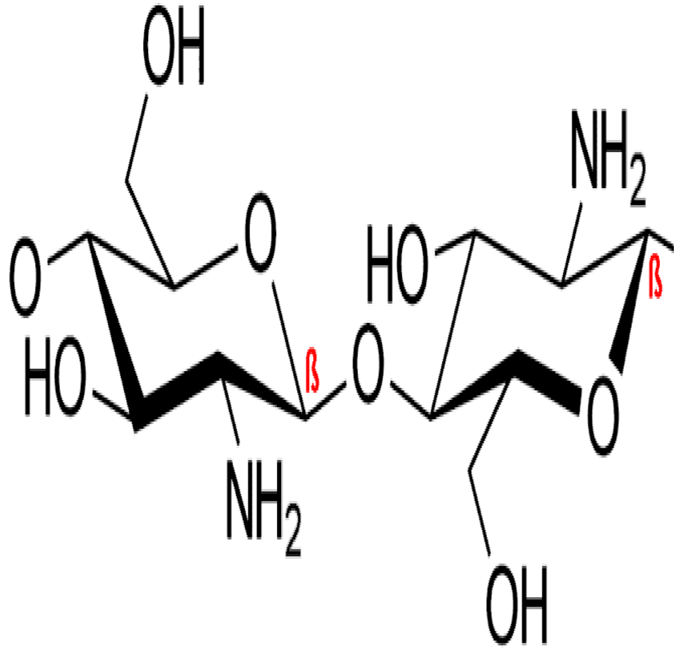
2.1. Materials
2.2. Films Preparations and Samples Treatments
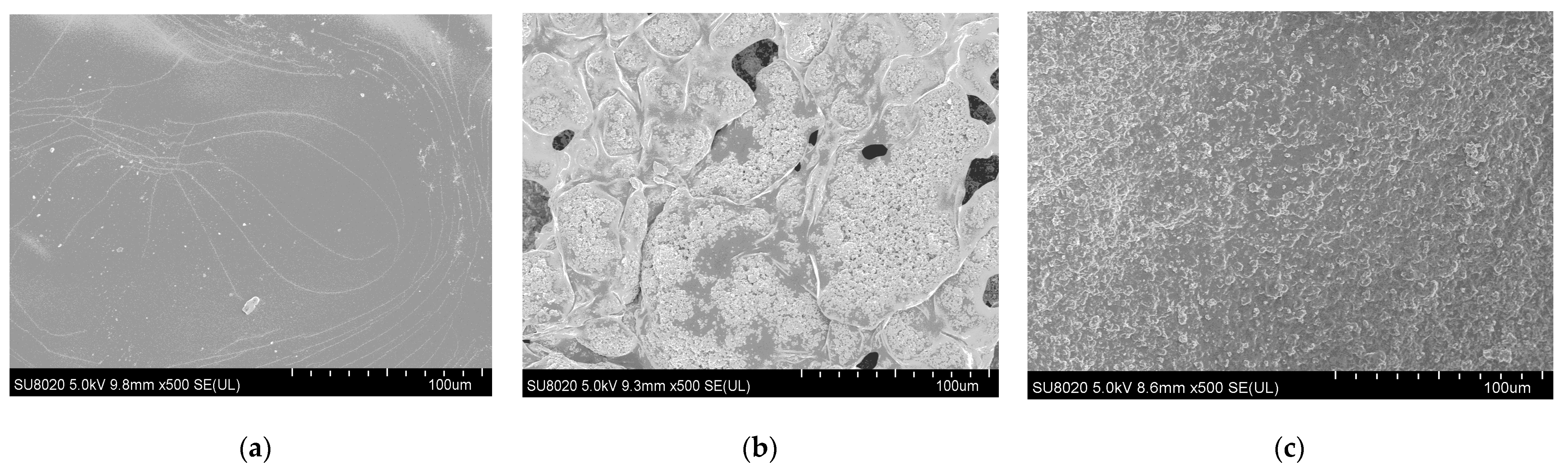
2.3. Scanning Electron Microscopy (SEM)
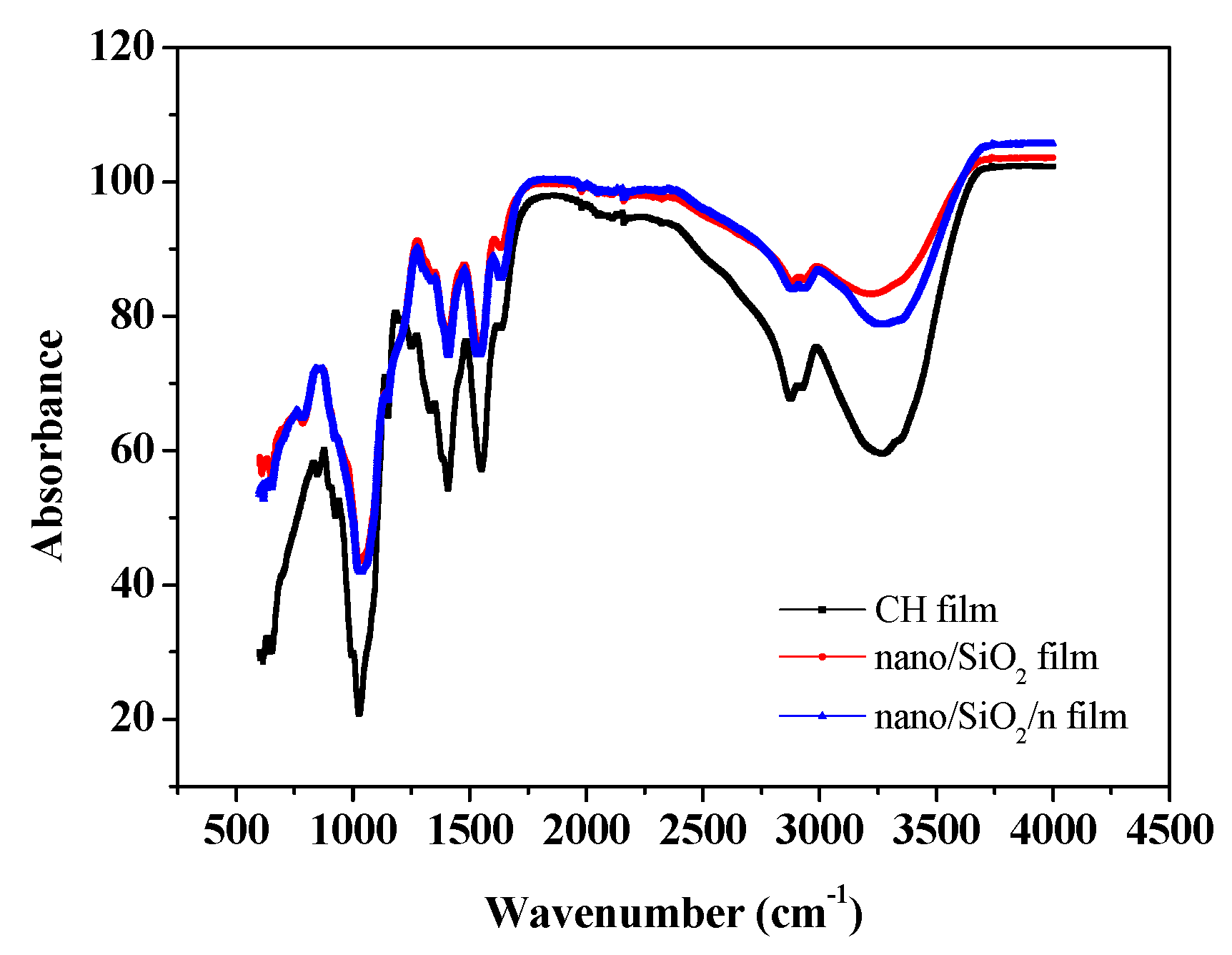
2.4. Fourier Transform Infrared (FTIR) Spectroscopy Measurements
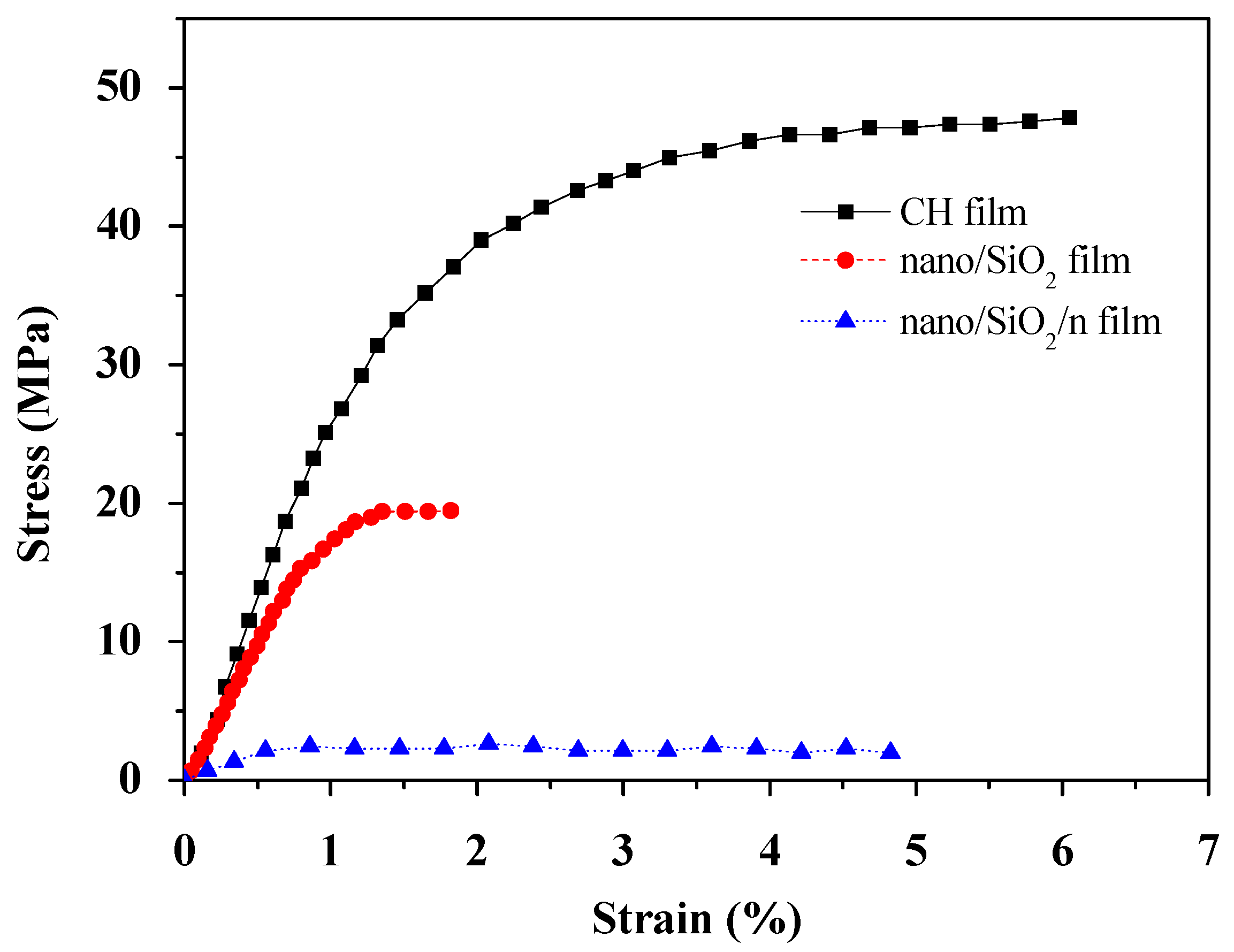
2.5. Tensile Strength Measurements
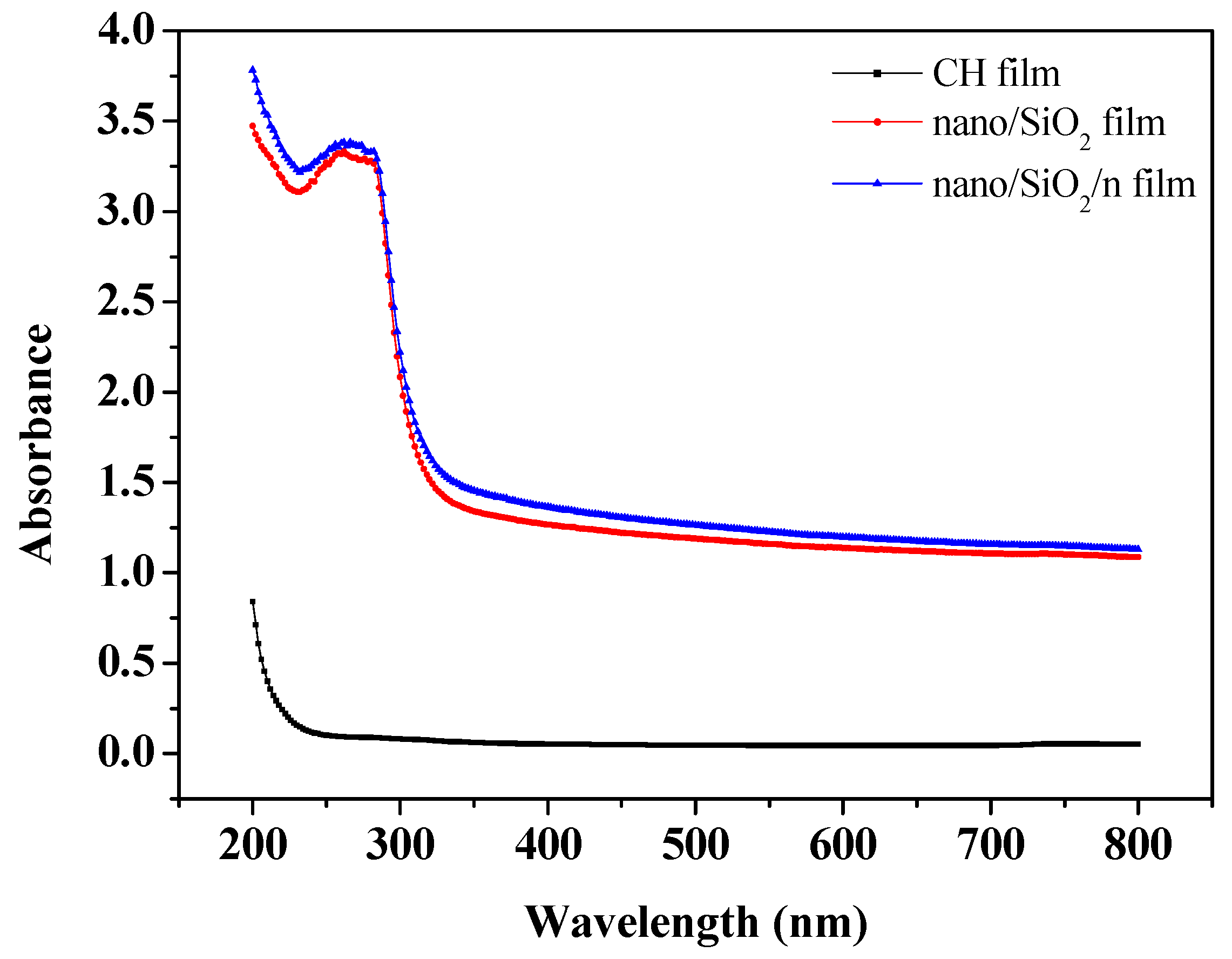
2.6. Optical Measurements
2.7. Thermal Measurements
2.8. X-ray Diffraction (XRD) Measurements
2.9. Turbidity, Partial Size, and Polydispersity Index (PDI) Measurements
2.10. Zeta Potential and Contact Angle Measurements
2.11. Antimicrobial Measurements
2.12. Statistical Analysis
3. Results and Discussion
3.1. SEM Study
3.2. FTIR Spectroscopic Study
3.3. Tensile Strength Analysis
3.4. Optical Properties
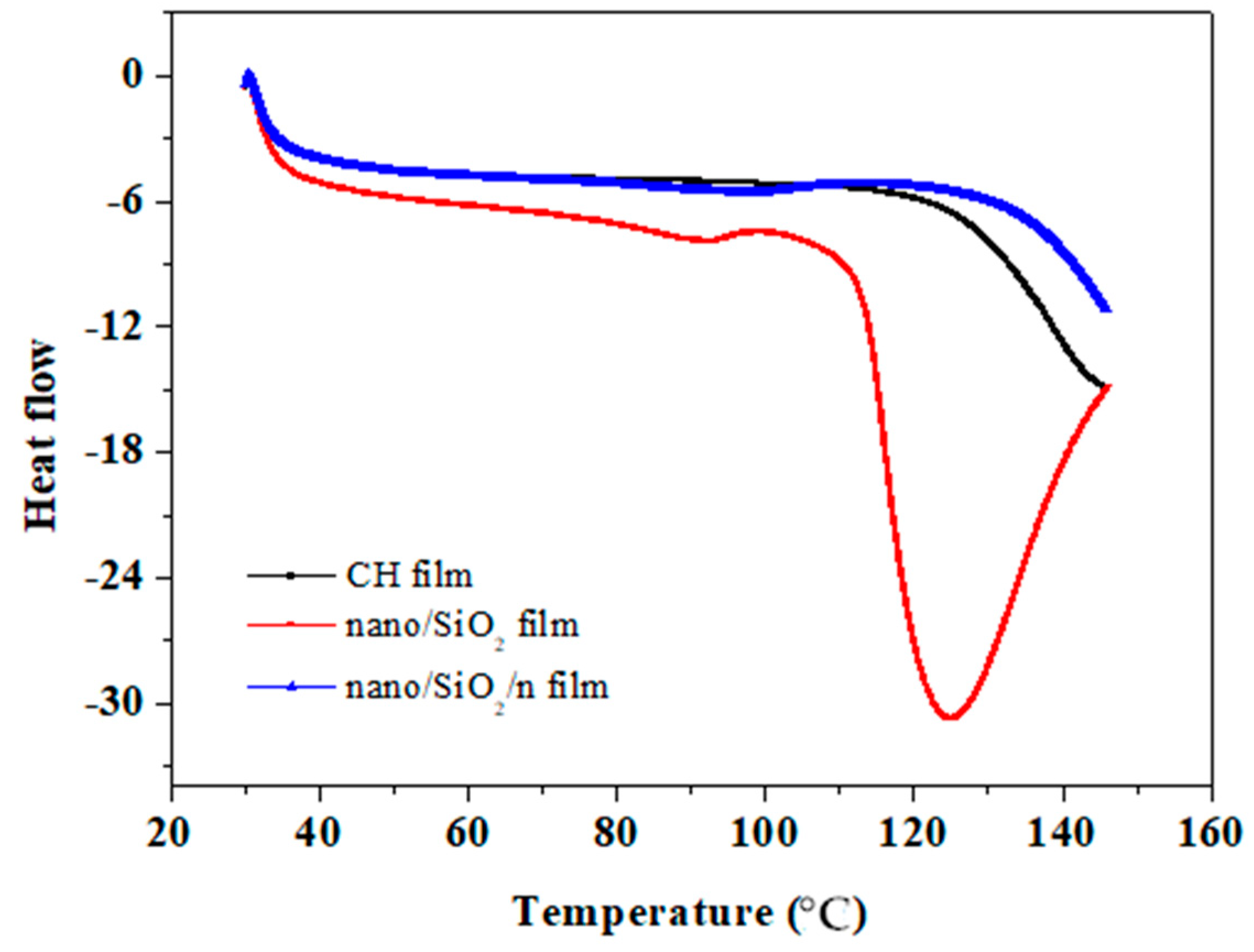
3.5. Thermal Stability Properties
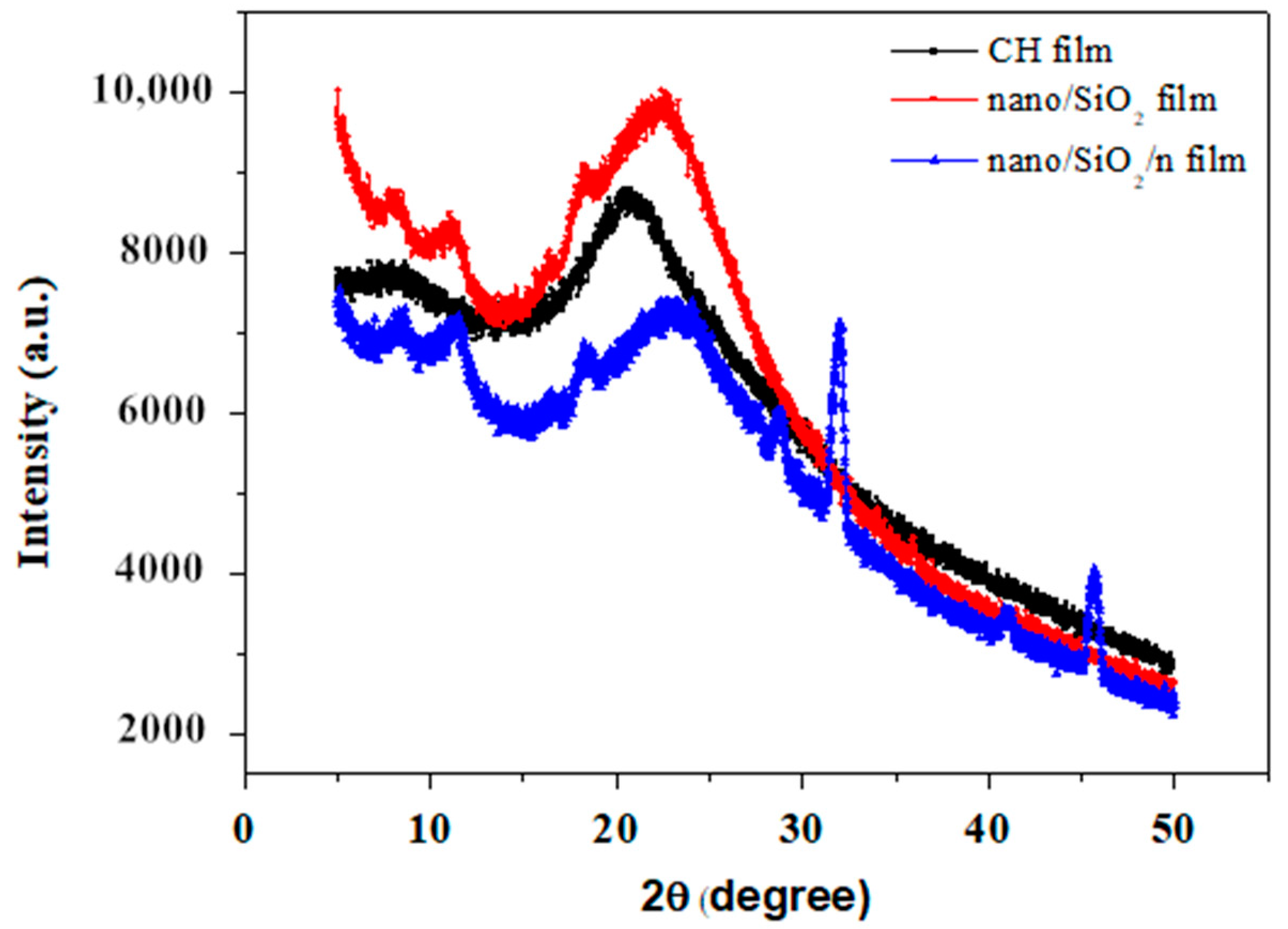
3.6. XRD Analysis
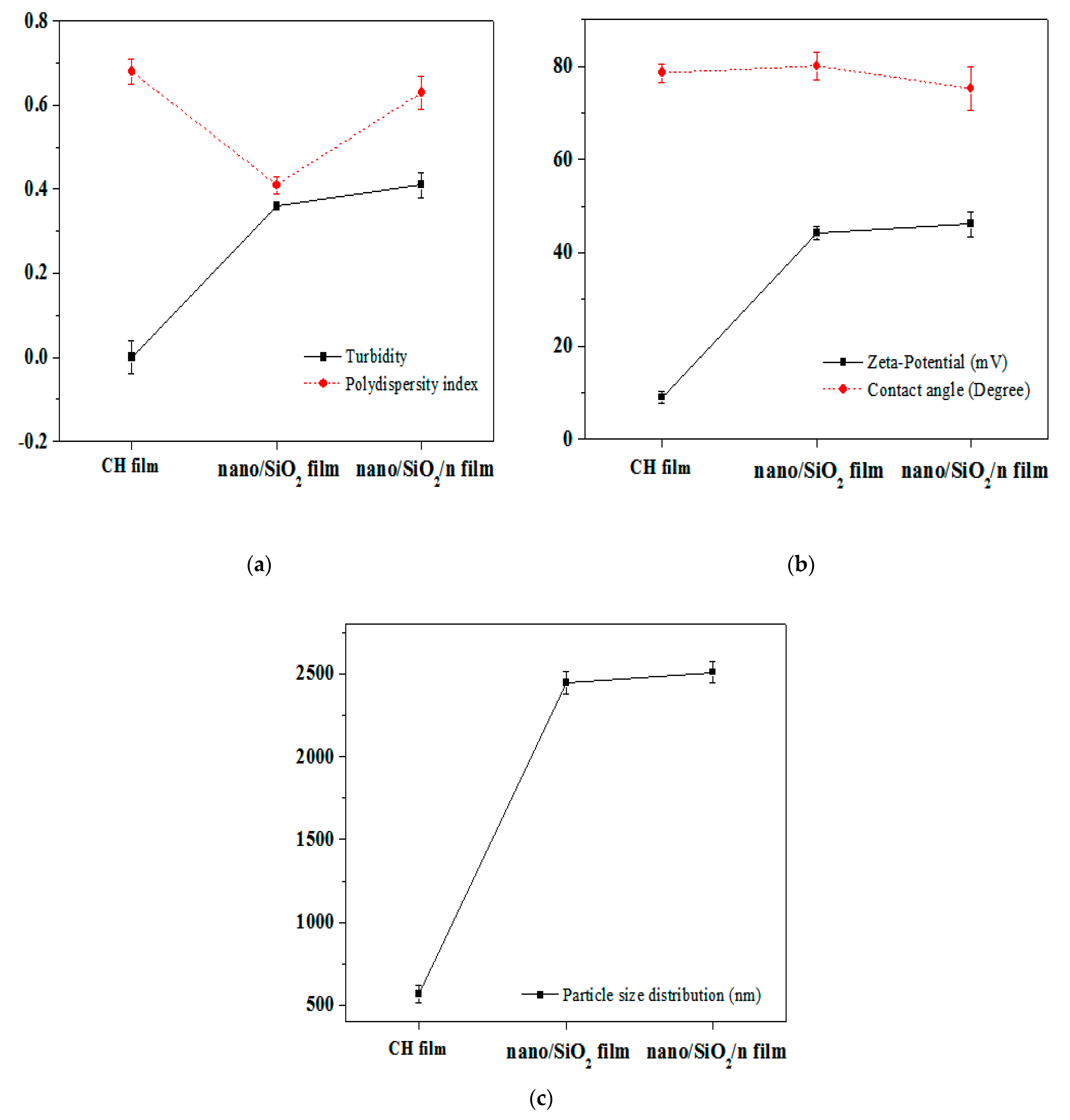
3.7. Performance Characterizes
3.8. Antimicrobial Application on Cantaloupe Fruit
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eldib, R. Application of Nano-coating and Chitosan Combination Films on Cantaloupe Preservation. Pak. J. Biol. Sci. 2020, 23, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Rokayya, S.; Jia, F.; Nie, X.; Xu, J.; Elhakem, A.; Almatrafi, M.; Benajiba, N.; Helal, M. Shelf-life, quality, safety evaluations of blueberry fruits coated with chitosan nano-material films. Sci. Rep. 2021, 11, 55. [Google Scholar] [CrossRef] [PubMed]
- Rokayya, S.; Khojah, E.; Elhakem, A.; Benajiba, N.; Chavali, M.; Vivek, K.; Iqbal, A.; Helal, M. Investigating the Nano-Films Effect on Physical, Mechanical Properties, Chemical Changes, and Microbial Load Contamination of White Button Mushrooms during Storage. Coatings 2021, 11, 44. [Google Scholar] [CrossRef]
- Sami, R.; Elhakem, A.; Alharbi, M.; Benajiba, N.; Almatrafi, M.; Abdelazez, A.; Helal, M. Evaluation of Antioxidant Activities, Oxidation Enzymes, and Quality of Nano-Coated Button Mushrooms (Agaricus bisporus) during Storage. Coatings 2021, 11, 149. [Google Scholar] [CrossRef]
- Qiao, G.; Xiao, Z.; Ding, W.; Rok, A. Effect of Chitosan/Nano-Titanium Dioxide/Thymol and Tween Films on Ready-to-Eat Cantaloupe Fruit Quality. Coatings 2019, 9, 828. [Google Scholar] [CrossRef]
- Eldib, R.; Khojah, E.; Elhakem, A.; Benajiba, N.; Helal, M. Chitosan, Nisin, Silicon Dioxide Nanoparticles Coating Films Effects on Blueberry (Vaccinium myrtillus) Quality. Coatings 2020, 10, 962. [Google Scholar] [CrossRef]
- Rokayya, S.; Jia, F.; Li, Y.; Nie, X.; Xu, J.; Han, R.; Yu, H.; Amanullah, S.; Almatrafi, M.M.; Helal, M. Application of nano-titanum dioxide coating on fresh Highbush blueberries shelf life stored under ambient temperature. LWT 2021, 137, 110422. [Google Scholar] [CrossRef]
- Sami, R.; Elhakem, A.; Alharbi, M.; Benajiba, N.; Almatrafi, M.; Jing, J.; Helal, M. Effect of Titanium Dioxide Nanocomposite Material and Antimicrobial Agents on Mushrooms Shelf-Life Preservation. Processes 2020, 8, 1632. [Google Scholar] [CrossRef]
- Zhou, Y.; Teng, F.; Tian, T.; Sami, R.; Wu, C.; Zhu, Y.; Zheng, L.; Jiang, L.; Wang, Z.; Li, Y. The impact of soy protein isolate-dextran conjugation on capsicum oleoresin (Capsicum annuum L.) nanoemulsions. Food Hydrocoll. 2020, 108, 105818. [Google Scholar] [CrossRef]
- Sami, R.; Elhakem, E.; Almushhin, A.; Alharbi, M.; Almatrafi, M.; Benajiba, N.; Fikry, M.; Helal, M. Enhancement in physicochemical parameters and microbial populations of mushrooms as influenced by nano-coating treatments. Sci. Rep. 2021, 11, 9715. [Google Scholar] [CrossRef] [PubMed]
- Hafez, E.M.; Osman, H.S.; Gowayed, S.M.; Okasha, S.A.; Omara, A.E.; Sami, R.; Abd El-Monem, A.M.; Abd El-Razek, U.A. Minimizing the Adversely Impacts of Water Deficit and Soil Salinity on Maize Growth and Productivity in Response to the Application of Plant Growth-Promoting Rhizobacteria and Silica Nanoparticles. Agronomy 2021, 11, 676. [Google Scholar] [CrossRef]
- Murthy, R.V.V.R.; Chavali, M.; Mohammad, F. Synergistic effect of nano-silica slurries for cementing oil and gas wells. Pet. Res. 2020, 5, 83–91. [Google Scholar] [CrossRef]
- Arumugam, H.; Krishnan, S.; Chavali, M.; Muthukaruppan, A. Cardanol based benzoxazine blends and bio-silica reinforced composites: Thermal and dielectric properties. New J. Chem. 2018, 42, 4067–4080. [Google Scholar] [CrossRef]
- Song, H.; Yuan, W.; Jin, P.; Wang, W.; Wang, X.; Yang, L.; Zhang, Y. Effects of chitosan/nano-silica on postharvest quality and antioxidant capacity of loquat fruit during cold storage. Postharvest Biol. Technol. 2016, 119, 41–48. [Google Scholar] [CrossRef]
- Alghdeir, M.; Mayya, K.; Dib, M.; Alghoraibi, I. Characterization of SiO2/ LDPE Composite Barrier Films. J. Mater. Environ. Sci. 2018, 9, 2042–2050. [Google Scholar]
- Prajitha, N.; Athira, S.S.; Mohanan, P.V. Bio-interactions and risks of engineered nanoparticles. Environ. Res. 2019, 172, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sun, Y.; Li, Y.; Tong, X.; Regenstein, J.M.; Huang, Y.; Ma, W.; Sami, R.; Qi, B.; Jiang, L. Effect of the condition of spray-drying on the properties of the polypeptide-rich powders from enzyme-assisted aqueous extraction processing. Dry. Technol. 2019, 37, 2105–2115. [Google Scholar] [CrossRef]
- Fortuni, B.; Fujita, Y.; Ricci, M.; Inose, T.; Aubert, R.; Lu, G.; Hutchison, J.A.; Hofkens, J.; Latterini, L.; Uji, I.H. A novel method for in situ synthesis of SERS-active gold nanostars on polydimethylsiloxane film. Chem. Commun. 2017, 53, 5121–5124. [Google Scholar] [CrossRef]
- Sami, R.; Almatrafi, M.; Elhakem, A.; Alharbi, M.; Benajiba, N.; Helal, M. Effect of Nano Silicon Dioxide Coating Films on the Quality Characteristics of Fresh-Cut Cantaloupe. Membranes 2021, 11, 140. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Wang, W.; Liu, L.; Wu, S.; Wei, Y.; Li, W. Effect of chitosan/nano-silica coating on the physicochemical characteristics of longan fruit under ambient temperature. J. Food Eng. 2013, 118, 125–131. [Google Scholar] [CrossRef]
- Kou, X.; He, Y.; Li, Y.; Chen, X.; Feng, Y.; Xue, Z. Effect of abscisic acid (ABA) and chitosan/nano-silica/sodium alginate composite film on the color development and quality of postharvest Chinese winter jujube (Zizyphus jujuba Mill. cv. Dongzao). Food Chem. 2019, 270, 385–394. [Google Scholar] [CrossRef]
- Rokayya, S.; Li, C.J.; Zhao, Y.; Li, Y.; Sun, C.H. Cabbage (Brassica oleracea L. var. capitata) phytochemicals with antioxidant and anti-inflammatory potential. Asian Pac. J. Cancer Prev. 2014, 14, 6657–6662. [Google Scholar] [CrossRef] [PubMed]
- Sami, R.; Khojah, E.; Elgarni, E.; Aljumayi, H. Morphological-Mechanical Proprieties of Five Different Tomato Varieties in Kingdom of Saudi Arabia for High Techniques in Harvesting, Handling and Manufacturing. Tikrit J. Agric. Sci. Vol. 2016, 16. ISSN-1813-1646. [Google Scholar]
- Rokayya, S.; Ebtihal, K. Evaluation of physical properties of okra (Abelmoschus esculentus L.) pods with different structural characteristics. Res. Crop. 2019, 20, 73–78. [Google Scholar] [CrossRef]
- Rokayya, S.; Khojah, E. Physical-mechanical Estimation of Pepper (Capsicum annuum L.) Fruit Varieties. J. Northeast. Agric. Univ. 2016, 23, 61–69. [Google Scholar] [CrossRef]
- Rokayya, S. Some aspects of physical-mechanical properties of apple (Malus domestica) cultivars for high techniques in manufacturing. J. Northeast. Agric. Univ. 2017, 24, 31–39. [Google Scholar]
- Ma, W.; Rokayya, S.; Xu, L.; Sui, X.; Jiang, L.; Li, Y. Physical-Chemical Properties of Edible Film Made from Soybean Residue and Citric Acid. J. Chem. 2018, 2018, 4026831. [Google Scholar] [CrossRef]
- Sami, R. Antioxidant Properties of Peptides from Soybean Meal Protein Hydrolysates Evaluated by Electron Spin Resonance Spectrometry. Adv. Environ. Biol. 2017, 11, 12–18. [Google Scholar]
- Rokayya, S.; Khojah, E.Y. The Extrusion Conditions Mechanism Effect on the Nutritional Value of Soybean Meal Protein. Adv. Environ. Biol. 2017, 11, 42–51. [Google Scholar]
- Bhattacharya, S.S.; Chaudhari, S.B. Change in physico-mechanical and thermal properties of polyamide/silica nanocomposite film. Int. J. Eng. Res. Dev. 2013, 7, 1–5. [Google Scholar]
- Daniyal, W.M.E.M.M.; Fen, Y.W.; Saleviter, S.; Chanlek, N.; Nakajima, H.; Abdullah, J.; Yusof, N.A. X-ray Photoelectron Spectroscopy Analysis of Chitosan–Graphene Oxide-Based Composite Thin Films for Potential Optical Sensing Applications. Polymers 2021, 13, 478. [Google Scholar] [CrossRef]
- Vo-An, Q.; Nguyen, T.C.; Nguyen, Q.T.; Vu, Q.T.; Truong, C.D.; Nguyen, T.L.; Ly, T.N.L.; Bach, L.G.; Thai, H. Novel Nanoparticle Biomaterial of Alginate/Chitosan Loading Simultaneously Lovastatin and Ginsenoside RB1: Characteristics, Morphology, and Drug Release Study. Int. J. Polym. Sci. 2021, 2021, 5214510. [Google Scholar] [CrossRef]
- Hu, M.; Xie, F.; Zhang, S.; Qi, B.; Li, Y. Effect of nanoemulsion particle size on the bioavailability and bioactivity of perilla oil in rats. J. Food Sci. 2021, 86, 206–214. [Google Scholar] [CrossRef]
- Mallakpour, S.; Khadem, E. Chitosan reinforced with modified CaCO3 nanoparticles to enhance thermal, hydrophobicity properties and removal of cu(II) and cd(II) ions. J. Polym. Res. 2017, 24, 86. [Google Scholar] [CrossRef]
- AOAC. Yeast and Molds Counts in Foods. Dry Rehydratable Film Method. Petrifilm Method; Official Method 997.02; AOAC International: Gaithersburg, MD, USA, 1998. [Google Scholar]
- Arl, D.; Rogé, V.; Adjeroud, N.; Pistillo, B.R.; Sarr, M.; Bahlawane, N.; Lenoble, D. SiO2 thin film growth through a pure atomic layer deposition technique at room temperature. RSC Adv. 2020, 10, 18073–18081. [Google Scholar] [CrossRef]
- Farhan, A.; Hani, N.M. Characterization of edible packaging films based on semi-refined kappa-carrageenan plasticized with glycerol and sorbitol. Food Hydrocoll. 2017, 64, 48–58. [Google Scholar] [CrossRef]
- Pandis, C.; Madeira, S.; Matos, J.; Kyritsis, A.; Mano, J.F.; Ribelles, J.L.G. Chitosan–silica hybrid porous membranes. Mater. Sci. Eng. C 2014, 42, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Junlabhut, P.; Boonruang, S.; Pecharapa, W. Optical Absorptivity Enhancement of SiO2 Thin Film by Ti and Ag Additive. Energy Procedia 2013, 34, 734–739. [Google Scholar] [CrossRef][Green Version]
- Sami, R.; Elhakem, A.; Alharbi, M.; Benajiba, N.; Fikry, M.; Helal, M. The combined effect of coating treatments to nisin, nano-silica, and chitosan on oxidation processes of stored button mushrooms at 4 °C. Sci. Rep. 2021, 11, 6031. [Google Scholar] [CrossRef]
- Ma, Q.; Liu, Y.; Dong, Z.; Wang, J.; Hou, X. Hydrophobic and nanoporous chitosan–silica composite aerogels for oil absorption. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- O’Callaghan, K.A.M.; Kerry, J.P. Preparation of low- and medium-molecular weight chitosan nanoparticles and their antimicrobial evaluation against a panel of microorganisms, including cheese-derived cultures. Food Control 2016, 69, 256–261. [Google Scholar] [CrossRef]
- Vivek, K.; Singh, S.S.; Sasikumar, R.; Sami, R. Consumer Preference Study on Combined Ultrasound and Sodium Hypochlorite Treated Fresh cut Kiwifruits Coated With Chitosan Using the Fuzzy Logic Approach. J. Microbiol. Biotechnol. Food Sci. 2021, e4054. [Google Scholar] [CrossRef]
- Pulicharla, R.; Marques, C.; Das, R.K.; Rouissi, T.; Brar, S.K. Encapsulation and release studies of strawberry polyphenols in biodegradable chitosan nanoformulation. Int. J. Biol. Macromol. 2016, 88, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Syamdidi, S.; Sidauruk, H.; Munandar, A.; Haryati, S. The Production of Nanoparticles Chitosan from Crustaceans Shell Using the Top-down Method. In E3S Web of Conferences; EDP Sciences: Les Ulis, France, 2020; Volume 147, p. 03025. [Google Scholar]
- Konecsni, K.; Low, N.H.; Nickerson, M.T. Chitosan-tripolyphosphate submicron particles as the carrier of entrapped rutin. Food Chem. 2012, 134, 1775–1779. [Google Scholar] [CrossRef] [PubMed]
- Syahidah, K.; Rosnah, S.; Noranizan, M.A.; Zaulia, O.; Ahmedov, A. Quality changes of fresh cut cantaloupe (Cucumis melo L. var Reticulatus cv. Glamour) in different types of polypropylene packaging. Int. Food Res. J. 2015, 22, 753–760. [Google Scholar]
- Koh, P.C.; Noranizan, M.A.; Karim, R.; Nur Hanani, Z.A. Microbiological stability and quality of pulsed light treated cantaloupe (Cucumis melo L. reticulatus cv. Glamour) based on cut type and light fluence. J. Food Sci. Technol. 2016, 53, 1798–1810. [Google Scholar] [CrossRef]
- Qiu, Y.; Fu, J.; Sun, B.; Ma, X. Sustainable nanocomposite films based on SiO2 and biodegradable poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBH) for food packaging. e-Polymers 2021, 21, 072–081. [Google Scholar] [CrossRef]
- Chen, W.; Jin, T.Z.; Gurtler, J.B.; Geveke, D.J.; Fan, X. Inactivation of Salmonella on whole cantaloupe by application of an antimicrobial coating containing chitosan and allyl isothiocyanate. Int. J. Food Microbiol. 2012, 155, 165–170. [Google Scholar] [CrossRef] [PubMed]
 | 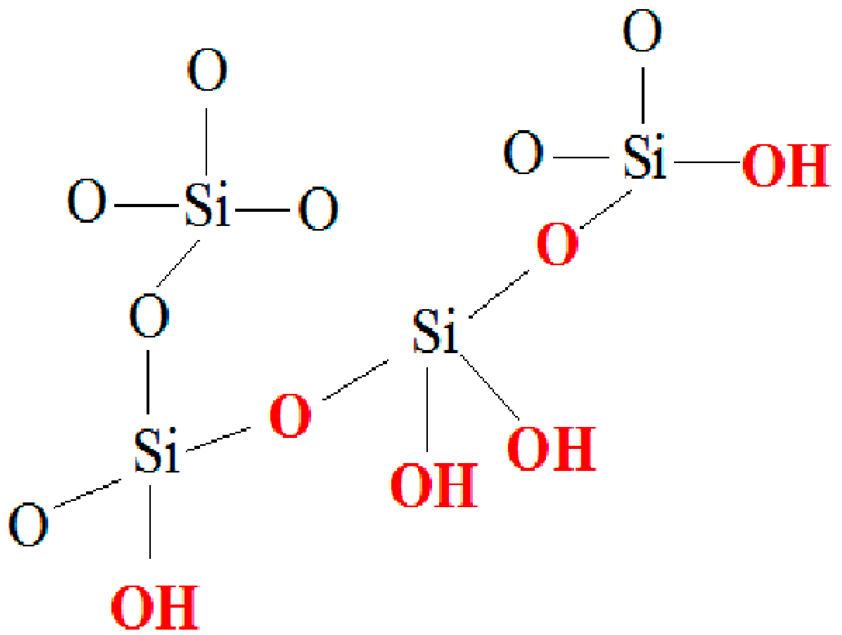 |
| (a) CAS Registry Number: 9012-76-4 Mol weight: 50,000–190,000 Da (based on viscosity) Viscosity: 20-300 cP, 1 wt.% in 1% acetic acid (25 °C, Brookfield)(lit.) Solubility: Dilute aqueous acid: soluble | (b) CAS Registry Number: 7631-86-9 Chemical formula: SiO2 Molar mass: 60.08 g/mol Density: 2.648 (α-quartz), 2.196 (amorphous) g·cm−3 Melting point: 1.713 °C (Amp) Thermal conductivity: 12 (|| c-axis), 6.8 (⊥axis), 1.4 (am.) W/(m⋅K) |
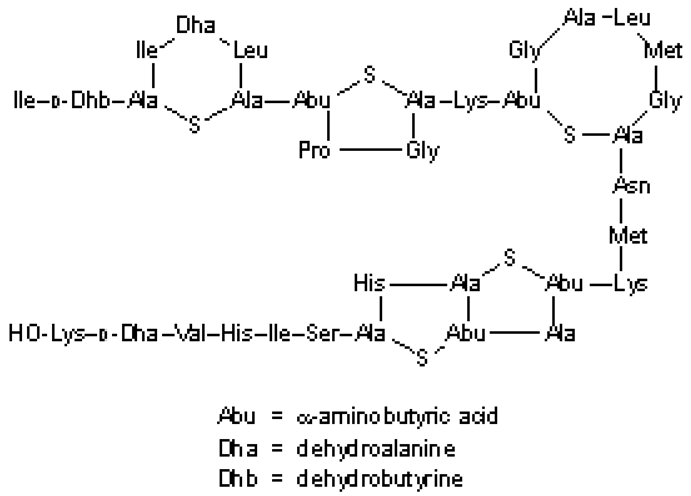 | |
| (c) CAS Registry Number:1414-45-5 Molar mass: 3354.07 g/mol Formula: C143H230N42O37S7 Boiling point: 2966 °C Classification: Bacteriocin Density: 1.402 g/mL | |
| Days | Control | CH Film | nano/SiO2 Film | nano/SiO2/n Film |
|---|---|---|---|---|
| 0 | 0.59 ± 0.29 a | 0.56 ± 0.25 b | 0.54 ± 0.21 c | 0.51 ± 0.22 d |
| 3 | 1.32 ± 0.31 a | 1.02 ± 0.25 b | 0.99 ± 0.37 b | 0.81 ± 0.08 c |
| 6 | 1.93 ± 0.17 a | 1.45 ± 0.22 c | 1.78 ± 0.31 b | 1.21 ± 0.40 d |
| 9 | 2.94 ± 0.31 a | 2.67 ± 0.26 b | 2.49 ± 0.40 c | 1.92 ± 0.31 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sami, R.; Khojah, E.; Elhakem, A.; Benajiba, N.; Helal, M.; Alhuthal, N.; Alzahrani, S.A.; Alharbi, M.; Chavali, M. Performance Study of Nano/SiO2 Films and the Antimicrobial Application on Cantaloupe Fruit Shelf-Life. Appl. Sci. 2021, 11, 3879. https://doi.org/10.3390/app11093879
Sami R, Khojah E, Elhakem A, Benajiba N, Helal M, Alhuthal N, Alzahrani SA, Alharbi M, Chavali M. Performance Study of Nano/SiO2 Films and the Antimicrobial Application on Cantaloupe Fruit Shelf-Life. Applied Sciences. 2021; 11(9):3879. https://doi.org/10.3390/app11093879
Chicago/Turabian StyleSami, Rokayya, Ebtihal Khojah, Abeer Elhakem, Nada Benajiba, Mahmoud Helal, Nawal Alhuthal, Sana A. Alzahrani, Mona Alharbi, and Murthy Chavali. 2021. "Performance Study of Nano/SiO2 Films and the Antimicrobial Application on Cantaloupe Fruit Shelf-Life" Applied Sciences 11, no. 9: 3879. https://doi.org/10.3390/app11093879
APA StyleSami, R., Khojah, E., Elhakem, A., Benajiba, N., Helal, M., Alhuthal, N., Alzahrani, S. A., Alharbi, M., & Chavali, M. (2021). Performance Study of Nano/SiO2 Films and the Antimicrobial Application on Cantaloupe Fruit Shelf-Life. Applied Sciences, 11(9), 3879. https://doi.org/10.3390/app11093879







