Spatial-Temporal Changes in Removal of Fecal Indicators and Diversity of Bacterial Communities in a Constructed Wetland with Ornamental Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Constructed Wetland
2.2. Physicochemical and Microbiological Analysis of Wastewater
2.3. Enumeration of Total Coliforms, E. coli, and Heterotrophic Bacteria Associated to Substrate and Plant Roots
2.4. Analysis of Bacterial Communities Colonizing the Substrate Surface
2.4.1. DNA Extraction and PCR Amplification
2.4.2. DGGE
2.5. Data Analysis
3. Results and Discussion
3.1. Physicochemical Data
3.2. Microbiological Data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Calheiros, C.S.C.; Bessa, V.S.; Mesquita, R.B.R.; Brix, H.; Rangel, A.O.S.S.; Castro, P.M.L. Constructed wetland with a polyculture of ornamental plants for wastewater treatment at a rural tourism facility. Ecol. Eng. 2015, 79, 1–7. [Google Scholar] [CrossRef]
- Calheiros, C.S.C.; Pereira, S.I.A.; Castro, P.M.L. Constructed wetlands as nature-based solutions. In An Introduction to Constructed Wetlands; Jespersen, A.N., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2020; pp. 47–75. ISBN 9781631172557. [Google Scholar]
- Stefanakis, A.I. Constructed wetlands: Description and benefits of an eco-tech water treatment system. In Impact of Water Pollution on Human Health and Environmental Sustainability; McKeown, E., Bugyi, G., Eds.; IGI Global: Hershey, PA, USA, 2016; pp. 281–303. ISBN 9781466695603. [Google Scholar]
- Calheiros, C.S.C.; Pereira, S.I.A.; Castro, P.M.L. Culturable bacteria associated to the rhizosphere and tissues of Iris pseudacorus plants growing in a treatment wetland for winery wastewater discharge. Ecol. Eng. 2018, 115, 67–74. [Google Scholar] [CrossRef]
- Masi, F.; Rizzo, A.; Regelsberger, M. The role of constructed wetlands in a new circular economy, resource oriented, and ecosystem services paradigm. J. Environ. Manag. 2018, 216, 275–284. [Google Scholar] [CrossRef]
- Hsu, C.-B.; Hsieh, H.L.; Yang, L.; Wu, S.H.; Chang, J.S.; Hsiao, S.C.; Su, H.C.; Yeh, C.H.; Ho, Y.S.; Lin, H.J. Biodiversity of constructed wetlands for wastewater treatment. Ecol. Eng. 2011, 37, 1533–1545. [Google Scholar] [CrossRef]
- Semeraro, T.; Giannuzzi, C.; Beccarisi, L.; Aretano, R.; de Marco, A.; Pasimeni, M.R.; Zurlini, G.; Petrosillo, I. A constructed treatment wetland as an opportunity to enhance biodiversity and ecosystem services. Ecol. Eng. 2015, 82, 517–526. [Google Scholar] [CrossRef]
- Moore, T.L.C.; Hunt, W.F. Ecosystem service provision by stormwater wetlands and ponds—A means for evaluation? Water Res. 2012, 46, 6811–6823. [Google Scholar] [CrossRef] [PubMed]
- Brix, H. Do macrophytes play a role in constructed treatment wetlands? Water Sci. Technol. 1997, 35, 11–17. [Google Scholar] [CrossRef]
- Vymazal, J. Plants used in constructed wetlands with horizontal subsurface flow: A review. Hydrobiologia 2011, 674, 133–156. [Google Scholar] [CrossRef]
- Kadlec, R.H.; Wallace, S. Treatment Wetlands, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2009; ISBN 9781566705264. [Google Scholar]
- Rehman, F.; Pervez, A.; Khattak, B.N.; Ahmad, R. Constructed Wetlands: Perspectives of the Oxygen Released in the Rhizosphere of Macrophytes. Clean Soil Air Water 2017, 45, 1–9. [Google Scholar] [CrossRef]
- Faulwetter, J.L.; Gagnon, V.; Sundberg, C.; Chazarenc, F.; Burr, M.D.; Brisson, J.; Camper, A.K.; Stein, O.R. Microbial processes influencing performance of treatment wetlands: A review. Ecol. Eng. 2009, 35, 987–1004. [Google Scholar] [CrossRef]
- Stottmeister, U.; Wießner, A.; Kuschk, P.; Kappelmeyer, U.; Kästner, M.; Bederski, O.; Müller, R.A.; Moormann, H. Effects of plants and microorganisms in constructed wetlands for wastewater treatment. Biotechnol. Adv. 2003, 22, 93–117. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, O. Constructed Wetlands Revisited: Microbial Diversity in the—Omics Era. Microb. Ecol. 2017, 73, 722–733. [Google Scholar] [CrossRef] [PubMed]
- Calheiros, C.S.C.; Duque, A.F.; Moura, A.; Henriques, I.S.; Correia, A.; Rangel, A.O.S.S.; Castro, P.M.L. Substrate effect on bacterial communities from constructed wetlands planted with Typha latifolia treating industrial wastewater. Ecol. Eng. 2009, 35, 744–753. [Google Scholar] [CrossRef]
- Calheiros, C.S.C.; Teixeira, A.; Pires, C.; Franco, A.R.; Duque, A.F.; Crispim, L.F.C.; Moura, S.C.; Castro, P.M.L. Bacterial community dynamics in horizontal flow constructed wetlands with different plants for high salinity industrial wastewater polishing. Water Res. 2010, 44, 5032–5038. [Google Scholar] [CrossRef] [PubMed]
- Calheiros, C.S.C.; Pereira, S.I.A.; Franco, A.R.; Castro, P.M.L. Diverse arbuscular mycorrhizal fungi (AMF) communities colonize plants inhabiting a constructedwetland for wastewater treatment. Water 2019, 11, 1535. [Google Scholar] [CrossRef]
- Arroyo, P.; Ansola, G.; de Miera, L.E.S. Effects of substrate, vegetation and flow on arsenic and zinc removal efficiency and microbial diversity in constructed wetlands. Ecol. Eng. 2013, 51, 95–103. [Google Scholar] [CrossRef]
- Guan, W.; Yin, M.; He, T.; Xie, S. Influence of substrate type on microbial community structure in vertical-flow constructed wetland treating polluted river water. Environ. Sci. Pollut. Res. 2015, 22, 16202–16209. [Google Scholar] [CrossRef]
- Long, Y.; Zhang, Z.; Pan, X.; Li, B.; Xie, S.; Guo, Q. Substrate influences on archaeal and bacterial assemblages in constructed wetland microcosms. Ecol. Eng. 2016, 94, 437–442. [Google Scholar] [CrossRef]
- Sánchez-Castro, I.; Ferrol, N.; Cornejo, P.; Barea, J.M. Temporal dynamics of arbuscular mycorrhizal fungi colonizing roots of representative shrub species in a semi-arid Mediterranean ecosystem. Mycorrhiza 2011, 22, 449–460. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, B.; Dai, X.; Li, S.; Lu, G.; Zhou, Y. Structure and function of the bacterial communities during rhizoremediation of hexachlorobenzene in constructed wetlands. Environ. Sci. Pollut. Res. 2017, 24, 11483–11492. [Google Scholar] [CrossRef]
- Stefanakis, A.I.; Akratos, C.S. Removal of Pathogenic Bacteria in Constructed Wetlands: Mechanisms and Efficiency. In Phytoremediation; Ansari, A., Gill, S., Gill, R., Lanza, G., Newman, L., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 327–346. [Google Scholar]
- Calheiros, C.S.C.; Ferreira, V.; Magalhães, R.; Teixeira, P.; Lima Castro, P.M. Presence of microbial pathogens and genetic diversity of Listeria monocytogenes in a constructed wetland system. Ecol. Eng. 2017, 102, 344–351. [Google Scholar] [CrossRef]
- Grover, M.; Ali, S.Z.; Sandhya, V.; Rasul, A.; Venkateswarlu, B. Role of microorganisms in adaptation of agriculture crops to abiotic stresses. World J. Microbiol. Biotechnol. 2011, 27, 1231–1240. [Google Scholar] [CrossRef]
- Wu, S.; Carvalho, P.N.; Müller, J.A.; Manoj, V.R.; Dong, R. Sanitation in constructed wetlands: A review on the removal of human pathogens and fecal indicators. Sci. Total Environ. 2016, 541, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Calheiros, C.S.C.; Pereira, S.I.A.; Brix, H.; Rangel, A.O.S.S.; Castro, P.M.L. Assessment of culturable bacterial endophytic communities colonizing Canna flaccida inhabiting a wastewater treatment constructed wetland. Ecol. Eng. 2017, 98, 418–426. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater; Clesceri, L., Greenberg, A., Eaton, A., Eds.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Pereira, S.I.A.; Castro, P.M.L. Phosphate-solubilizing rhizobacteria enhance Zea mays growth in agricultural P-deficient soils. Ecol. Eng. 2014, 73, 526–535. [Google Scholar] [CrossRef]
- Muyzer, G.; de Waal, E.C.; Uitterlinden, A.G. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 1993, 59, 695–700. [Google Scholar] [CrossRef]
- Eddy, M. Wastewater Engineering: Treatment and Reuse; Tchobanoglous, G., Burton, F.L., Eds.; McGraw-Hill Publishing: New York, NY, USA, 2003; ISBN 0070418780. [Google Scholar]
- Shingare, R.P.; Thawale, P.R.; Raghunathan, K.; Mishra, A.; Kumar, S. Constructed wetland for wastewater reuse: Role and efficiency in removing enteric pathogens. J. Environ. Manag. 2019, 246, 444–461. [Google Scholar] [CrossRef] [PubMed]
- Vymazal, J. Removal of enteric bacteria in constructed treatment wetlands with emergent macrophytes: A review. J. Environ. Sci. Health 2005, 40, 1355–1367. [Google Scholar] [CrossRef]
- Vymazal, J.; Kröpfelová, L. Wastewater treatment in constructed wetlands with horizontal sub-surface flow. In Environmental Pollution; Alloway, B.J., Trevors, J.T., Eds.; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Hazra, M.; Avishek, K.; Pathak, G. Developing an Artificial Wetland System for Wastewater Treatment: A Designing Perspective. Int. J. Environ. Prot. 2011, 1, 8–18. [Google Scholar]
- Elfanssi, S.; Ouazzani, N.; Latrach, L.; Hejjaj, A.; Mandi, L. Phytoremediation of domestic wastewater using a hybrid constructed wetland in mountainous rural area. Int. J. Phytoremediat. 2018, 20, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Zurita, F.; Carreón-Álvarez, A. Performance of three pilot-scale hybrid constructed wetlands for total coliforms and Escherichia coli removal from primary effluent—A 2-year study in a subtropical climate. J. Water Health 2015, 13, 446–458. [Google Scholar] [CrossRef] [PubMed]
- Truu, J.; Nurk, K.; Juhanson, J.; Mander, Ü. Variation of miorobiologioal parameters within planted soil filter for domestic wastewater treatment. J. Environ. Sci. Health 2005, 40, 1191–1200. [Google Scholar] [CrossRef] [PubMed]
- Calheiros, C.S.C.; Duque, A.F.; Moura, A.; Henriques, I.S.; Correia, A.; Rangel, A.O.S.S.; Castro, P.M.L. Changes in the bacterial community structure in two-stage constructed wetlands with different plants for industrial wastewater treatment. Bioresour. Technol. 2009, 100, 3228–3235. [Google Scholar] [CrossRef] [PubMed]

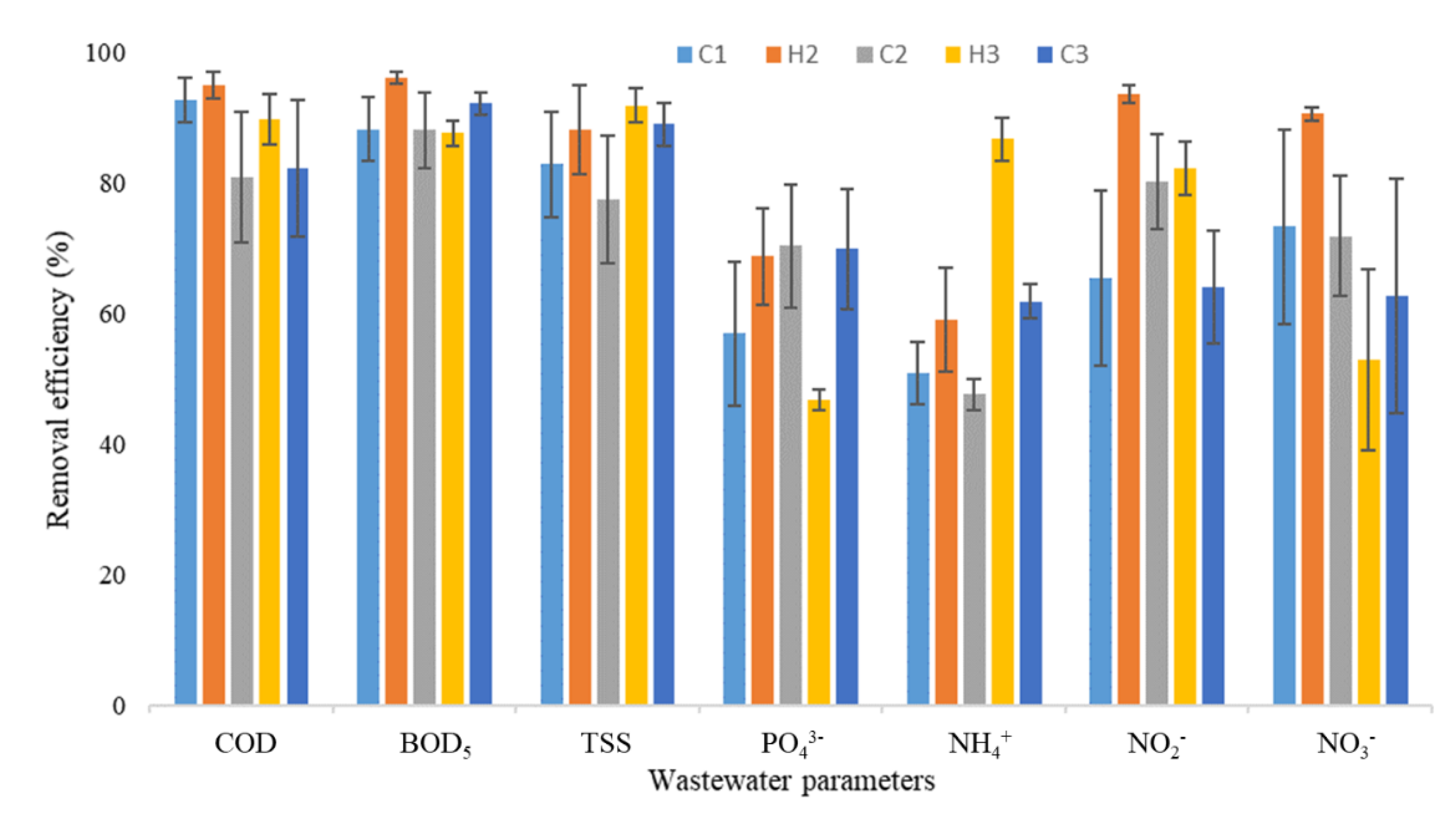
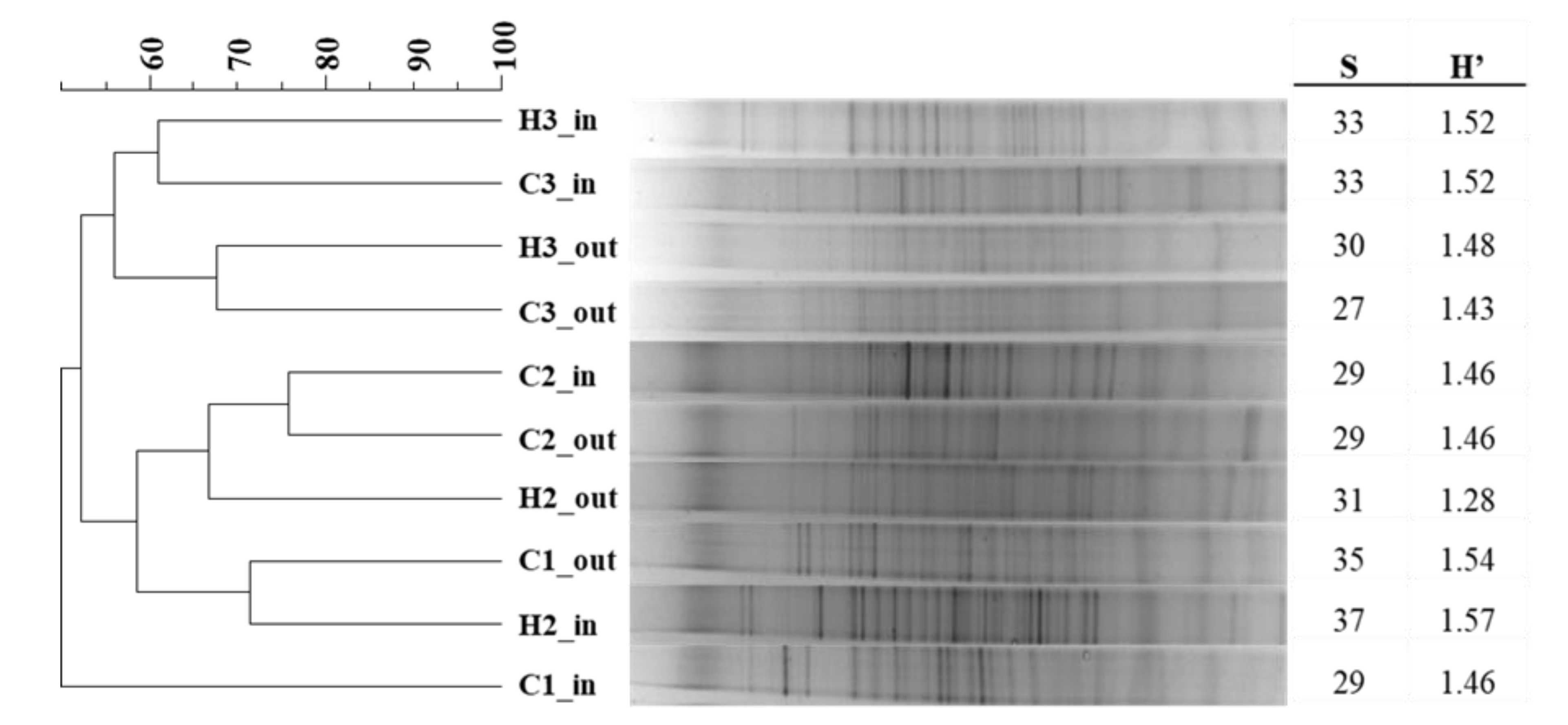
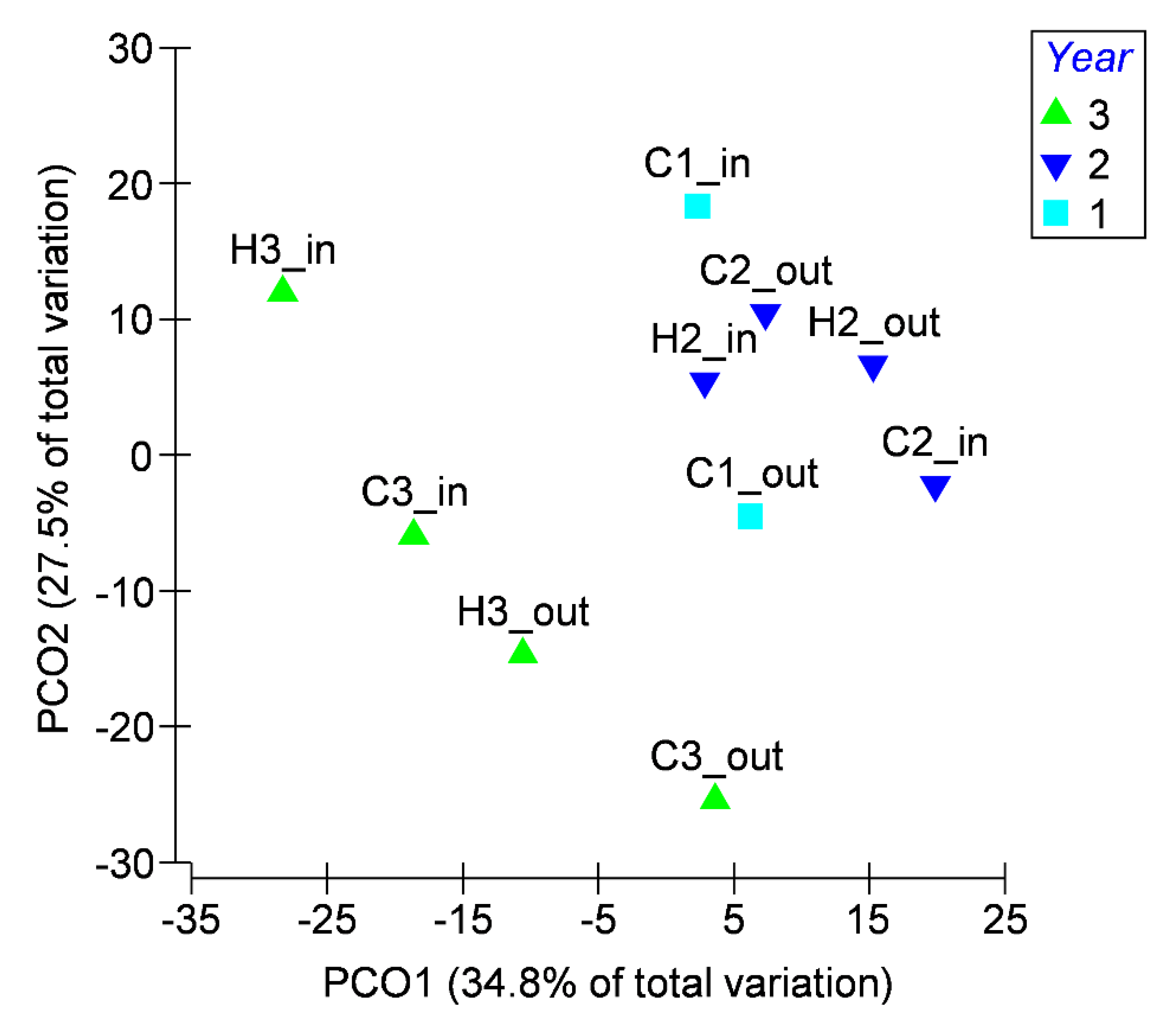
| Season/Year | pH | EC | COD | BOD | TSS | |||||
| (µS/cm) | (mg/L) | (mg/L) | (mg/L) | |||||||
| Inlet | Outlet | Inlet | Outlet | Inlet | Outlet | Inlet | Outlet | Inlet | Outlet | |
| C1 | 6.70 ± 0.23 | 7.69 ± 0.09 | 365 ± 176 | 251 ± 86 | 563 ± 337 | 11 ± 4 | 165 ± 109 | 7 ± 3 | 101 ± 44 | 8 ± 3 |
| H2 | 6.75 ± 0.17 | 7.38 ± 0.18 | 1047 ± 414 | 706 ± 104 | 691 ± 203 | 26 ± 5 | 281 ± 47 | 10 ± 1 | 287 ± 115 | 14 ± 3 |
| C2 | 7.41 ± 0.12 | 7.33 ± 0.03 | 1002 ± 151 | 648 ± 135 | 443 ± 175 | 136 ± 110 | 184 ± 94 | 38 ± 32 | 66 ± 20 | 12 ± 4 |
| H3 | 7.33 ± 0.07 | 7.07 ± 0.33 | 816 ± 100 | 559 ± 53 | 385 ± 25 | 37 ± 11 | 186 ± 34 | 24 ± 7 | 113 ± 25 | 7 ± 1 |
| C3 | 7.34 ± 0.12 | 6.81 ± 0.04 | 818 ± 138 | 643 ± 185 | 508 ± 137 | 38 ± 6 | 247 ± 80 | 23 ± 8 | 72 ± 28 | 6 ± 1 |
| Season/Year | PO43− | NH4+ | NO2− | NO3− | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (mg/L) | (mg/L) | (mg/L) | (mg/L) | |||||||
| Inlet | Outlet | Inlet | Outlet | Inlet | Outlet | Inlet | Outlet | |||
| C1 | 26.15 ± 24.02 | 3.47 ± 2.31 | 19.16 ± 11.74 | 7.91 ± 3.92 | 2.10 ± 1.77 | 0.13 ± 0.07 | 9.85 ± 5.54 | 0.95 ± 0.43 | ||
| H2 | 26.64 ± 6.91 | 7.13 ± 1.41 | 60.22 ± 29.69 | 27.07 ± 14.10 | 2.18 ± 0.28 | 0.13 ± 0.03 | 21.26 ± 2.67 | 1.92 ± 0.06 | ||
| C2 | 18.57 ± 4.23 | 5.15 ± 2.11 | 27.26 ± 24.70 | 14.24 ± 12.84 | 1.83 ± 1.27 | 0.14 ± 0.02 | 15.08 ± 5.75 | 3.07 ± 1.19 | ||
| H3 | 15.75 ± 1.98 | 8.39 ± 1.12 | 44.65 ± 15.11 | 7.37 ± 4.37 | 0.63 ± 0.16 | 0.12 ± 0.06 | 3.42 ± 2.12 | 0.76 ± 0.12 | ||
| C3 | 25.85 ± 5.58 | 6.38 ± 0.79 | 51.77 ± 17.06 | 20.87 ± 7.40 | 0.31 ± 0.07 | 0.10 ± 0.02 | 9.16 ± 3.40 | 5.00 ± 2.93 | ||
| Season | Total Coliforms (log10 CFU/mL) | Escherichiacoli (log10 CFU/mL) | ||
|---|---|---|---|---|
| Inlet | Outlet | Inlet | Outlet | |
| C1 | 5.1 ± 0.5 | 3.4 ± 0.5 | 2.7 ± 0.6 | NC |
| H2 | 4.8 ± 0.3 | 3.5 ± 0.2 | 3.5 ± 0.3 | 2.5 ± 0.3 |
| C2 | 4.6 ± 0.1 | 2.8 ± 0.2 | 3.0 ± 0.3 | NC |
| H3 | 5.1 ± 0.6 | 2.8 ± 0.6 | 2.9 ± 0.3 | 2.3 ± 0.5 |
| C3 | 4.3 ± 0.3 | 2.7 ± 0.1 | 3.1 ± 0.3 | NC |
| Total Coliforms (log10 CFU/g) | Escherichia coli (log10 CFU/g) | HB (log10 CFU/g) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CW Inlet Zone | CW Outlet Zone | CW Inlet Zone | CW Outlet Zone | CW Inlet Zone | CW Outlet Zone | |||||||
| Substrate | Root | Substrate | Root | Substrate | Root | Substrate | Root | Substrate | Root | Substrate | Root | |
| C1 | 4.5 | 5.1 | 4.0 | 3.7 | 3.8 | 4.9 | NC | NC | 7.6 | 7.6 | 5.7 | 7.3 |
| H2 | 6.6 | 6.7 | 4.7 | 5.6 | 3.6 | 4.6 | 2.5 | 3.6 | 7.6 | 6.7 | 5.5 | 6.0 |
| C2 | 4.8 | 6.8 | NC | NC | 3.7 | 3.5 | NC | NC | 5.5 | 4.7 | 3.7 | 5.0 |
| H3 | 6.6 | 6.5 | 5.7 | 5.7 | 4.3 | 4.7 | 3.4 | 2.1 | 7.4 | 7.3 | 5.5 | 6.5 |
| C3 | 3.6 | 5.8 | 3.5 | 4.1 | 2.9 | 3.8 | NC | NC | 3.8 | 4.7 | 3.4 | 3.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calheiros, C.S.C.; Pereira, S.I.A.; Franco, A.R.; Castro, P.M.L. Spatial-Temporal Changes in Removal of Fecal Indicators and Diversity of Bacterial Communities in a Constructed Wetland with Ornamental Plants. Appl. Sci. 2021, 11, 3875. https://doi.org/10.3390/app11093875
Calheiros CSC, Pereira SIA, Franco AR, Castro PML. Spatial-Temporal Changes in Removal of Fecal Indicators and Diversity of Bacterial Communities in a Constructed Wetland with Ornamental Plants. Applied Sciences. 2021; 11(9):3875. https://doi.org/10.3390/app11093875
Chicago/Turabian StyleCalheiros, Cristina S. C., Sofia I. A. Pereira, Albina R. Franco, and Paula M. L. Castro. 2021. "Spatial-Temporal Changes in Removal of Fecal Indicators and Diversity of Bacterial Communities in a Constructed Wetland with Ornamental Plants" Applied Sciences 11, no. 9: 3875. https://doi.org/10.3390/app11093875
APA StyleCalheiros, C. S. C., Pereira, S. I. A., Franco, A. R., & Castro, P. M. L. (2021). Spatial-Temporal Changes in Removal of Fecal Indicators and Diversity of Bacterial Communities in a Constructed Wetland with Ornamental Plants. Applied Sciences, 11(9), 3875. https://doi.org/10.3390/app11093875