Which Is the Motion State of a Droplet on an Inclined Hydrophilic Rough Surface in Gravity: Pinned or Sliding?
Abstract
1. Introduction
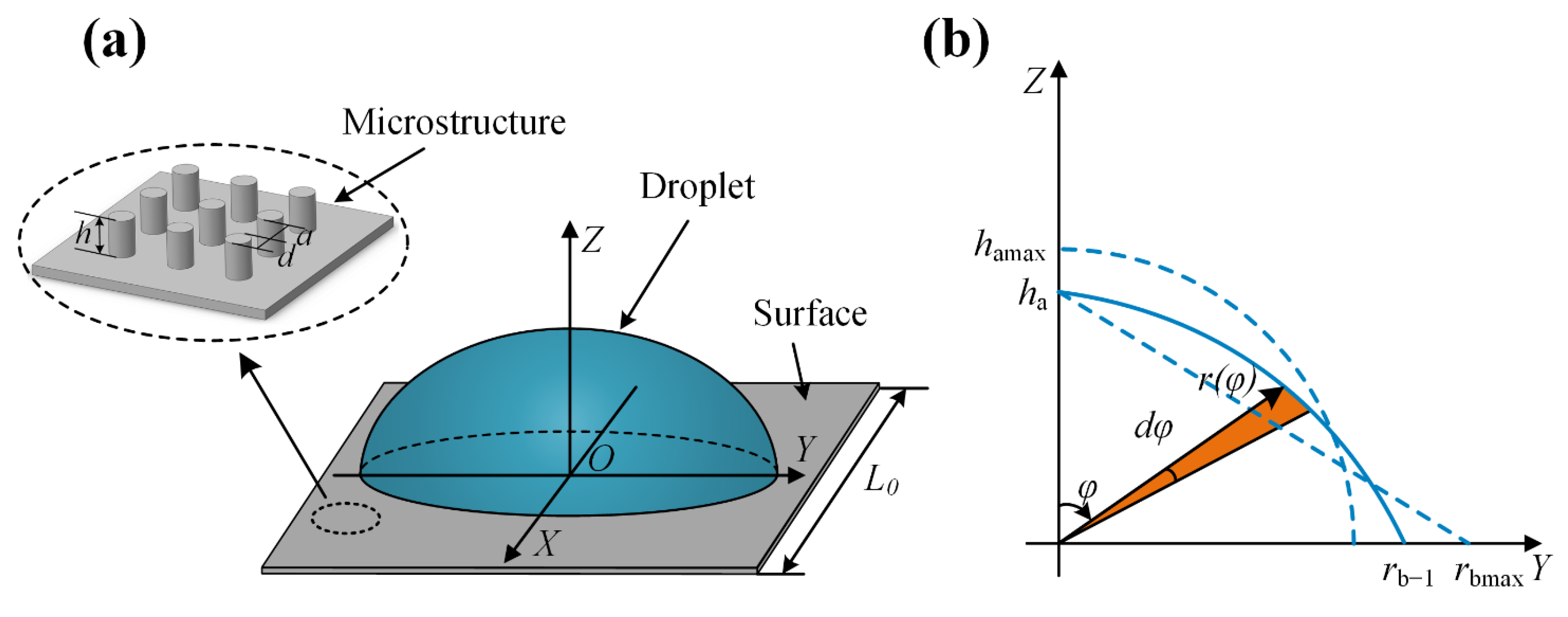
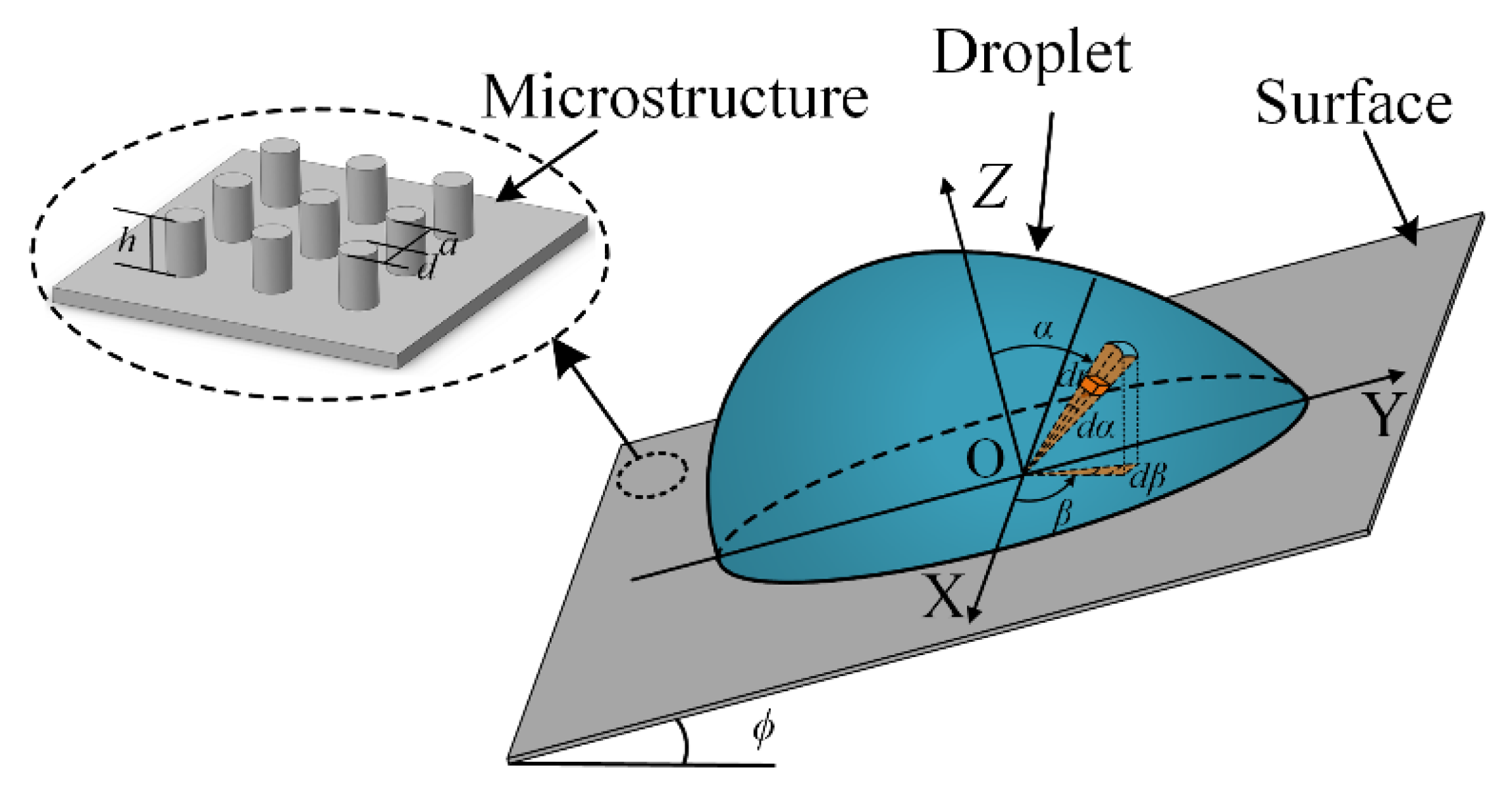
2. Theoretical Model
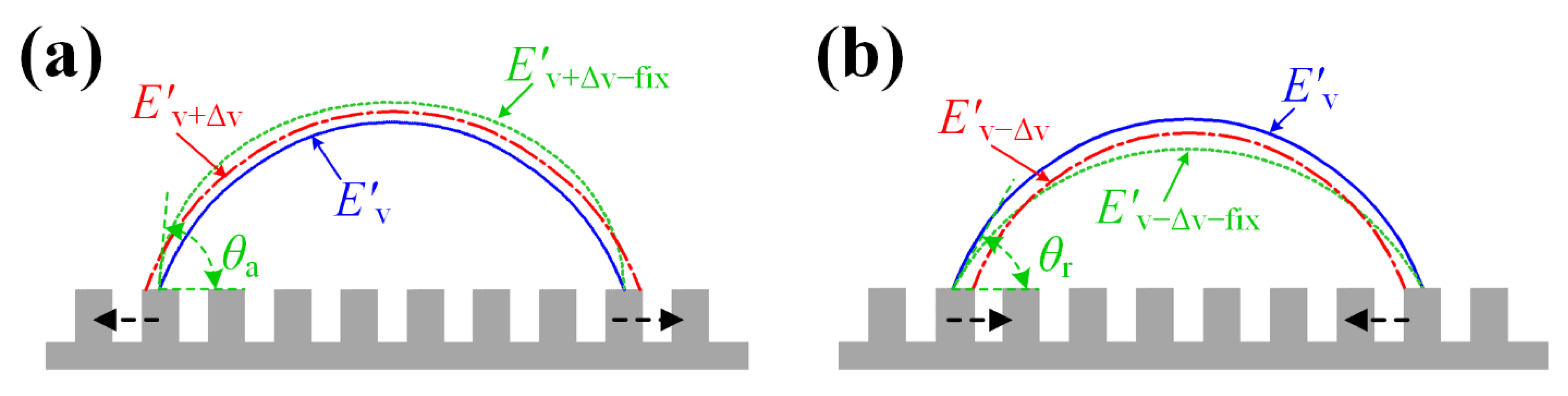
2.1. Model 1 for the and of a Droplet on the Hydrophilic Rough Surface
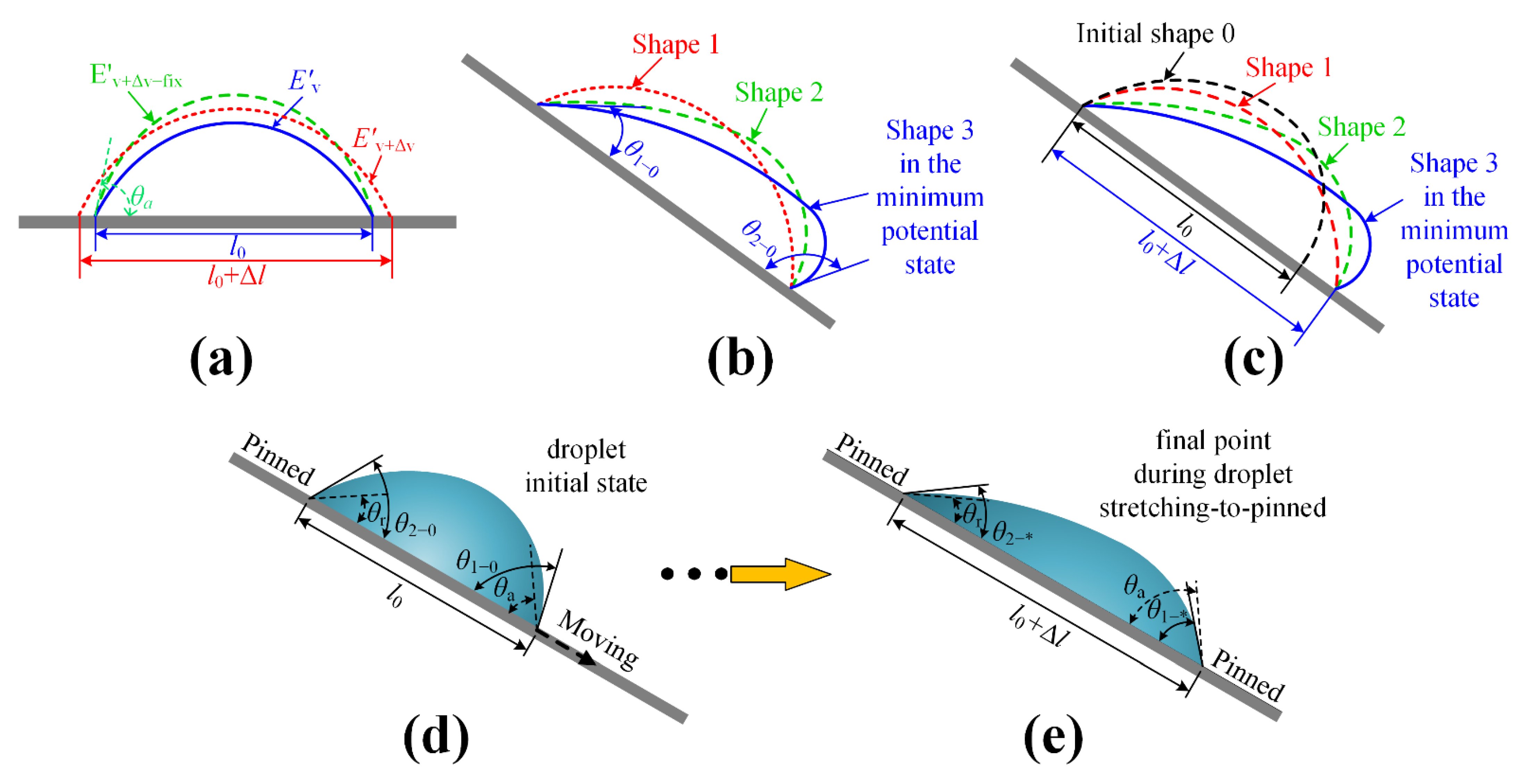
2.2. Model 2 for the Initial Front Contact Angle and the Initial Rear Contact Angle When a Droplet Begins to Stay on an Inclined, Hydrophilic Rough Surface
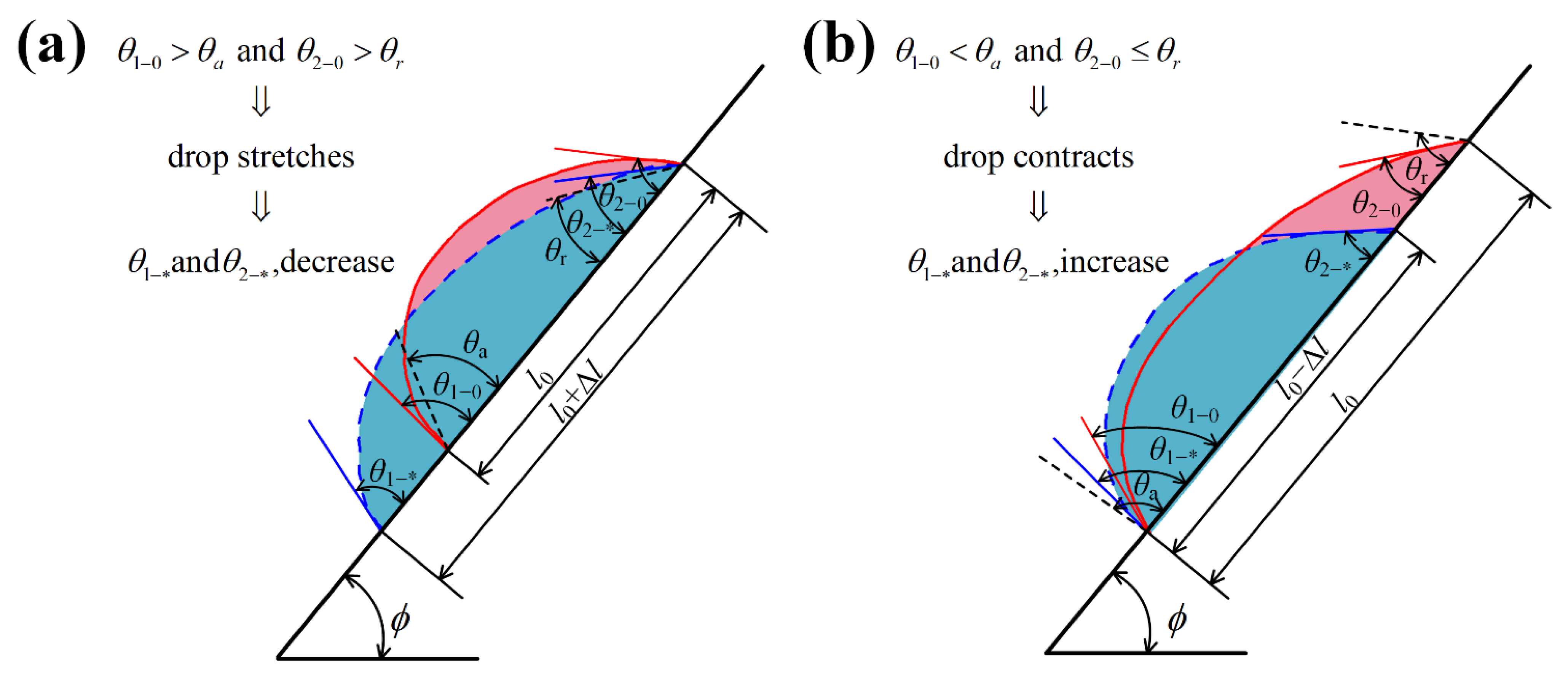
2.3. Model 3 for the Dynamic Front Contact Angles and the Dynamic Rear Contact Angles When a Droplet Evolves Its Contact Line Length on an Inclined, Hydrophilic Rough Surface
2.4. The Prediction Method of the Droplet: Pinned or Sliding
- Step 1: We used Model 1 to calculate the advancing angle and the receding angle ;
- Step 2: We used Model 2 to calculate the initial droplet profile , the initial droplet front contact angle , and the initial droplet rear contact angle ;
- Step 3: We used , , , and to first judge the motion state of the droplet:
- (1)
- If and , the droplet is pinned;
- (2)
- If and , the droplet is sliding;
- (3)
- If and , the droplet is contracting—we then went to Step 4 and further judged the motion state of the droplet;
- (4)
- If and , the droplet is stretching—we then went to Step 5 and further judged the motion state of the droplet.
- Step 4: For the contracting droplet, we used Model 3 to calculate out and . By constraining the contact line length , we calculated every and corresponding to every contact line length during the droplet contracting period. Then, we calculated when . Subsequently, we made the judgment that the droplet is contracting-to-pinned if , and the droplet is contracting-to-sliding if ;
- Step 5: For the stretching droplet, we used Model 3 to calculate out and . By constraining the contact line length , we calculated every and corresponding to every contact line length during the droplet stretching period. Then, we calculated when . Subsequently, we made the judgment as to that the droplet is stretching-to-pinned if , and the droplet is stretching-to-sliding if .
3. Experiments
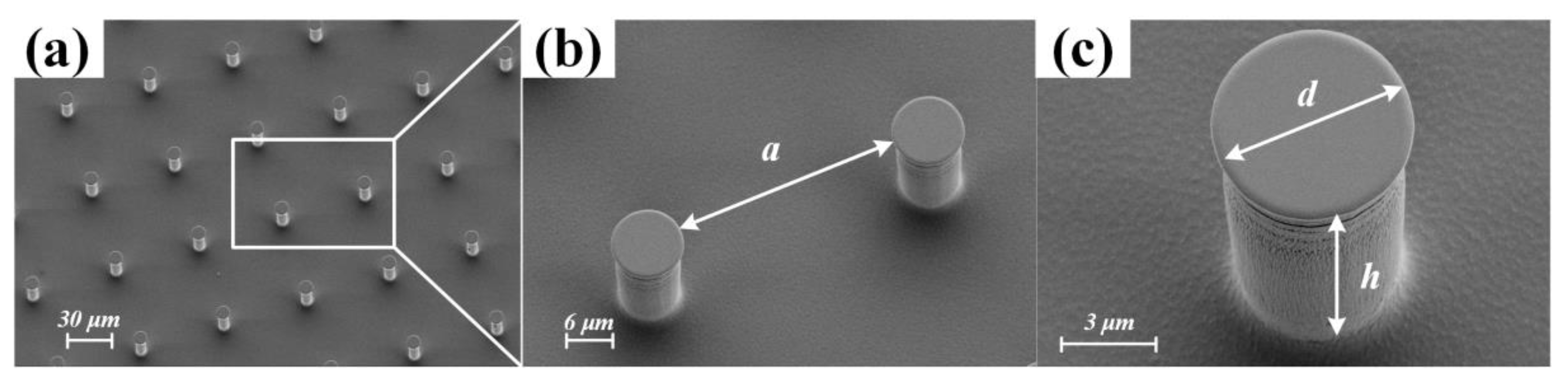
3.1. SiO2 Rough Surface Fabrication and Measurement
3.1.1. SiO2 Rough Surface Fabrication
3.1.2. SiO2 Rough Surface Measurement
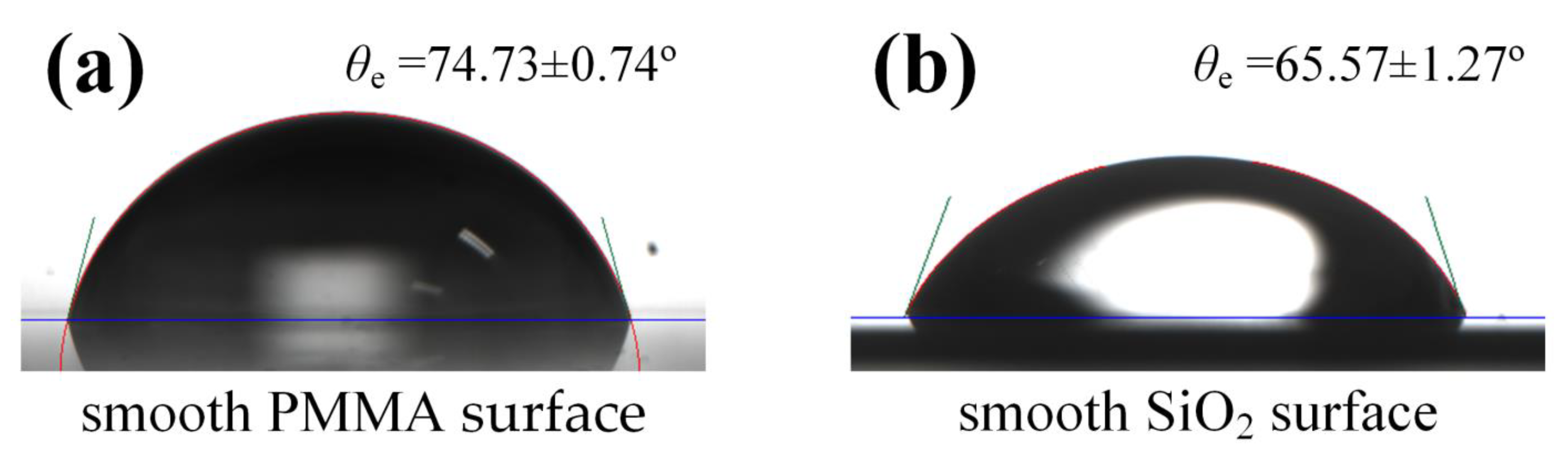
3.2. Characterization of Droplet Equilibrium Contact Angles
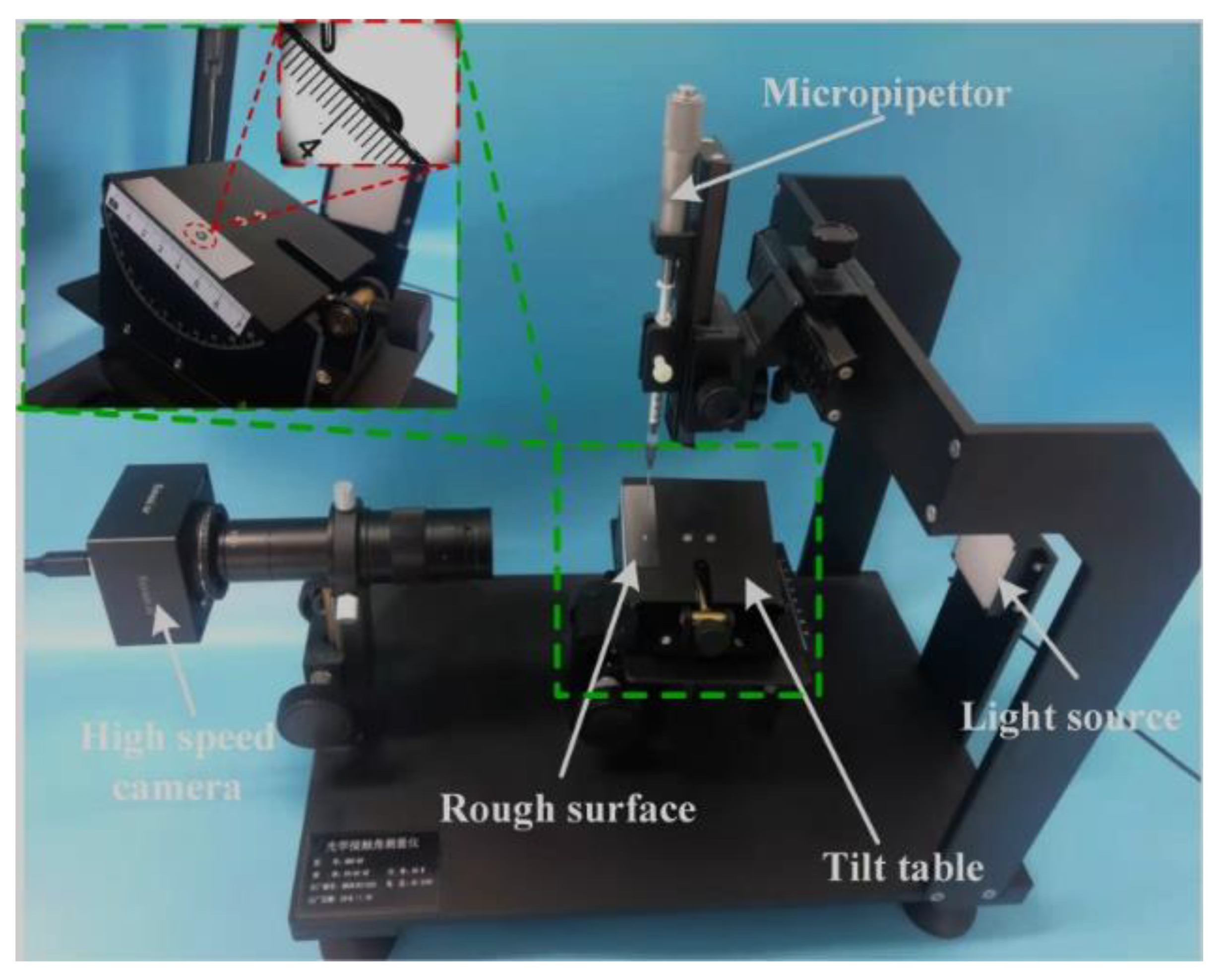
3.3. Characterization of Droplet Motion on Inclined Surfaces
4. Results and Discussion
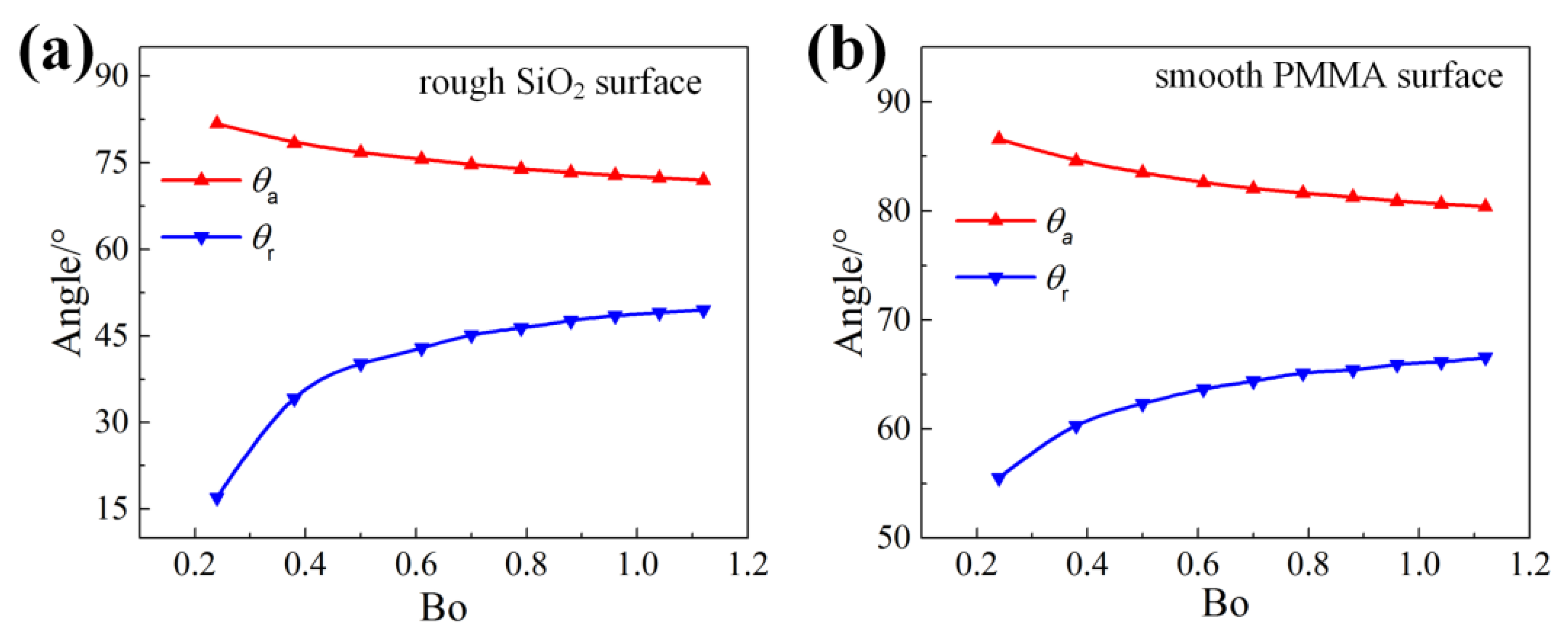
4.1. The Advancing and Receding Contact Angles(/) Change with the Droplet Volume
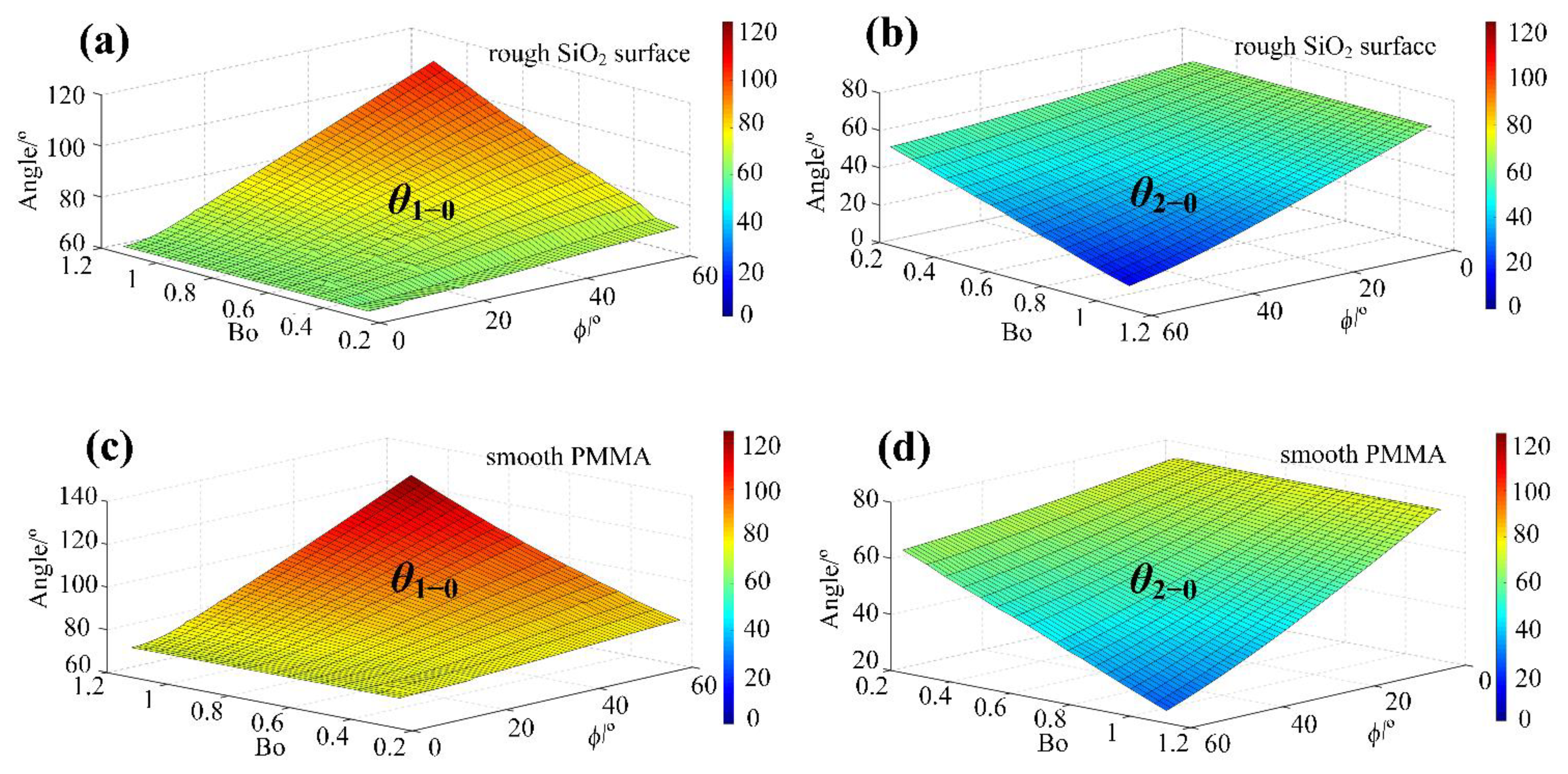
4.2. The Initial Front and Rear Contact Angles (/) Change with the Droplet Volume and the Surface Tilt Angle
4.3. Prediction Results Compared with Experiments
4.3.1. Droplets on an inclined Rough SiO2 Surface
4.3.2. Droplets on an Inclined, Smooth PMMA Surface
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ye, Y.; Liu, Z.; Liu, W.; Zhang, D.; Zhao, H.; Wang, L.; Li, X. Superhydrophobic oligoaniline-containing electroactive silica coating as pre-process coating for corrosion protection of carbon steel. Chem. Eng. J. 2018, 348, 940–951. [Google Scholar] [CrossRef]
- Wang, K.; Hou, D.; Wang, J.; Wang, Z.; Tian, B.; Liang, P. Hydrophilic surface coating on hydrophobic PTFE membrane for robust anti-oil-fouling membrane distillation. Appl. Surf. Sci. 2018, 450, 57–65. [Google Scholar] [CrossRef]
- Eykens, L.; Rose, K.; Dubreuil, M.; De Sitter, K.; Dotremont, C.; Pinoy, L.; Van Der Bruggen, B. Functionalization of a Hydrophilic Commercial Membrane Using Inorganic-Organic Polymers Coatings for Membrane Distillation. Appl. Sci. 2017, 7, 637. [Google Scholar] [CrossRef]
- Yang, W.; Huang, H.; Yan, W. Thermal Lattice Boltzmann Simulation of Evaporating Thin Liquid Film for Vapor Generation. Appl. Sci. 2018, 8, 798. [Google Scholar] [CrossRef]
- Esmaeilnezhad, E.; Karimian, M.; Choi, H.J. Synthesis and thermal analysis of hydrophobic iron oxide nanoparticles for improving in-situ combustion efficiency of heavy oils. J. Ind. Eng. Chem. 2019, 71, 402–409. [Google Scholar] [CrossRef]
- Gunay, A.A.; Sett, S.; Oh, J.; Miljkovic, N. Steady Method for the Analysis of Evaporation Dynamics. Langmuir 2017, 33, 12007–12015. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, D.; Li, Y.; Zhang, J.; Ye, S.; Cui, J.; Chen, L.; Wang, Z.; Butt, H.-J.; Vollmer, D.; et al. Surface charge printing for programmed droplet transport. Nat. Mater. 2019, 18, 936–941. [Google Scholar] [CrossRef]
- Wijshoff, H. Drop dynamics in the inkjet printing process. Curr. Opin. Colloid Interface Sci. 2018, 36, 20–27. [Google Scholar] [CrossRef]
- Chauhan, P.; Kumar, A.; Bhushan, B. Self-cleaning, stain-resistant and anti-bacterial superhydrophobic cotton fabric prepared by simple immersion technique. J. Colloid Interface Sci. 2019, 535, 66–74. [Google Scholar] [CrossRef]
- Li, J.; Wei, Y.; Huang, Z.; Wang, F.; Yan, X.; Wu, Z. Electrohydrodynamic behavior of water droplets on a horizontal super hydrophobic surface and its self-cleaning application. Appl. Surf. Sci. 2017, 403, 133–140. [Google Scholar] [CrossRef]
- Li, Q.; Liu, H.; Zhang, S.; Zhang, D.; Liu, X.; He, Y.; Mi, L.; Zhang, J.; Liu, C.; Shen, C.; et al. Superhydrophobic Electrically Conductive Paper for Ultrasensitive Strain Sensor with Excellent Anticorrosion and Self-Cleaning Property. ACS Appl. Mater. Interfaces 2019, 11, 21904–21914. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Sun, N.; Nielsen, S.O.; Stogin, B.B.; Wang, J.; Yang, S.; Wong, T.-S. Hydrophilic directional slippery rough surfaces for water harvesting. Sci. Adv. 2018, 4, eaaq0919. [Google Scholar] [CrossRef]
- Knapczyk-Korczak, J.; Ura, D.P.; Gajek, M.; Marzec, M.M.; Berent, K.; Bernasik, A.; Chiverton, J.P.; Stachewicz, U. Fiber-Based Composite Meshes with Controlled Mechanical and Wetting Properties for Water Harvesting. ACS Appl. Mater. Interfaces 2019, 12, 1665–1676. [Google Scholar] [CrossRef]
- Lu, J.; Ngo, C.-V.; Singh, S.C.; Yang, J.; Xin, W.; Yu, Z.; Guo, C. Bioinspired Hierarchical Surfaces Fabricated by Femtosecond Laser and Hydrothermal Method for Water Harvesting. Langmuir 2019, 35, 3562–3567. [Google Scholar] [CrossRef]
- Bokharaei, M.; Saatchi, K.; Häfeli, U.O. A single microfluidic chip with dual surface properties for protein drug delivery. Int. J. Pharm. 2017, 521, 84–91. [Google Scholar] [CrossRef]
- Liu, X.; Li, H.; Jia, W.; Chen, Z.; Xu, D. Selection of aptamers based on a protein microarray integrated with a microfluidic chip. Lab Chip 2016, 17, 178–185. [Google Scholar] [CrossRef]
- Biočanin, M.; Bues, J.; Dainese, R.; Amstad, E.; Deplancke, B. Simplified Drop-seq workflow with minimized bead loss using a bead capture and processing microfluidic chip. Lab Chip 2019, 19, 1610–1620. [Google Scholar] [CrossRef]
- Marsh, J.A.; Garoff, S. Dynamic contact angles and hydrodynamics near a moving contact line. Phys. Rev. Lett. 1993, 70, 2778–2781. [Google Scholar] [CrossRef]
- Dussan, E.B.; Chow, R.T.-P. On the ability of drops or bubbles to stick to non-horizontal surfaces of solids. J. Fluid Mech. 1983, 137, 1–29. [Google Scholar] [CrossRef]
- Ngan, C.G. On the dynamics of liquid spreading on solid surfaces. J. Fluid Mech. 1989, 209, 191. [Google Scholar] [CrossRef]
- Dussan, E.B. On the ability of drops or bubbles to stick to non-horizontal surfaces of solids. Part 2. Small drops or bubbles having contact angles of arbitrary size. J. Fluid Mech. 1985, 151. [Google Scholar] [CrossRef]
- Varagnolo, S.; Ferraro, D.; Fantinel, P.; Pierno, M.; Mistura, G.; Amati, G.; Biferale, L.; Sbragaglia, M. Stick-Slip Sliding of Water Drops on Chemically Heterogeneous Surfaces. Phys. Rev. Lett. 2013, 111, 066101. [Google Scholar] [CrossRef]
- Xu, H.; Clarke, A.; Rothstein, J.; Poole, R. Viscoelastic drops moving on hydrophilic and superhydrophobic surfaces. J. Colloid Interface Sci. 2018, 513, 53–61. [Google Scholar] [CrossRef]
- Yonemoto, Y.; Suzuki, S.; Uenomachi, S.; Kunugi, T. Sliding behaviour of water-ethanol mixture droplets on inclined low-surface-energy solid. Int. J. Heat Mass Transf. 2018, 120, 1315–1324. [Google Scholar] [CrossRef]
- Bommer, S.; Scholl, H.; Seemann, R.; Kanhaiya, K.; Sheraton, M.V.; Verma, N. Depinning of Drops on Inclined Smooth and Topographic Surfaces: Experimental and Lattice Boltzmann Model Study. Langmuir 2014, 30, 11086–11095. [Google Scholar] [CrossRef]
- Furmidge, C. Studies at phase interfaces. I. The sliding of liquid drops on solid surfaces and a theory for spray retention. J. Colloid Sci. 1962, 17, 309–324. [Google Scholar] [CrossRef]
- Miwa, M.; Nakajima, A.; Fujishima, A.; Hashimoto, K.; Watanabe, T. Effects of the Surface Roughness on Sliding Angles of Water Droplets on Superhydrophobic Surfaces. Langmuir 2000, 16, 5754–5760. [Google Scholar] [CrossRef]
- Man, X.; Doi, M. Ring to Mountain Transition in Deposition Pattern of Drying Droplets. Phys. Rev. Lett. 2016, 116, 066101. [Google Scholar] [CrossRef]
- Xu, X.; Di, Y.; Doi, M. Variational method for liquids moving on a substrate. Phys. Fluids 2016, 28, 087101. [Google Scholar] [CrossRef]
- Doi, M. Onsager’s variational principle in soft matter. J. Phys. Condens. Matter 2011, 23, 284118. [Google Scholar] [CrossRef] [PubMed]
- Maglio, M.; Legendre, D. Numerical Simulation of Sliding Drops on an Inclined Solid Surface. In Computational and Experimental Fluid Mechanics with Applications to Physics, Engineering and the Environment; Sigalotti, L.D.G., Klapp, J., Sira, E., Eds.; Environmental Science and Engineering; Springer International Publishing: Cham, Switzerland, 2014; pp. 47–69. [Google Scholar]
- Dupont, J.-B.; Legendre, D. Numerical simulation of static and sliding drop with contact angle hysteresis. J. Comput. Phys. 2010, 229, 2453–2478. [Google Scholar] [CrossRef]
- Legendre, D.; Maglio, M. Numerical simulation of spreading drops. Colloids Surf. A Physicochem. Eng. Asp. 2013, 432, 29–37. [Google Scholar] [CrossRef]
- Luo, M.; Gupta, R.; Frechette, J. Modulating Contact Angle Hysteresis to Direct Fluid Droplets along a Homogenous Surface. ACS Appl. Mater. Interfaces 2012, 4, 890–896. [Google Scholar] [CrossRef]
- Peng, Z.; Moghtaderi, B.; Doroodchi, E. Suspension stability of slurry Taylor flow: A theoretical analysis. Chem. Eng. Sci. 2017, 174, 459–471. [Google Scholar] [CrossRef]
- Peng, Z.; Gai, S.; Barma, M.; Rahman, M.M.; Moghtaderi, B.; Doroodchi, E. Experimental study of gas-liquid-solid flow characteristics in slurry Taylor flow-based multiphase microreactors. Chem. Eng. J. 2021, 405, 126646. [Google Scholar] [CrossRef]
- Whyman, G.; Bormashenko, E.; Stein, T. The rigorous derivation of Young, Cassie–Baxter and Wenzel equations and the analysis of the contact angle hysteresis phenomenon. Chem. Phys. Lett. 2008, 450, 355–359. [Google Scholar] [CrossRef]
- Bormashenko, E.; Musin, A.; Zinigrad, M. Evaporation of droplets on strongly and weakly pinning surfaces and dynamics of the triple line. Colloids Surf. A Physicochem. Eng. Asp. 2011, 385, 235–240. [Google Scholar] [CrossRef]
- Dong, J.; Jin, Y.; Dong, H.; Liu, J.; Ye, S. Numerical Study for a Large-Volume Droplet on the Dual-Rough Surface: Apparent Contact Angle, Contact Angle Hysteresis, and Transition Barrier. Langmuir 2018, 34, 8119–8127. [Google Scholar] [CrossRef]
| Surface Tilt Angle/° | Droplet Volume/μL | /° | /° | /° | /° | Initial Motion State | /° | /° | Final Motion State |
|---|---|---|---|---|---|---|---|---|---|
| 39 | 40 | 75.61 | 42.91 | 77.71 | 46.43 | stretching | 75.48 | 44.05 | stretching-to-pinned |
| Tilt Angle/° | /° | /° | /° | /° | Initial Motion State | /° | /° | Final Motion State |
|---|---|---|---|---|---|---|---|---|
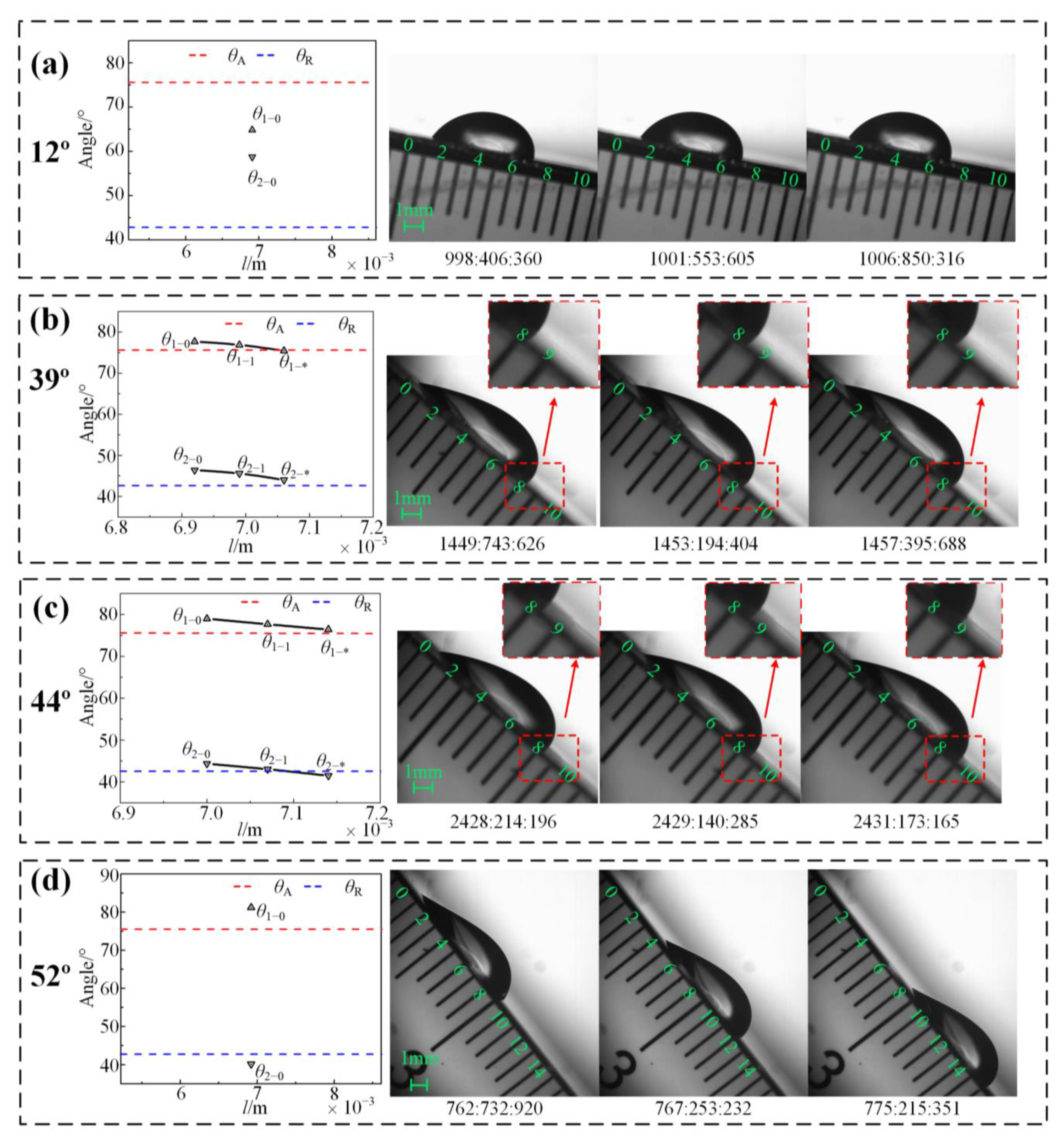
| 12 | 75.61 | 42.91 | 64.85 | 58.74 | Pinned | - | - | Pinned |
| 39 | 75.61 | 42.91 | 77.71 | 46.43 | Stretching | 75.48 | 44.05 | Stretching-to-pinned |
| 44 | 75.61 | 42.91 | 79.01 | 44.39 | Stretching | 76.42 | 41.51 | Stretching-to-sliding |
| 52 | 75.61 | 42.91 | 81.21 | 40.19 | Sliding | - | - | Sliding |
| Tilt Angle/° | /° | /° | /° | /° | Initial Motion State | /° | /° | Final Motion State |
|---|---|---|---|---|---|---|---|---|
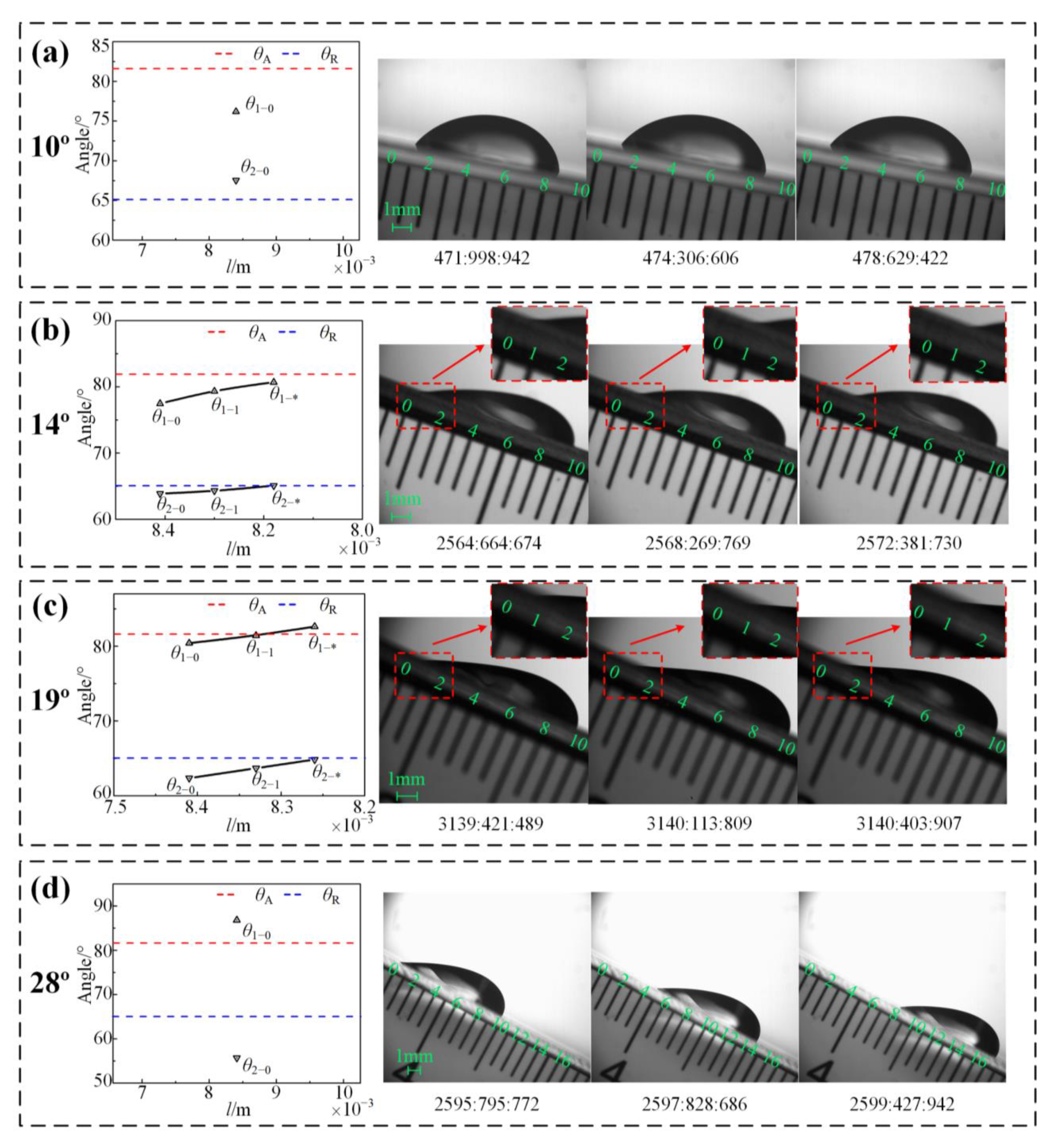
| 10 | 81.62 | 65.10 | 76.20 | 67.56 | Pinned | - | - | Pinned |
| 14 | 81.62 | 65.10 | 77.48 | 63.89 | Contracting | 80.71 | 65.13 | Contracting-to-pinned |
| 19 | 81.62 | 65.10 | 80.42 | 62.37 | Contracting | 82.64 | 64.86 | Contracting-to-sliding |
| 28 | 81.62 | 65.10 | 86.85 | 55.71 | Sliding | - | - | Sliding |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, J.; Guo, Y.; Jiao, L.; Si, C.; Bian, Y.; Zhang, Z.; Hu, J. Which Is the Motion State of a Droplet on an Inclined Hydrophilic Rough Surface in Gravity: Pinned or Sliding? Appl. Sci. 2021, 11, 3734. https://doi.org/10.3390/app11093734
Dong J, Guo Y, Jiao L, Si C, Bian Y, Zhang Z, Hu J. Which Is the Motion State of a Droplet on an Inclined Hydrophilic Rough Surface in Gravity: Pinned or Sliding? Applied Sciences. 2021; 11(9):3734. https://doi.org/10.3390/app11093734
Chicago/Turabian StyleDong, Jian, Youhai Guo, Long Jiao, Chao Si, Yinbo Bian, Zheng Zhang, and Jianliang Hu. 2021. "Which Is the Motion State of a Droplet on an Inclined Hydrophilic Rough Surface in Gravity: Pinned or Sliding?" Applied Sciences 11, no. 9: 3734. https://doi.org/10.3390/app11093734
APA StyleDong, J., Guo, Y., Jiao, L., Si, C., Bian, Y., Zhang, Z., & Hu, J. (2021). Which Is the Motion State of a Droplet on an Inclined Hydrophilic Rough Surface in Gravity: Pinned or Sliding? Applied Sciences, 11(9), 3734. https://doi.org/10.3390/app11093734






