Abstract
(1) Background: The development of a biocompatible material for direct additive manufacturing of maxillofacial extraoral prosthesis is still a challenging task. The aim of the present study was to obtain a photocurable PDMS, with nano TiO2 inclusions, for directly 3D printing of extraoral, maxillofacial prosthesis. The biocompatibility of the newly obtained nanocomposite was also investigated; (2) Methods: 2.5% (m/m) titania nanoparticles (TiO2) oxide anatase and a photoinitiator, benzophenone (BF) 4.5% were added to commercially available PDMS for maxillofacial soft prostheses manufacturing. The three different samples (PDMS, PDMS-BF and PDMS-BF-TiO2) were assessed by dielectric curing analysis (DEA) based on their viscosities and curing times. In vitro micronucleus test (MNvit) was performed for genotoxicity assessment and three concentrations of each compounds (2 mg/L, 4 mg/L and 8 mg/L) were tested in duplicate and compared to a control; (3) Results: The nanocomposite PDMS-BP-TiO2 was fully reticulated within a few minutes under UV radiation, according to the dielectric analysis. PDMS-BF-TiO2 nanocomposite showed the lowest degree of cyto- and genotoxicity; (4) Conclusions: In the limits of the present study, the proposed ex situ preparation of a PDMS-BP-TiO2 offers an easy, simple, and promising technique that could be successfully used for 3D printing medical applications.
1. Introduction
Maxillofacial prostheses, used for the rehabilitation of patients with congenital or acquired disabilities, are classified as extraoral (replacing nose, eye, orbit, ear, or face parts), intraoral (replacing parts of the maxilla, middle face, and mandible) and complex (replacing extraoral and intraoral anatomical parts) [1]. The psychological impact of physical disfigurement, whether it is congenital, developed, or acquired, is extremely severe, impacting on quality of life; therefore, achieving the highest level of prosthetic realism and function with maxillofacial prosthesis, especially with extraoral prosthesis, is extremely important [2].
Maxillofacial prosthesis has different characteristics, depending on the type of defect needing to be restored (missing soft or hard tissue), requiring different types of materials. For extraoral prosthesis, the material used needs to mimic soft-tissue characteristics in terms of visual and tactile properties, being simultaneously physically and chemically stable, as well as having microbiological resistance and biocompatibility [3].
Polydimethylsiloxane (PDMS) is presently the first option as material for soft maxillofacial prosthesis production due to its versatility, soft-tissue-like properties, ease of manipulation, chemical inertness, durability, and good biocompatibility. Moreover, PDMS presents a few other desirable characteristics such as hydrophobicity, thermal stability, low reactivity, resistance to ozone, and UV radiation due to the particular polymeric backbone with Si-O-Si bonds [4]. Its structural features transform PDMS into a structure with a highly flexible backbone (Figure 1), formed by the large Si atoms and oxygen atoms with smaller size connected through a 1.65 Å bound. The PDMS backbone structure is also characterized by two different bond angles: for Si-O-Si—143° and O-Si-O—110° [5]. Consequently, the dynamic flexibility of the PDMS chains increase significantly, offering pliability and softness similar to human skin.
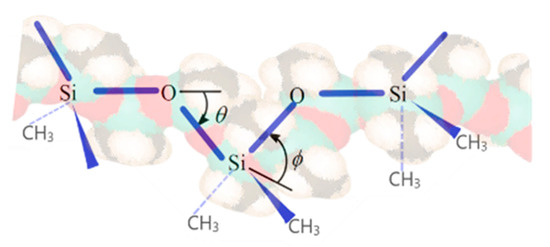
Figure 1.
Polydimethylsiloxane (PDMS) backbone.
As maxillofacial prosthesis are not worn while sleeping, removal and attachment must be straightforward [2], therefore mechanical properties, such as tensile strength, tear strength, and elongation at break are important, mainly around the thin edges of the prosthetic where adhesive need to be used for bonding with the skin [3]. Regarding this aspect, PDMS has some significant disadvantages as the low mechanical properties [6]. These could be overcome by inserting reinforcing additives, such as silica [7]. Silica particles with a modified surface could interact with Si-O from PDMS, generating an in situ reinforced polymeric matrix with significantly improved mechanical properties.
Prostheses are often used by patients for many hours each day, so tissue compatibility issues must be considered when selecting a material. Also, the humidity and temperature at the skin-prosthetic interface is a perfect environment for the proliferation of opportunistic bacteria and fungi, leading to a biofilm formation, not only on the epithesis surface but it could also penetrate in the material structure [2,8]. For good biocompatibility and for its antibacterial properties [9], titanium oxide nanoparticles were extensively studied, as the generated structures and compounds are stable and with photochemical properties [10,11]. However, to our knowledge, improving PDMS structure with nanotitania was not yet addressed.
For significant improvements in the capabilities of maxillofacial prostheses, mainly with respect to their esthetics, attachment, function, cost and resistance [2] not only the materials need to be enhanced but also the manufacturing technique. A review of the recent developments in maxillofacial prosthesis manufacturing [1] highlighted the progress registered in patients data acquisition and design software for a simplified and predictable digitalized protocol. Manufacturing prosthesis based on digital designs were carried out directly, by printing the prosthesis itself and indirectly by printing prosthesis prototypes or molds [1]. Most of the epithesis (extraoral maxillofacial prosthesis) were manufactured indirectly using regular silicone in a 3D printed mold, and for the ones obtained directly, the 3D-printed silicones with suitable prosthetic properties are currently under development [12,13].
Therefore, the aim of the present study was to obtain a photocurable silicone, with nano TiO2 inclusions, for directly 3D printing of extraoral, soft, maxillofacial prosthesis. The biocompatibility of the newly obtained nanocomposite was also investigated.
2. Materials and Methods
2.1. Materials and Equipment for Preparing PDMS-Nano TiO2 with Benzophenone as Photoinitiator
The polydimethylsiloxane (PDMS) with silanol functional termination used is presented as a two-component kit (base and curing agent). It is a silicone used for conventionally manufacturing of extraoral maxillofacial prosthesis (Multisil Epithetik, Bredent, Senden, Germany), normally hardening in 30 min at 60 °C or in 12 h at room temperature. The combining ratio base to curing agent was 1:1 (m/m). Benzophenone (white crystalline powder) was purchased from Sigma-Aldrich (Sigma-Aldrich, Merck KGaA, Darmstadt, Germany), and was dissolved in chloroform (Sigma-Aldrich, Merck KGaA, Darmstadt, Germany). The benzophenone solution was then added to the PDMS and mixed for 30 min. As the mixing procedure could generate air bubbles, the obtained mixture was then degassed for another 15 min using an ultrasonic bath (Elmasonic S10 H, Elma Schmidbauer GmbH, Singen, Germany). The benzophenone was added in different amounts, the optimal ratio required addition of 4.5% reported to PDMS. Titania nanoparticles (Titanium (IV) oxide anatase, <25 nm particle size, from Sigma-Aldrich, Merck KGaA, Darmstadt, Germany) were dispersed in PDMS. The nanoparticles have been added in a ratio of 2.5% (m/m). The ultrapure water used for washings was obtained with a Millipore System (MerckMillipore, Merck KGaA, Darmstadt, Germany). The UV–visible absorption spectrum of the nanotitania photocatalyst was recorded using a UV-Vis JASCO V-560 (JASCO GmbH, Germany) spectrophotometer in the range 200–900 nm with high resolution (1 nm) using quartz cuvettes of 10 mm.
The nanocomposite was prepared through the blending route, representing one of the most straightforward approaches, as follows (Figure 2). Nanoparticles were dispersed into the polymer solution, and then the mixture was casted into a mold or a glass substrate. Finally, the nanocomposite film was obtained after a subsequent post-curing stage. Upon applying such a preparation method, a polymeric matrix of polydimethylsiloxane (PDMS), incorporating semiconductor oxide nanoparticles, was obtained.
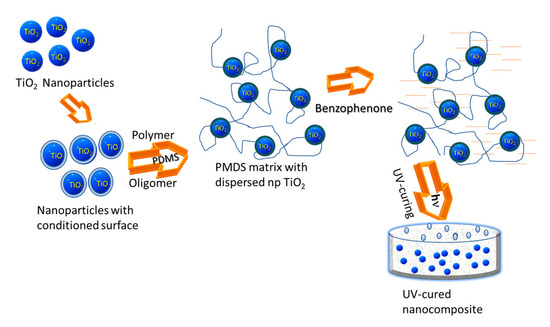
Figure 2.
Preparation of the PDMS-TiO2 nanocomposites applying a blending route. PDMS—polydimethylsiloxane.
2.2. Electrochemical Investigation of the Nanocomposite Polymer
For the electrochemical investigations, the prepared mixture PDMS-benzophenone-titanium dioxide nanoparticles (PDMS-BP-TiO2) was casted on the sensing surface of a coated sensor (Coated Tool Mountable Comb Electrode, TMCc, 0.5 mm electrode spacing) from Netzsch-Gerätebau GmbH (Selb, Germany). The formed film thickness was 1.5 mm. The electrical parameters were obtained with a dielectric analyzer—DEA 288 Ionic Analyzer (Netzsch-Gerätebau GmbH, Selb, Germany) applying 10 mHz and 100 Hz frequencies. Determinations of the ionic viscosity and loss factor during the generation of the reticulated polymeric deposition for PDMS, mixture PDMS and benzophenone (PDMS-BP), and for PDMS-BP-TiO2, were performed. The study included different working conditions: open atmosphere for PDMS, under UV irradiation for PDMS-BP, and PDMS-BP-TiO2 at a light intensity of 120 mW/cm2. Also, for comparison, the behavior of the PDMS-BP-TiO2 mixture at temperature was studied. Therefore, the PDMS-BP-TiO2 mixture was cast directly on an interdigitated electrode’s surface (mini-IDEX, Netzsch-Gerätebau GmbH, Selb, Germany), connected through an adapter box to a multifunctional lab furnace (Netzsch-Gerätebau GmbH, Selb, Germany).
2.3. Genotoxicity Evaluation
In vitro micronucleus test (MNvit) was performed following OECD (Organization for Economic Cooperation and Development) 487 guidelines (OECD, 487) [14]. The study was carried out using human peripheral lymphocytes from fresh blood samples. Peripheral blood was taken from a middle-aged, non-smoking healthy male who volunteered for this study, signed an informed consent and had not been recently exposed to genotoxic agents (chemical substances, ionizing radiations, bacterial/viral infections). Approximately, 10 mL of blood was collected using heparin-based anticoagulant. About 0.5 mL of heparinized blood was transferred in a tube containing 4.5 mL PB-MAX (GIBCO, Thermo Fisher Scientific, Waltham, MA, USA) Karyotyping medium supplemented with phytohemagglutinin, bovine serum albumin (BSA), amino acids, and antibiotics, all purchased from Merck (KGaA, Darmstadt, Germany). The lymphocytes were incubated at 37 °C in an atmosphere containing 5% CO2 for 48 h. Tested materials were fragmented into small pieces and inoculated in the human lymphocytes culture 48 h after the culture was initiated. They were previously sterilized being exposed to UV for 15 min.
In our previous studies [10,15,16] we found that samples with high concentrations of compounds or nanomaterials in lymphocyte culture cannot be used for genotoxicity assessment because they exceed the maximum allowed concentrations for a correct assessment without false positive results. There is also agglutination and precipitation of the culture. For this purpose, three concentrations of each compound, PDMS, PDMS-BP, and PDMS-BP-TiO2, were tested: 2 mg/L, 4 mg/L and 8 mg/L. A sample (free of compounds and TiO2 nanoparticles) was used as negative control. Each sample was tested in duplicate. The cultures were sacrificed 24 h after compounds were added, a period of time which is considered sufficient for the cells to undergo cell division, so that potential DNA damage in the form of micronuclei in interphase cells could be identified. The culture was developed without adding cytochalasin B (CytoB is a chemical agent used to block cytokinesis because it inhibits actin assembly). In the next steps potassium chloride solution 0.075M was used for hypotonization for 45 min at 37 °C, then cells were fixed in 3:1 methanol/acetic acid solution. Microscope slides were prepared, stained using Giemsa solution. Digital images were obtained using an Olympus BX40 fluorescence microscope (Olympus Life Science, Waltham, MA, USA) and processed with QuickPhoto Micro 2.3 software (PROMICRA, s.r.o., Prague, Czech Republic). Micronuclei were scored in 1000 cells per culture, two cultures per concentrations.
2.4. Statistical Analysis
All data were synthetized in Excel tables, compared and analyzed using Origin Lab Pro 2019 (OriginLab Corporation, Northampton, MA, USA) software. Mann–Whitney U nonparametric test was used to determine statistical significance of micronuclei incidence (‰) for the three concentrations (2 mg/L, 4 mg/L and 8 mg/L) of each compound, PDMS, PDMS-BP, and PDMS-BP-TiO2, when compared to the negative control (p < 0.05).
3. Results
A schematic diagram, Jablonski diagram [17], (Figure 3) of the photoinitiator’s possible action on the polymeric system highlights the excited chemical species’ deactivation ways when an intersystem crossing (ISC) process generating triplet excitation state occurs. During the photochemical reaction, such ISC process appears due to the forbidden transition of the excited chemical species directly on a triplet state.
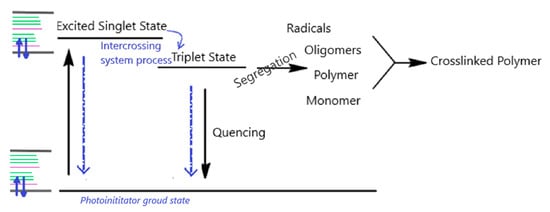
Figure 3.
Deactivation pathways.
BP is excited to singlet state when absorbing a photon, then converts to a triplet state (ISC) and subsequently relaxes from the triplet state through a possible extraction reaction of hydrogen from the hydroxyl group on PDMS (segregation stage on the diagram, in Figure 3). Thus, a radical site could be created on PDMS, generating, in the meantime, the BP ketyl radical [18] and depleting BP. When there are not available H-donors, the triplet state of excited BP could relax to its ground singlet state through a phosphorescence emission (quenching stage, Figure 3), thus regenerating the photo activating BP species [19]. If the BP concentration in the system is high enough, then the depletion effects are not significant, as only the BP molecules reacting with PDMS are consumed.
Considering the photochemical reaction’s intimate behavior, the UV-curing initiation rate could be fine-tuned through the radiation intensity modification. This is a welcome advantage of photocuring compared to thermal curing. The photoinitiator concentration influences the overall reaction rate, but its level depends on the application specificity, as it depends on the thickness of the generated shapes. For each photocurable system, it should be determined an optimal initiator concentration, because, above this value, the photocuring process does not progress to its completeness.
The proposed procedure used a PDMS variety with silanol termination. The groups Si-OH could react with the nanotitania hydroxyl groups present on its surface to generate Si-O-Ti bonds stabilizing the nanocomposite material and assuring a better dispersion of nano-TiO2 in the polymeric matrix.
The nanocomposites obtained were employed to study their processability under UV radiation, in the view of their usage with a stereolithography (SLA) 3D printer.
Figure 4 presents the ionic viscosity/log (ionic viscosity) for the studied PDMS mixtures under the mentioned conditions in Section 2.2.
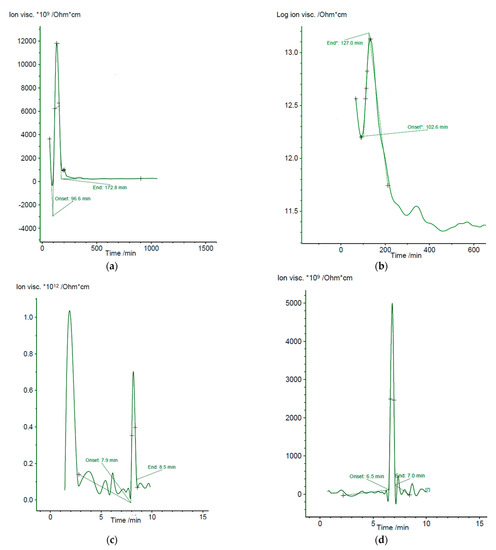
Figure 4.
Evolution of electrochemical parameters of various PDMS formulations during the film-forming process. (a) Ionic viscosity of polydimethylsiloxane cured in opened air (10 mHz); (b) Ionic viscosity of polydimethylsiloxane-benzophenone mixture cured in opened air (10 mHz); (c) Ionic viscosity of polydimethylsiloxane -benzophenone-titanium dioxide nanoparticles thermally cured (1 Hz); (d) Ionic viscosity of polydimethylsiloxane -benzophenone-titanium dioxide nanoparticles UV cured (50 mHz).
The polymeric film’s electrochemical parameters (ionic viscosity, impedance, conductivity, permeability, loss factor) evolve abruptly during composite polymeric film-forming (ambient evaporation), after which the values are flattened, which suggests that the film-forming has ended. Through the dielectric analysis, the evolution of the PDMS crosslinking process (curing) was followed up, considering that it starts with the phase transformation, gelation, when the viscous polymeric matrix becomes a gel, and which is marked on the recorded DEA curves as the “onset” value. Practically, the formation of fully reticulated PDMS is considered terminated at the moment when the “end” value is reached on the ion viscosity curve. Consequently, from the perspective of PDMS processing, it is essential to identify the debut (gelation—“onset” point) and termination (crosslinking—“end” point) to follow the curing progression. The “onset” (gelation point) and “end” (end of curing) values varied significantly between the studied PDMS formulations. Through the dielectric cure monitoring, the composite cure state or thermoset in real time was determined under experimental conditions. In general, the change of ionic viscosity is directly dependent on the viscosity change before and after gelation, offering an overall assessment throughout curing process. As observed from Figure 4, initially the ion viscosity decreases due to the higher fluidity at the thermoset when the material is consequently less resistive (at 96.62 min for PDMS, 102.60 min for PDMS-BP, 7.9 min for PDMS-BP-TiO2 thermally cured, and 6.5 min for PDMS-BP-TiO2 UV cured). Afterwards, the reaction rate increased and an increase in ion viscosity due to polymerization was recorded.
When the reticulation reaction accelerates, ion viscosity increased as the nanocomposite was denser. However, the viscosity increased tremendously at the gelation point; when the crosslinking and network generation started, the ion viscosity still recorded the experimental data on the cure state over the gel point. When the crosslinking was extensive, the respective reaction rate decreased, and consequently, the ion viscosity decreased till its slope become null and the cure was complete (at 172.8 min for PDMS, 127.0 min for PDMS-BP, 8.5 min for PDMS-BP-TiO2 thermally cured, and 7.0 min for PDMS-BP-TiO2 UV cured).
The nanocomposite developed for further usage in stereolithography application was prepared using benzophenone and TiO2 nanoparticles, and consequently, the mixed material was irradiated with 360 nm UV radiation. The reason behind using UV radiation with this wavelength is the sensitivity of both benzophenone and nano-TiO2 to radiations below 365 nm [20,21]. The UV spectra of the photoinitiator has absorption peaks at 258 nm [22,23], while the used photocatalyst filler (TiO2) presents absorption peak at 336 nm, and a tail at 370 nm, as shown in Figure 5.
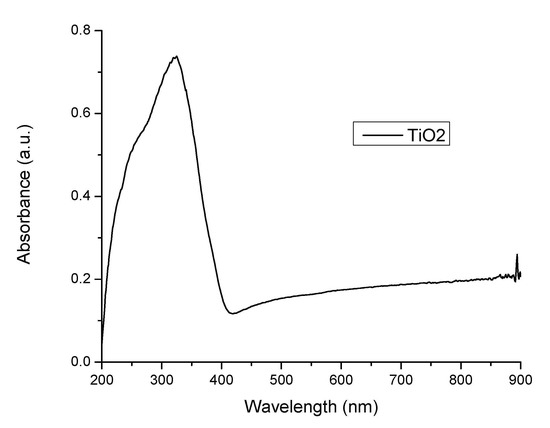
Figure 5.
UV spectrum of nanotitania.
The determinations for PDMS-BP-TiO2 composite were conducted in the opened atmosphere and normal lightening, using a conventional portable UV lamp. The optimum proportion of the components in the PDMS-BP-TiO2 material, namely 1:4.5:2.5, was established based on the homogeneity of the uncured mixture and its curing time. The experimental results presented in Figure 4 clearly shows that the UV cured PDMS-BP-TiO2 sample is reticulated in very short time compared to the samples without UV treatment.
Genotoxicity Evaluation
The evaluation of genotoxicity was performed by the in vitro micronucleus test, recommended by the OECD [14].
The purpose of this test is to detect micronuclei in the cytoplasm of interphase cells and to score their incidence. This test detects the clastogenic and aneugenic activity of some chemical agents. The micronucleus assay has several advantages over the metaphase analysis, which is performed in the OECD Guidelines 473 and 475, to measure chromosome aberrations. Because micronuclei in interphase cells can be assessed much more objectively than chromosomal aberrations in metaphase cells MN vit the preparations can be scored much more quickly [14]. This makes it practical to score thousands of cells per treatment. Finally, as micronuclei may contain whole (lagging) chromosomes, there is the potential to detect aneuploidy-inducing agents that are currently very difficult to study in conventional chromosomal aberration tests. MNvit is the most widely used and reliable test for assessing genotoxic potential of a chemical agent [24].
As the lymphocyte cultures were developed without CytoB it was necessary to measure the RICC (Relative Increase in Cell Count) index to demonstrate the fact the cells in the culture had undergone cell division and to avoid false negative responses.
According to RICC index, cytotoxicity was calculated for each concentration (Table 1).

Table 1.
Cytotoxicity (%) of PDMS, PDMS-B and PDMS-B-TiO2 in lymphocyte cell culture according to RICC index (±SD).
Comparative to negative control sample (Figure 6), the MN incidence analysis for the PDMS concentration of 2 mg/L and 4 mg/L indicated a relative high degree of genotoxicity with atypical and deformed nuclei, numerous acentric fragments, some of them linked by chromatin bridges (Figure 7a,b).
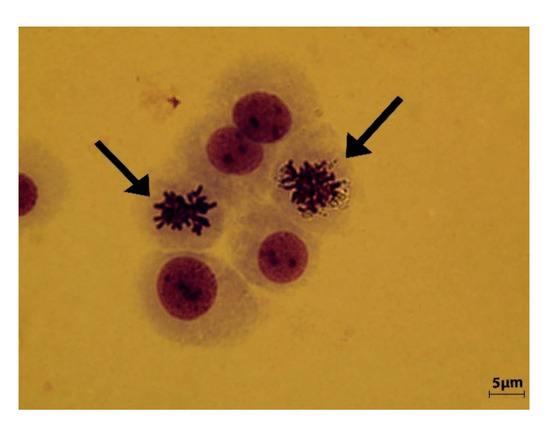
Figure 6.
Negative control sample. Interphase nuclei with normal morphology and dividing metaphase chromosomes (arrow) are observed.
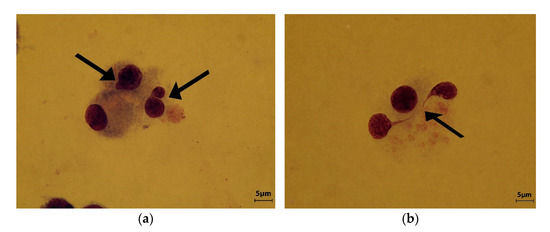
Figure 7.
(a) Polydimethylsiloxane (PDMS) concentration 2 mg/L Micronucleus and atypical dividing nucleus are indicated by arrows; (b) PDMS concentration 4 mg/L. A dividing nucleus with abnormally bridge, suggesting a nuclear stickiness (arrow).
For the concentration of 8 mg/L PDMS the micronuclei incidence could not be scored as the genetic material was damaged, the cytotoxicity exceeded the maximum allowed value (Table 2), turbidity, and precipitation visible by naked eye were found. If the cytotoxicity exceeds 60% it results in the appearance of micronuclei as an adverse effect of cytotoxicity, so the incidence of MN as a result of genotoxic capacity cannot be assessed. This concentration of 8 mg/L of PDMS was too high to allow for micronuclei scoring. Both genotoxic capacity and cytotoxicity of PDMS determined by higher concentration and by the fact that this compound could directly interact with DNA [25], which synergistically resulted in changing the quality and integrity of genetic material.

Table 2.
Micronuclei incidence (‰) in lymphocyte cells culture.
PDMS-BF compound at 2 mg/L and 4 mg/L showed a relative high degree of genotoxicity (Figure 8a,b) but at 8 mg/L the MN incidence analysis indicated a high degree of genotoxicity and the presence of gross lesions in the genetic material. Atypical and deformed nuclei were common in this sample (Figure 8c,d).
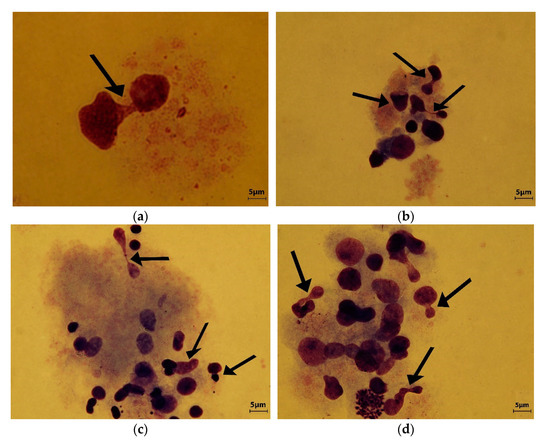
Figure 8.
(a) Polydimethylsiloxane—benzophenone (PDMS-BF) concentration 2 mg/L. Atypical dividing nucleus are indicated by arrow; (b) PDMS-BF 4 mg/L. Deformed and genetically unbalanced nuclei are indicated by arrows.; (c,d) Different analyzed area of PDMS-BF 8 mg/L. Many atypical and deformed nuclei lead to the micronuclei formation and aneugenicity (arrows).
A statistically significant increase in micronuclei incidence was observed from all the tested concentrations of PDMS and PDMS-BF, compared to the negative control (Table 2).
Surprising results were obtained for PDMS-BF-TiO2 nanocomposite. For all three tested concentrations, the incidence of micronuclei was very low, and correlated with the RICC index showed a low degree of cyto- and genotoxicity (Figure 9a–c, and Table 2). No statistically significant micronuclei incidence compared to the negative control was observed for all tested concentrations of PDMS-BF-TiO2 nanocomposite (Table 2).
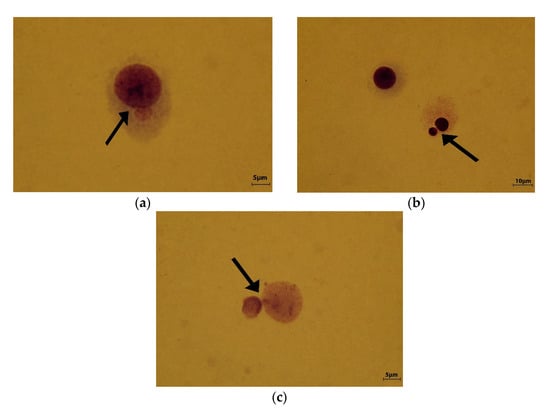
Figure 9.
Polydimethylsiloxane—benzophenone—titanium dioxide nanoparticles (PDMS-BF-TiO2) (a) concentration 2 mg/L; (b) 4 mg/L; (c) 8 mg/L (arrows indicate micronuclei).
4. Discussion
The development of a biocompatible material for direct additive manufacturing of maxillofacial extraoral prosthesis is still a challenging task. Some attempts were made: Jindal et al. developed direct printing materials and technique by customizing a fused deposition modelling printer by replacing the heated nozzle and print bed, and making it suitable for printing two-component customized room temperature vulcanized silicones [26,27]. Unkovskiy et al. presented a complete digital workflow for the manufacture of a nasal prosthesis from a pure solvent-free silicone (ACEO Silicone General Purpose; Wacker Chemie AG, Germany) with a drop-on-demand 3D printer (ACEO; Wacker Chemie AG, Burghausen, Germany) [12]. However, none of the tested materials had the approval for clinical application at the time of the studies.
The aim of the present research was to obtain a photocurable PDMS with improved characteristics to be used for soft maxillofacial prosthesis manufacturing using Vat photo-polymerization (stereolithography) as 3D printing technique.
To the author’s knowledge, this is the first attempt for improving a medical silicon by adding TiO2 nanoparticles.
Also, among the proposed 3D printable silicone for extraoral prostheses manufacturing, few studies focused on the new material’s biocompatibility.
Lately, many applications of photosensitive materials polymerizing under UV radiation have emerged in the areas where 3D printing procedure became important. Commonly, the photosensitive polymers suppose a mixture of monomers or oligomers and a suitable photoinitiator that triggers a radicalic or cationic reticulation upon UV-exposure (Figure 3). The short cure time at room temperature and minimum energy requirements could be mentioned among the UV-curing advantages. The UV-curing process depends on the photoinitiator’s behavior and characteristics. Thus, the amount of photosensitive compound conditions the crosslinking rate, while its efficiency and absorption wavelength influence the initiation rate. The free radicals, eventually cations, are generated in a system where exists a photoinitiator compound, following the conversion of the absorbed photons’ energy to chemical energy [28].
The successful application of this procedure supposes a sustained rationale behind the right choice of the necessary cosolvents, design of the nanocomposite matrix that could result in a homogeneous polymeric phase, and increased compatibility.
For assessing genotoxicity, the sensitivity and specificity of in vitro tests should be a priority to increase predictability and to avoid other unnecessary in vivo tests. In vitro micronucleus testing is a complex method because it can be performed on target cells, even in others than those recommended under standard conditions, including human cells [29].
Three type of compounds were comparatively assessed: PDMS, PDMF-BF, and PDMS-BF-TiO2 and compared with a negative control sample. All three concentrations of PDMS-BF-TiO2 favor the assessment of the genotoxic capacity. When referring to PDMS-BF-TiO2 and negative control, low DNA damage was found, with no statistically significant micronuclei incidence in PDMS-BF-TiO2 compared to the negative control (Table 2), probably due to TiO2 nanoparticles stabilization.
It is well known that metallic nanoparticles have cytotoxic/genotoxic potential, but in this particular case, the combination of the silicon composite and the TiO2 nanoparticles resulted in a more stable compound, less reactive to the genetic material.
Toxicological information on the engineered compounds especially those commonly used in medical practice, is very important in terms of risk assessment and management, as these are either already in use or may be applied in the future in numerous other fields of interest. In this context, the development of new nanomaterials could reduce the risk of interaction with genetic material, thus preventing toxic and allergic effects. For maxillofacial soft prosthesis, this particular characteristic is extremely important, as some of these devices are in intimate contact with tissues, most of the time fragile and inflamed.
PDMS is silicon-based organic polymer extensively used as material for maxillofacial epithesis manufacturing, but very little is known about cyto- and genotoxic effect. Chakrabarti et al. noticed that the use of this silicone, even in relatively low concentrations, affects cell viability and produces genotoxic effects [30]. PDMS attacks proteins and salts [31] disturbing even the chromatin conformation and structure resulting in cell division disorder and aneugenic effects. Our results reinforce this concern regarding genotoxic capacity, especially at doses over 4 mg/L.
The modification of PDMS surfaces using benzophenone could reduce the risk of genotoxicity but very little compare to PDMS and only at higher doses, over 4 mg/L. Both polymers, PDMS and PDMS-BF, registered a statistically significant increase in micronuclei incidence compared to the negative control (Table 2).
The study or the genotoxicity of the PDMS-BF compound was performed for the first time in the present study. To the author’s knowledge, there are no data in the published literature on this subject.
The surprising preliminary results obtained by modifying the PDMS-BF surfaces with TiO2 nanoparticles showed a stabilization of the compound, becoming a neutral material without major interactions affecting the genetic material of cellular homeostasis even at concentrations exceeding 4 mg/L.
Our previous results showed that TiO2 nanoparticles incorporated in poly(methylmethacrylate) could stabilize the interactions between DNA and PMMA, rendering it less reactive to the genetic material [15].
Also, our previous studies on including nanotitania in PMMA for 3D printed dentures revealed the antibacterial characteristics of the obtained polymer [9], as well as other improved properties including color stability, assessed under clinical conditions [32,33,34,35].
To overcome the limitations of 3D printing direct manufacturing of silicone polymers for soft maxillofacial prosthesis manufacturing, a nanocomposite PDMS with photoinitiator (BF) and TiO2 nanoparticles was proposed, and its genotoxicity was assessed. Further studies are required to evaluate mechanical resistance and long-term maintenance in clinical environment of the 3D printed prosthesis.
Although the present investigation introduced the procedure for obtaining a rapid prototyping photopolymerizable PDMS-nano TiO2 with promising outputs, some limitations still exist. The actual study limits the type of stereolithography-based 3D printers that could be used, as some of the common devices available on the market are working with UV radiation with wavelengths over 360 nm. Therefore, further studies with the use of photoinitiators with maximum absorbance between 365–390 nm will be initiated. This will allow the manufacturing of extraoral maxillofacial prosthesis using commercially available printers, in most of the dental laboratories, at affordable cost.
Also, following the 3D printing procedure, to obtain the final prosthesis, a post-processing procedure is required: the product needs to be cleaned with isopropanol, dried, the printing supports are gently removed and then a final curing procedure is applied. Final cleaning and curing are also meant to remove the excess of photoinitiator and unreticulated monomer. Moreover, for the extraoral prosthesis, manual cosmetic adjustments are mandatory.
The biocompatibility assessment in the present study was done prior to the postprocessing procedure. Consequently, further investigations on the proposed material will be performed under specific working conditions.
5. Conclusions
In the limits of the present study, the proposed ex situ preparation of a PDMS-BP-TiO2 offers an easy, simple, and promising technique that could be successfully used for 3D printing medical applications.
PDMS-BF-TiO2 nanocomposite showed a low degree of cyto- and genotoxicity, making it a good candidate for digitally manufacturing soft-tissue maxillofacial prosthesis.
Author Contributions
Conceptualization, C.M.C., E.E.T. and L.B.; methodology, E.E.T. and C.M.C.; software, C.M.C.; validation, M.B., L.B., and V.S.P.; formal analysis, D.C.P., V.S.P., M.B.; investigation, E.E.T., D.C.P., and L.B.; resources, C.M.C. and V.S.P.; data curation, M.B.; writing—original draft preparation, E.E.T., L.B., V.S.P. and C.M.C.; writing—review and editing, C.M.C.; visualization, L.B.; supervision, E.E.T.; project administration, C.M.C.; funding acquisition, M.B. and V.S.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of “Carol Davila” University of Medicine and Pharmacy (98/2016).
Informed Consent Statement
Informed consent was obtained from the subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cristache, C.M.; Tudor, I.; Moraru, L.; Cristache, G.; Lanza, A.; Burlibasa, M. Digital Workflow in Maxillofacial Prosthodontics—An Update on Defect Data Acquisition, Editing and Design Using Open-Source and Commercial Available Software. Appl. Sci. 2021, 11, 973. [Google Scholar] [CrossRef]
- Cruz, R.L.J.; Ross, M.T.; Powell, S.K.; Woodruff, M.A. Advancements in Soft-Tissue Prosthetics Part A: The Art of Imitating Life. Front. Bioeng. Biotechnol. 2020, 8. [Google Scholar] [CrossRef]
- Powell, S.K.; Cruz, R.L.J.; Ross, M.T.; Woodruff, M.A. Past, Present, and Future of Soft-Tissue Prosthetics: Advanced Polymers and Advanced Manufacturing. Adv. Mater. 2020, 32. [Google Scholar] [CrossRef]
- Lin, Y.; He, D.; Hu, H.; Yi, P.; Liu, X.; Huang, J.; Wu, S.; Li, G. Preparation and Properties of Polydimethylsiloxane (PDMS)/Polyethylene Glycol (PEG)-Based Amphiphilic Polyurethane Elastomers. ACS Appl. Bio Mater. 2019, 2, 4377–4384. [Google Scholar] [CrossRef]
- Sidorov, T.A. Asymmetry of Si-O-Si bridge bond and atom groups in silicates. Russ. J. Inorg. Chem. 2007, 52, 532–542. [Google Scholar] [CrossRef]
- Qiu, H.; Hölken, I.; Gapeeva, A.; Filiz, V.; Adelung, R.; Baum, M. Development and Characterization of Mechanically Durable Silicone-Polythiourethane Composites Modified with Tetrapodal Shaped ZnO Particles for the Potential Application as Fouling-Release Coating in the Marine Sector. Materials 2018, 11, 2413. [Google Scholar] [CrossRef]
- Kamitakahara, M.; Kawashita, M.; Miyata, N.; Kokubo, T.; Nakamura, T. Bioactivity and mechanical properties of polydimethylsiloxane (PDMS)-CaO-SiO2 hybrids with different PDMS contents. J. SolGel Sci. Technol. 2001, 21, 75–81. [Google Scholar] [CrossRef]
- Ariani, N.; Vissink, A.; Van Oort, R.P.; Kusdhany, L.; Djais, A.; Rahardjo, T.B.W.; Van Der Mei, H.C.; Krom, B.P. Microbial biofilms on facial prostheses. Biofouling 2012, 28, 583–591. [Google Scholar] [CrossRef]
- Totu, E.E.; Nechifor, A.C.; Nechifor, G.; Aboul-Enein, H.Y.; Cristache, C.M. Poly (methyl methacrylate) with TiO 2 nanoparticles inclusion for stereolitographic complete denture manufacturing—The fututre in dental care for elderly edentulous patients? J. Dent. 2017, 59, 68–77. [Google Scholar] [CrossRef]
- Cristache, C.M.; Totu, E.E.; Burlibasa, M.; Tanase, G.; Iorgulescu, G.; Burlibasa, L. Preliminary Study on Genotoxicity Assessment of an Innovative Topical Treatment for Periodontal Disease. Rev. Chim. 2020, 71, 145–150. [Google Scholar] [CrossRef]
- Nechifor, G.; Totu, E.E.; Nechifor, A.C.; Constantin, L.; Constantin, A.M.; Cărăuşu, M.E.; Isildak, I. Added value recyclability of glass fiber waste as photo-oxidation catalyst for toxic cytostatic micropollutants. Sci. Rep. 2020, 10, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Unkovskiy, A.; Spintzyk, S.; Brom, J.; Huettig, F.; Keutel, C. Direct 3D printing of silicone facial prostheses: A preliminary experience in digital workflow. J. Prosthet. Dent. 2018, 120, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Eggbeer, D.; Bibb, R.J.; Evans, P.L.; Ji, L. Evaluation of direct and indirect additive manufacture of maxillofacial prostheses. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2012, 226, 718–728. [Google Scholar] [CrossRef] [PubMed]
- Test No. 487: In Vitro Mammalian Cell Micronucleus Test. In OECD Guidelines for the Testing of Chemicals; Organization for Economic Cooperation and Development-OECD: Paris, France, 2010.
- Totu, E.E.; Cristache, C.M.; Isildak, I.; Yildirim, R.; Burlibasa, M.; Nigde, M.; Burlibasa, L. Preliminary Studies on Citotoxicity and Genotoxicity Assessment of the PMMA-TiO2 Nanocompozites for Stereolithographic Complete Dentures Manufacturing. Rev. Chim. 2018, 69, 1160–1165. [Google Scholar] [CrossRef]
- Burlibaşa, L.; Chifiriuc, M.C.; Lungu, M.V.; Lungulescu, E.M.; Mitrea, S.; Sbarcea, G.; Popa, M.; Măruţescu, L.; Constantin, N.; Bleotu, C.; et al. Synthesis, physico-chemical characterization, antimicrobial activity and toxicological features of AgZnO nanoparticles. Arab. J. Chem. 2020, 13, 4180–4197. [Google Scholar] [CrossRef]
- Xu, H.; Chen, R.; Sun, Q.; Lai, W.; Su, Q.; Huang, W.; Liu, X. Recent progress in metal–Organic complexes for optoelectronic applications. Chem. Soc. Rev. 2014, 43, 3259–3302. [Google Scholar] [CrossRef]
- Goda, T.; Konno, T.; Takai, M.; Moro, T.; Ishihara, K. Biomimetic phosphorylcholine polymer grafting from polydimethylsiloxane surface using photo-induced polymerization. Biomaterials 2006, 27, 5151–5160. [Google Scholar] [CrossRef]
- Silva, R.S.; Nicodem, D.E. Deuterium isotope effects on the photoreduction of 9,10-phenanthrenequinone and benzophenone by 2-propanol. J. Photochem. Photobiol. A Chem. 2008, 194, 76–80. [Google Scholar] [CrossRef]
- Basu, M.; Sarkar, S.; Pande, S.; Jana, S.; Sinha, A.K.; Sarkar, S.; Pradhan, M.; Pal, A.; Pal, T. Hydroxylation of benzophenone with ammonium phosphomolybdate in the solid state via UV photoactivation. Chem. Commun. R. Soc. Chem. 2009, 46, 7191–7193. [Google Scholar] [CrossRef]
- Dalai, S.; Pakrashi, S.; Kumar, R.S.S.; Chandrasekaran, N.; Mukherjee, A. A comparative cytotoxicity study of TiO2 nanoparticles under light and dark conditions at low exposure concentrations. Toxicol. Res. 2012, 1, 116–130. [Google Scholar] [CrossRef]
- Oh, K.W.; Choi, H.-M.; Kim, J.M.; Park, J.H.; Park, I.S. A novel antimicrobial photosensitizer: Hydroxyethyl Michler’s ketone. Text. Res. J. 2013, 84, 808–818. [Google Scholar] [CrossRef]
- Aldrich Polymer Products Applicaton & Reference Information. Available online: https://www.sigmaaldrich.com/content/dam/sigma-aldrich/docs/Aldrich/General_Information/photoinitiators.pdf (accessed on 10 April 2021).
- Corvi, R.; Madia, F. In vitro genotoxicity testing–Can the performance be enhanced? Food Chem. Toxicol. 2017, 106, 600–608. [Google Scholar] [CrossRef]
- Liu, D.; Perdue, R.K.; Sun, L.; Crooks, R.M. Immobilization of DNA onto Poly (dimethylsiloxane) Surfaces and Application to a Microelectrochemical Enzyme-Amplified DNA Hybridization Assay. Langmuir 2004, 20, 5905–5910. [Google Scholar] [CrossRef]
- Jindal, S.K.; Sherriff, M.; Waters, M.G.; Coward, T.J. Development of a 3D printable maxillofacial silicone: Part I Optimization of polydimethylsiloxane chains and cross-linker concentration. J. Prosthet. Dent. 2016, 116, 617–622. [Google Scholar] [CrossRef][Green Version]
- Jindal, S.K.; Sherriff, M.; Waters, M.G.; Smay, J.E.; Coward, T.J. Development of a 3D printable maxillofacial silicone: Part II Optimization of moderator and thixotropic agent. J. Prosthet. Dent. 2018, 119, 299–304. [Google Scholar] [CrossRef]
- Chatani, S.; Kloxin, C.J.; Bowman, C.N. The power of light in polymer science: Photochemical processes to manipulate polymer formation, structure, and properties. Polym. Chem. 2014, 5, 2187–2201. [Google Scholar] [CrossRef]
- Benameur, L.; Lei, W.; Botta, A. Genotoxicity of nanoparticles. In Encyclopedia of Nanotechnology; Springer: Dordrecht, The Netherlands, 2015. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Ghosh, I.; Mukherjee, A. Poly(dimethylsiloxane) Induces Cytotoxicity and Genotoxicity in Human Lymphocytes. Proc. Zool. Soc. 2020, 73, 82–85. [Google Scholar] [CrossRef]
- De Smet, N.; Rymarczyk-Machal, M.; Schacht, E. Modification of polydimethylsiloxane surfaces using benzophenone. J. Biomater. Sci. Polym. Ed. 2009, 20, 2039–2053. [Google Scholar] [CrossRef]
- Cristache, C.M.; Totu, E.E.; Iorgulescu, G.; Pantazi, A.; Dorobantu, D.; Nechifor, A.C.; Isildak, I.; Burlibasa, M.; Nechifor, G.; Enachescu, M. Eighteen Months Follow-Up with Patient-Centered Outcomes Assessment of Complete Dentures Manufactured Using a Hybrid Nanocomposite and Additive CAD/CAM Protocol. J. Clin. Med. 2020, 9, 324. [Google Scholar] [CrossRef]
- Totu, E.E.; Cristache, C.M.; Isildak, S.; Tavukcuoglu, O.; Pantazi, A.; Enachescu, M.; Buga, R.; Burlibasa, M.; Totu, T. Structural Investigations on Poly(methyl methacrylate) Various Composites Used for Stereolithographyc Complete Dentures. Mater. Plast. 2018, 55, 616–619. [Google Scholar] [CrossRef]
- Cristache, C.M.; Oancea, L.; Didilescu, A.C.; Burlibasa, M.; Totu, E.E. Color Changes and Stainability of Complete Dentures Manufactured Using PMMA-TiO 2 Nanocomposite and 3D Printing Technology-one Year Evaluation. Rev. Chim. 2018, 69, 463–468. [Google Scholar] [CrossRef]
- Cristache, C.M.; Totu, E.E.; Grosu, A.R.; Ene, O.; Beuran, I.A.; Burlibasa, M. Nanocomposite for rapid prototyped complete denture eighteen months follow-up on clinical performance. Rev. Chim. 2019, 70, 387–392. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).