Anticancer Efficacy of Nonthermal Plasma Therapy Combined with PD-L1 Antibody Conjugated Gold Nanoparticles on Oral Squamous Cell Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Culture of Cells
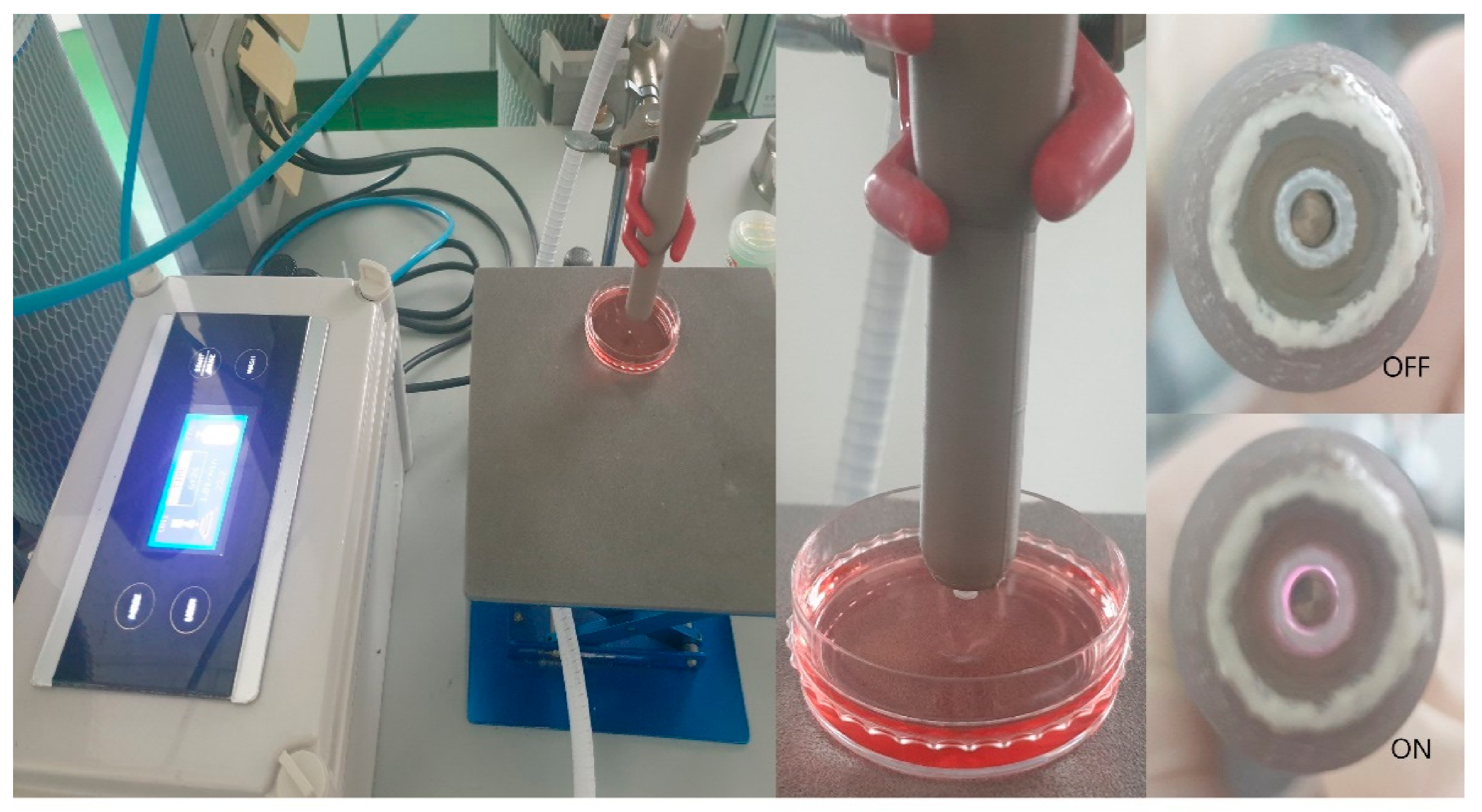
2.3. Nonthermal Atmospheric Pressure Plasma Supplier
2.4. Preparation of PD-L1 Antibody-Conjugated Gold Nanoparticles
2.5. Cell Viability Assay
2.6. LIVE/DEAD™ Viability/Cytotoxicity Assay
2.7. Immunocytochemistry
2.8. Western Blot Analysis
2.9. Statistical Analysis
3. Results
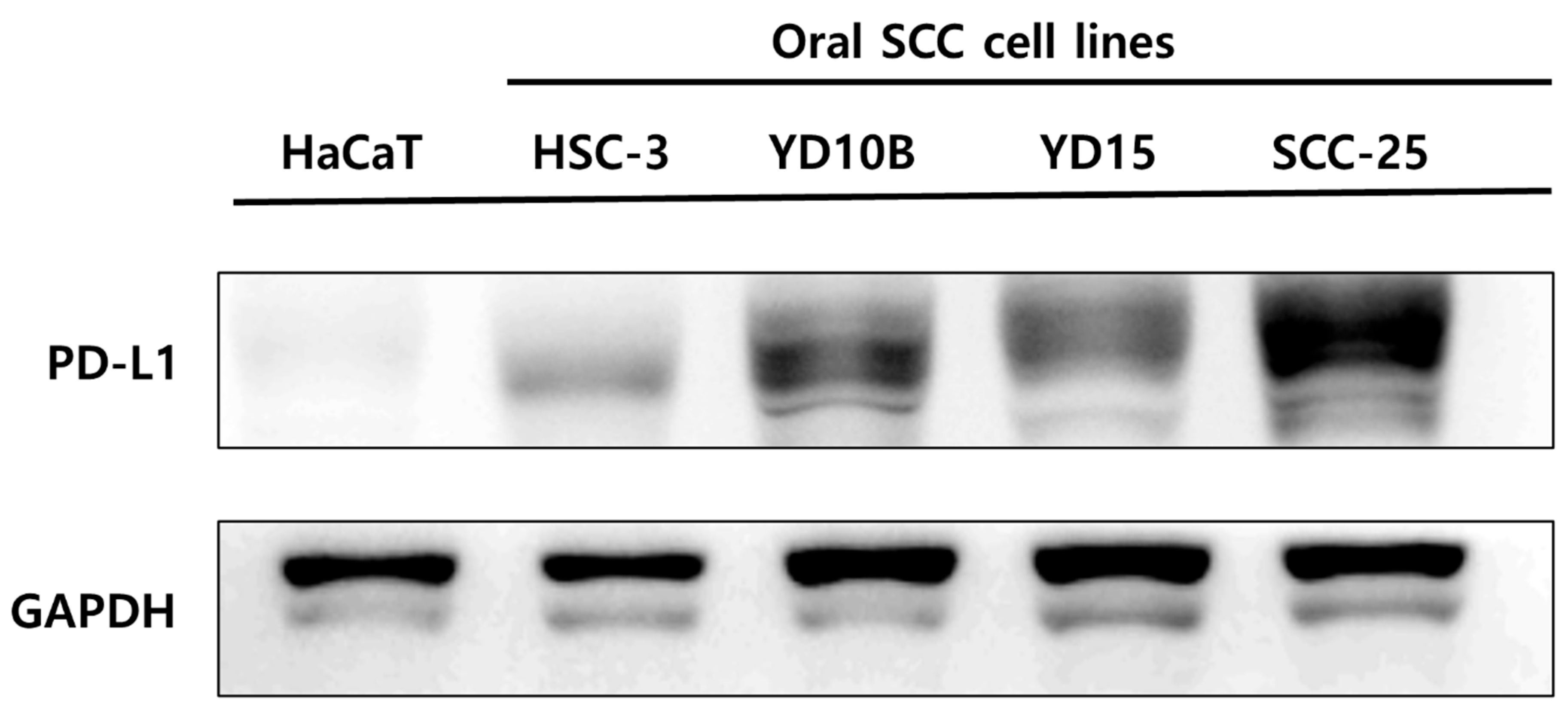
3.1. Comparison of PD-L1 Expression between Oral Squamous Cell Carcinoma Cells and Nonmalignant Keratinocyte Cells
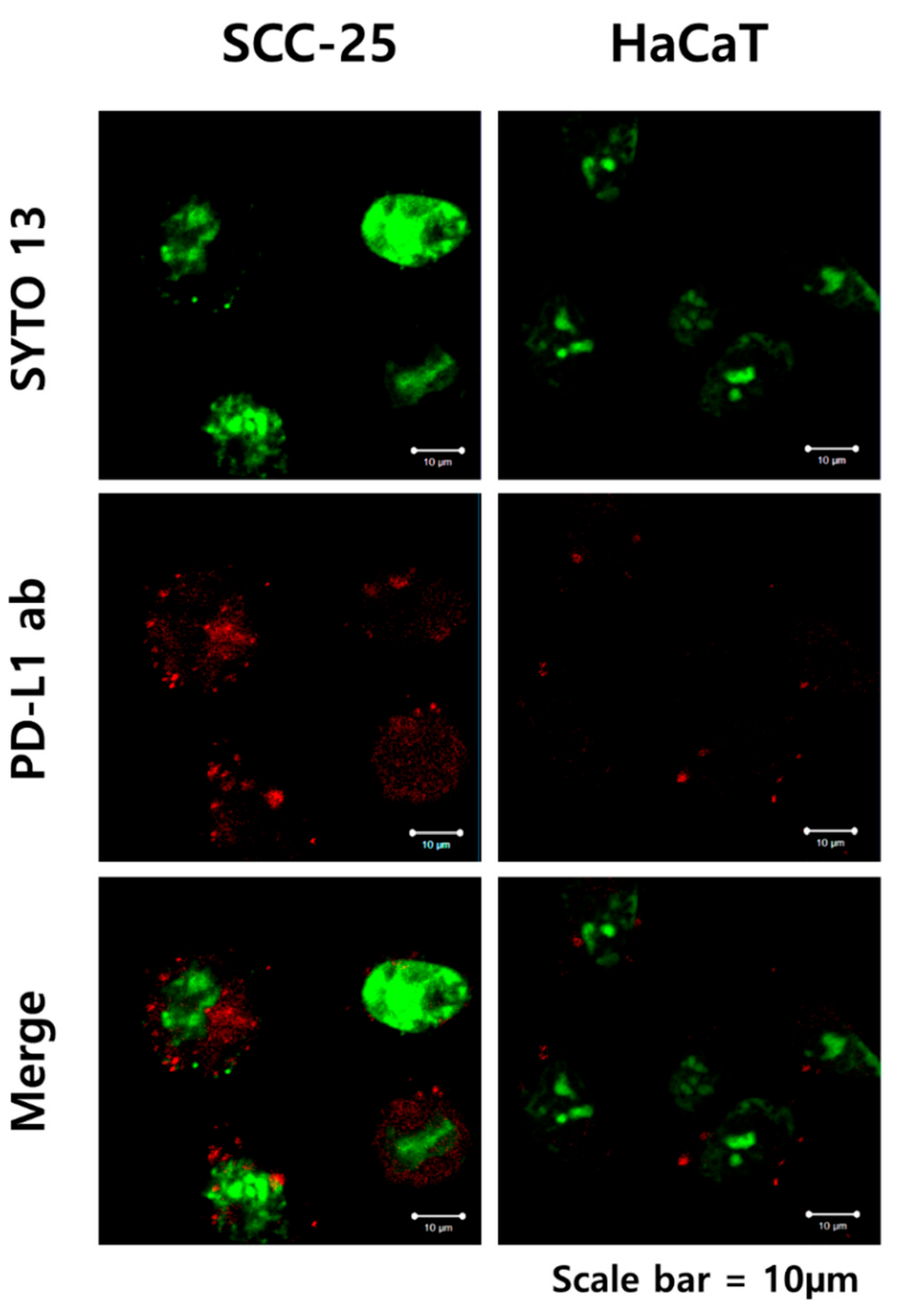
3.2. The Binding of PD-L1 Antibody + GNP in SCC-25 Cells
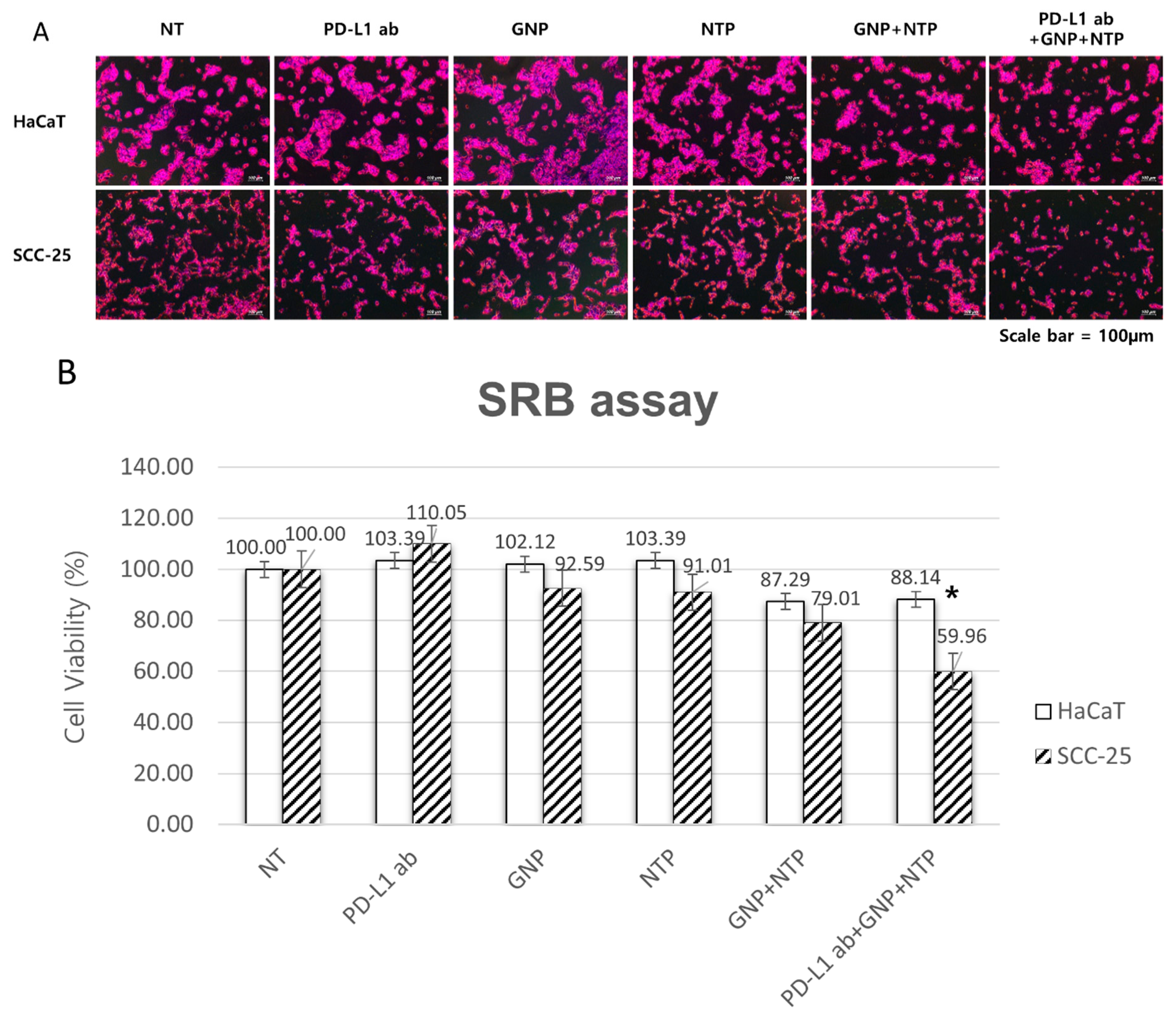
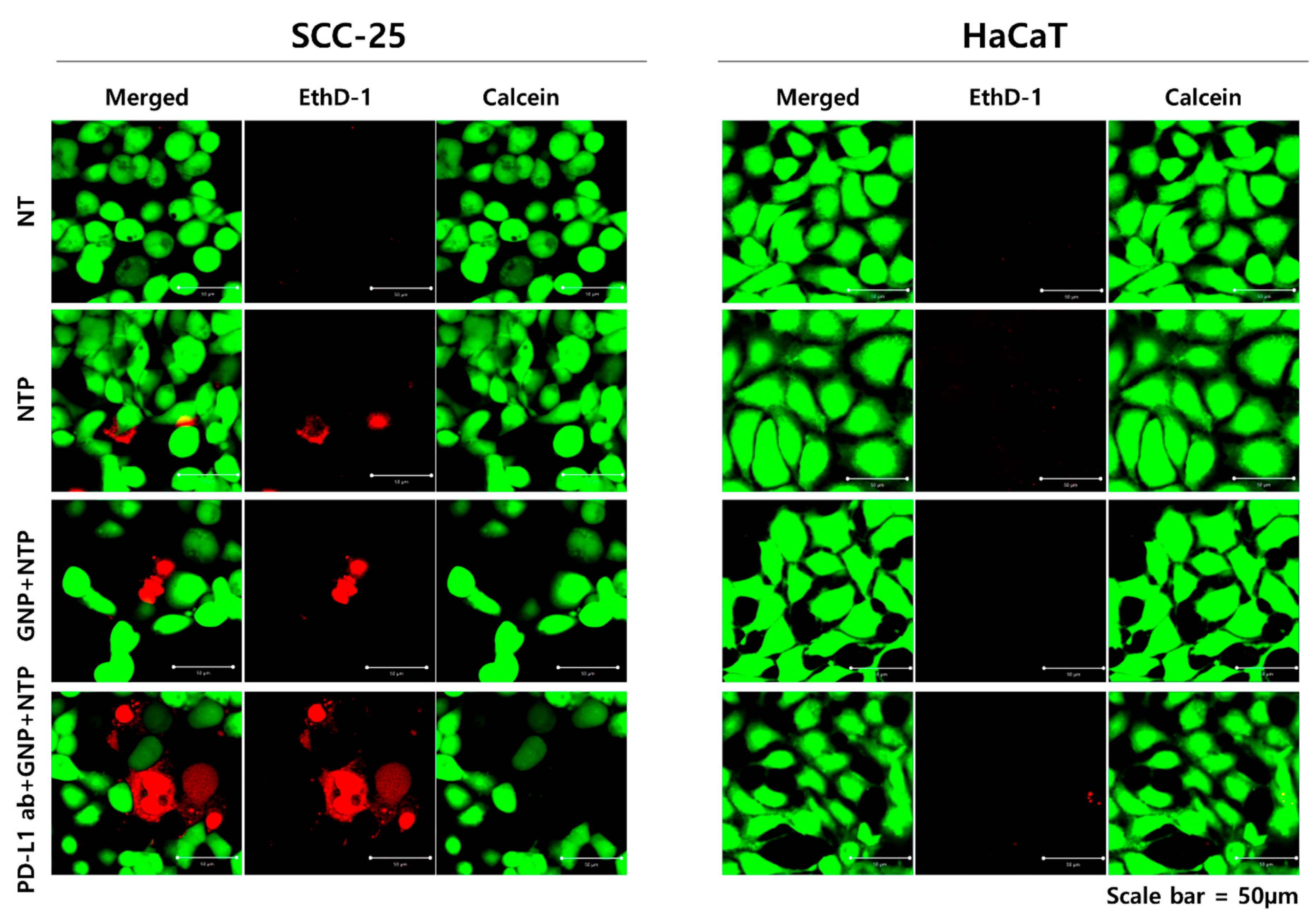
3.3. Selective Induction of Cancer Cell Death by PD-L1 ab + GNP and Nonthermal Plasma
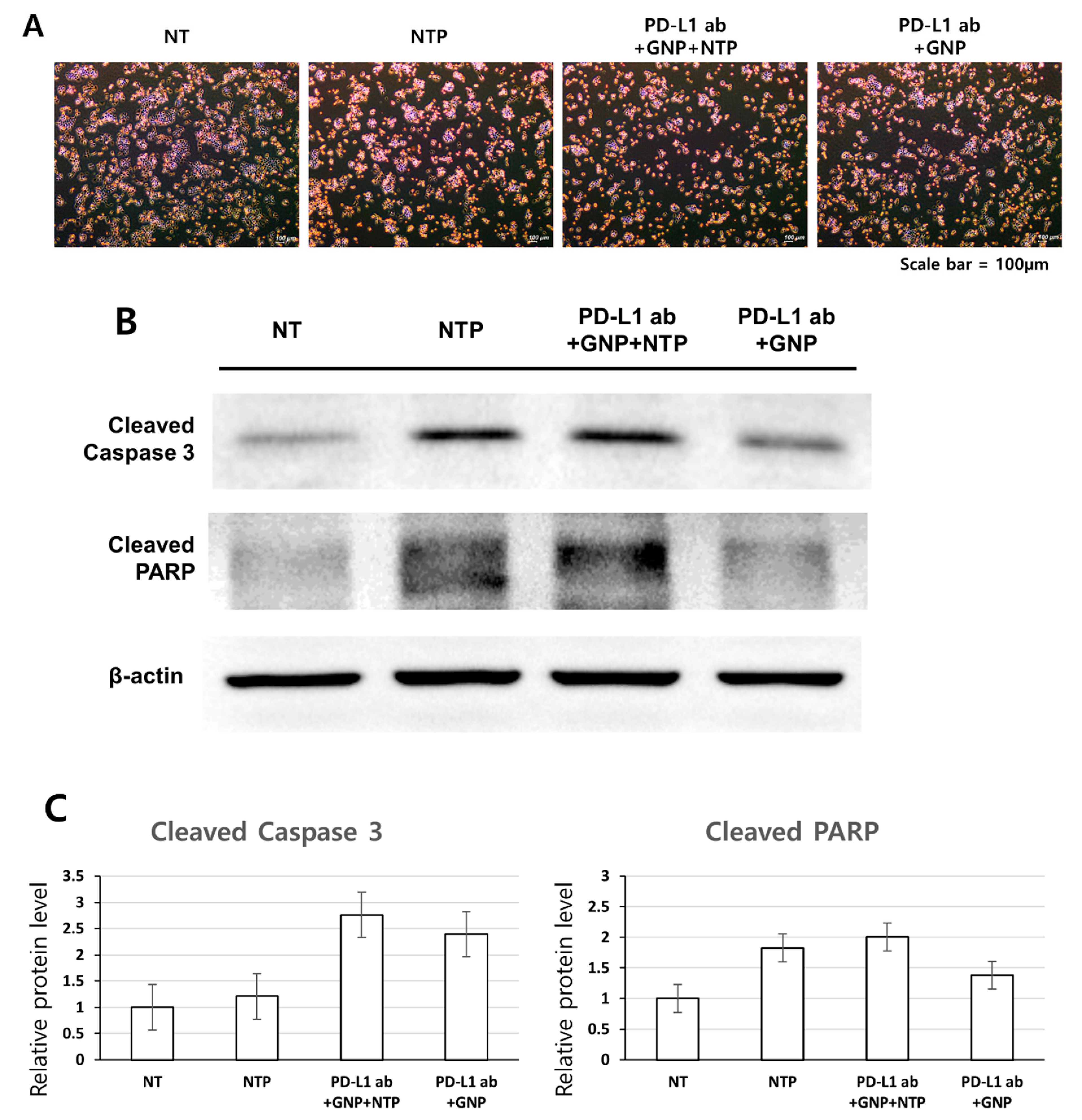
3.4. Induction of Apoptosis in SCC-25 Cells by the Treatment of PD-L1 ab + GNP + NTP
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hong, S.; Won, Y.-J.; Park, Y.R.; Jung, K.-W.; Kong, H.-J.; Lee, E.S. Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2017. Cancer Res. Treat. 2020, 52, 335–350. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.Q.M. Head and Neck Cancer. N. Engl. J. Med. 2020, 382, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.B.; Andersen, P.E.; Fernandes, R.P. Oral, Head and Neck Oncology and Reconstructive Surgery; Elsevier: St. Louis, MO, USA, 2017. [Google Scholar]
- Deng, R.; Bumbaca, D.; Pastuskovas, C.V.; Boswell, C.A.; West, D.; Cowan, K.J.; Chiu, H.; McBride, J.; Johnson, C.; Xin, Y.; et al. Preclinical pharmacokinetics, pharmacodynamics, tissue distribution, and tumor penetration of anti-PD-L1 monoclonal antibody, an immune checkpoint inhibitor. MAbs 2016, 8, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Mellman, I.; Coukos, G.; Dranoff, G. Cancer immunotherapy comes of age. Nature 2011, 480, 480–489. [Google Scholar] [CrossRef]
- Patel, S.A.; Minn, A.J. Combination Cancer Therapy with Immune Checkpoint Blockade: Mechanisms and Strategies. Immunity 2018, 48, 417–433. [Google Scholar] [CrossRef]
- Alsaab, H.O.; Sau, S.; Alzhrani, R.; Tatiparti, K.; Bhise, K.; Kashaw, S.K.; Iyer, A.K. PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, Combinations, and Clinical Outcome. Front. Pharm. 2017, 8, 561. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, W.; Xu, Z.P.; Gu, W. PD-L1 Distribution and Perspective for Cancer Immunotherapy-Blockade, Knockdown, or Inhibition. Front. Immunol 2019, 10, 2022. [Google Scholar] [CrossRef]
- O’Donnell, J.S.; Smyth, M.J.; Teng, M.W.L. Acquired resistance to anti-PD1 therapy: Checkmate to checkpoint blockade? Genome Med. 2016, 8. [Google Scholar] [CrossRef]
- Li, X.Y.; Liu, J.W.; Zhang, W.Y.; Wu, Y.; Li, J.J.; Foda, M.F.; Han, H.Y. Biogenic Hybrid Nanosheets Activated Photothermal Therapy and Promoted Anti-PD-L1 Efficacy for Synergetic Antitumor Strategy. ACS Appl. Mater. Interfaces 2020, 12, 29122–29132. [Google Scholar] [CrossRef]
- Ott, P.A.; Hodi, F.S.; Kaufman, H.L.; Wigginton, J.M.; Wolchok, J.D. Combination immunotherapy: A road map. J. Immunother. Cancer 2017, 5, 15. [Google Scholar] [CrossRef]
- Mahoney, K.M.; Rennert, P.D.; Freeman, G.J. Combination cancer immunotherapy and new immunomodulatory targets. Nat. Rev. Drug Discov. 2015, 14, 561–584. [Google Scholar] [CrossRef]
- Nam, J.; Son, S.; Park, K.S.; Zou, W.; Shea, L.D.; Moon, J.J. Cancer nanomedicine for combination cancer immunotherapy. Nat. Rev. Mater. 2019, 4, 398–414. [Google Scholar] [CrossRef]
- Bahrami, B.; Hojjat-Farsangi, M.; Mohammadi, H.; Anvari, E.; Ghalamfarsa, G.; Yousefi, M.; Jadidi-Niaragh, F. Nanoparticles and targeted drug delivery in cancer therapy. Immunol. Lett. 2017, 190, 64–83. [Google Scholar] [CrossRef]
- Daraee, H.; Eatemadi, A.; Abbasi, E.; Aval, S.F.; Kouhi, M.; Akbarzadeh, A. Application of gold nanoparticles in biomedical and drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 410–422. [Google Scholar] [CrossRef]
- Guo, L.R.; Yan, D.D.; Yang, D.F.; Li, Y.J.; Wang, X.D.; Zalewski, O.; Yan, B.F.; Lu, W. Combinatorial Photothermal and Immuno Cancer Therapy Using Chitosan-Coated Hollow Copper Sulfide Nanoparticles. ACS Nano. 2014, 8, 5670–5681. [Google Scholar] [CrossRef]
- Abadeer, N.S.; Murphy, C.J. Recent Progress in Cancer Thermal Therapy Using Gold Nanoparticles. J. Phys. Chem. C 2016, 120, 4691–4716. [Google Scholar] [CrossRef]
- Shivaprasad, G.; Shristi, S.; Yogendra, N.; Sanjay, G.; Usha, Y.N. Combination Therapy and Nanoparticulate Systems: Smart Approaches for the Effective Treatment of Breast Cancer. Pharmaceutics 2020, 12, 524. [Google Scholar]
- Huang, X.; El-Sayed, M.A. Plasmonic photo-thermal therapy (PPTT). Alex. J. Med. 2011, 47, 1–9. [Google Scholar] [CrossRef]
- Guerrero-Preston, R.; Ogawa, T.; Uemura, M.; Shumulinsky, G.; Valle, B.L.; Pirini, F.; Ravi, R.; Sidransky, D.; Keidar, M.; Trink, B. Cold atmospheric plasma treatment selectively targets head and neck squamous cell carcinoma cells. Int. J. Mol. Med. 2014, 34, 941–946. [Google Scholar] [CrossRef]
- Metelmann, H.-R.; Seebauer, C.; Miller, V.; Fridman, A.; Bauer, G.; Graves, D.B.; Pouvesle, J.-M.; Rutkowski, R.; Schuster, M.; Bekeschus, S.; et al. Clinical experience with cold plasma in the treatment of locally advanced head and neck cancer. Clin. Plasma Med. 2018, 9, 6–13. [Google Scholar] [CrossRef]
- Xu, Z.M.; Lan, Y.; Ma, J.; Shen, J.; Han, W.; Hu, S.H.; Ye, C.B.; Xi, W.H.; Zhang, Y.D.; Yang, C.J.; et al. Applications of atmospheric pressure plasma in microbial inactivation and cancer therapy: A brief review. Plasma Sci. Technol. 2020, 22, 103001. [Google Scholar] [CrossRef]
- Choi, B.B.R.; Choi, J.H.; Hong, J.W.; Song, K.W.; Lee, H.J.; Kim, U.K.; Kim, G.C. Selective Killing of Melanoma Cells With Non-Thermal Atmospheric Pressure Plasma and p-FAK Antibody Conjugated Gold Nanoparticles. Int. J. Med. Sci. 2017, 14, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.G.; Keidar, M.; Ostrikov, K. Plasmas meet nanoparticles—where synergies can advance the frontier of medicine. J. Phys. D Appl. Phys. 2011, 44, 174018. [Google Scholar] [CrossRef]
- Brullé, L.; Vandamme, M.; Riès, D.; Martel, E.; Robert, E.; Lerondel, S.; Trichet, V.; Richard, S.; Pouvesle, J.-M.; Le Pape, A. Effects of a Non Thermal Plasma Treatment Alone or in Combination with Gemcitabine in a MIA PaCa2-luc Orthotopic Pancreatic Carcinoma Model. PLoS ONE 2012, 7, e52653. [Google Scholar] [CrossRef]
- Dai, X.F.; Yu, L.H.; Zhao, X.J.; Ostrikov, K. Nanomaterials for oncotherapies targeting the hallmarks of cancer. Nanotechnology 2020, 31, 392001. [Google Scholar] [CrossRef]
- Singh, P.; Pandit, S.; Mokkapati, V.R.S.S.; Garg, A.; Ravikumar, V.; Mijakovic, I. Gold Nanoparticles in Diagnostics and Therapeutics for Human Cancer. Int. J. Mol. Sci. 2018, 19, 1979. [Google Scholar] [CrossRef]
- Xing, J.Q.; Xin, B.Q.; Xia, H. Preparation of Programmed Death-Ligand 1 Antibody Nanoparticles and Their Lung Cancer Targeting Therapeutic Effects. J. Nanosci. Nanotechnol. 2020, 20, 6033–6039. [Google Scholar] [CrossRef]
- Sengupta, S. Cancer Nanomedicine: Lessons for Immuno-Oncology. Trends Cancer 2017, 3, 551–560. [Google Scholar] [CrossRef]
- Connor, D.M.; Broome, A.-M. Gold Nanoparticles for the Delivery of Cancer Therapeutics. Adv. Cancer Res. 2018, 139, 163–184. [Google Scholar]
- Brousell, S.C.; Liu, Y.; Maccarini, P.F.; Palmer, G.M.; Etienne, W.; Zhao, Y.L.; Lee, C.T.; Vo-Dinh, T.; Inman, B.A. Synergistic Immuno-Photothermal Nanotherapy (Symphony): A Novel Treatment for Localized and Metastatic Bladder Cancer. J. Urol. 2017, 197, 1315. [Google Scholar] [CrossRef]
- Shevtsov, M.; Zhou, Y.; Khachatryan, W.; Multhoff, G.; Gao, H.L. Recent Advances in Gold Nanoformulations for Cancer Therapy. Curr. Drug Metab. 2018, 19, 768–780. [Google Scholar] [CrossRef]
- Choi, B.B.; Choi, Y.S.; Lee, H.J.; Lee, J.K.; Kim, U.K.; Kim, G.C. Nonthermal Plasma-Mediated Cancer Cell Death; Targeted Cancer Treatment. J. Therm. Sci. Technol. 2012, 7, 399–404. [Google Scholar] [CrossRef]
- Jeon, H.J.; Choi, B.B.R.; Park, K.H.; Hwang, D.S.; Kim, U.K.; Kim, G.C. Induction of Melanoma Cell-Selective Apoptosis Using Anti-HER2 Antibody-Conjugated Gold Nanoparticles. Yonsei Med. J. 2019, 60, 509–516. [Google Scholar] [CrossRef]
- Lin, A.; Truong, B.; Pappas, A.; Kirifides, L.; Oubarri, A.; Chen, S.; Lin, S.; Dobrynin, D.; Fridman, G.; Fridman, A.; et al. Uniform Nanosecond Pulsed Dielectric Barrier Discharge Plasma Enhances Anti-Tumor Effects by Induction of Immunogenic Cell Death in Tumors and Stimulation of Macrophages. Plasma Process. Polym. 2015, 12, 1392–1399. [Google Scholar] [CrossRef]
- Miller, V.; Lin, A.; Fridman, A. Why Target Immune Cells for Plasma Treatment of Cancer. Plasma Chem. Plasma Process. 2016, 36, 259–268. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Jang, Y.-S.; Choi, J.-H.; Ryu, M.; Kim, G.-C.; Byun, J.-H.; Hwang, D.-S.; Kim, U.-K. Anticancer Efficacy of Nonthermal Plasma Therapy Combined with PD-L1 Antibody Conjugated Gold Nanoparticles on Oral Squamous Cell Carcinoma. Appl. Sci. 2021, 11, 4559. https://doi.org/10.3390/app11104559
Park J, Jang Y-S, Choi J-H, Ryu M, Kim G-C, Byun J-H, Hwang D-S, Kim U-K. Anticancer Efficacy of Nonthermal Plasma Therapy Combined with PD-L1 Antibody Conjugated Gold Nanoparticles on Oral Squamous Cell Carcinoma. Applied Sciences. 2021; 11(10):4559. https://doi.org/10.3390/app11104559
Chicago/Turabian StylePark, Jinyoung, Yoon-Seo Jang, Jeong-Hae Choi, Miheon Ryu, Gyoo-Cheon Kim, June-Ho Byun, Dae-Seok Hwang, and Uk-Kyu Kim. 2021. "Anticancer Efficacy of Nonthermal Plasma Therapy Combined with PD-L1 Antibody Conjugated Gold Nanoparticles on Oral Squamous Cell Carcinoma" Applied Sciences 11, no. 10: 4559. https://doi.org/10.3390/app11104559
APA StylePark, J., Jang, Y.-S., Choi, J.-H., Ryu, M., Kim, G.-C., Byun, J.-H., Hwang, D.-S., & Kim, U.-K. (2021). Anticancer Efficacy of Nonthermal Plasma Therapy Combined with PD-L1 Antibody Conjugated Gold Nanoparticles on Oral Squamous Cell Carcinoma. Applied Sciences, 11(10), 4559. https://doi.org/10.3390/app11104559







