Measurement of Gas Exchange on Excised Grapevine Leaves Does Not Differ from In Situ Leaves, and Potentially Shortens Sampling Time
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Experiment Testing Accuracy of Gas Exchange Measurement on Excised Grape Leaves
2.2. Analysis of Leaf Gas Exchange Data from Past Field Experiments
2.3. Data Analysis
3. Results and Discussion
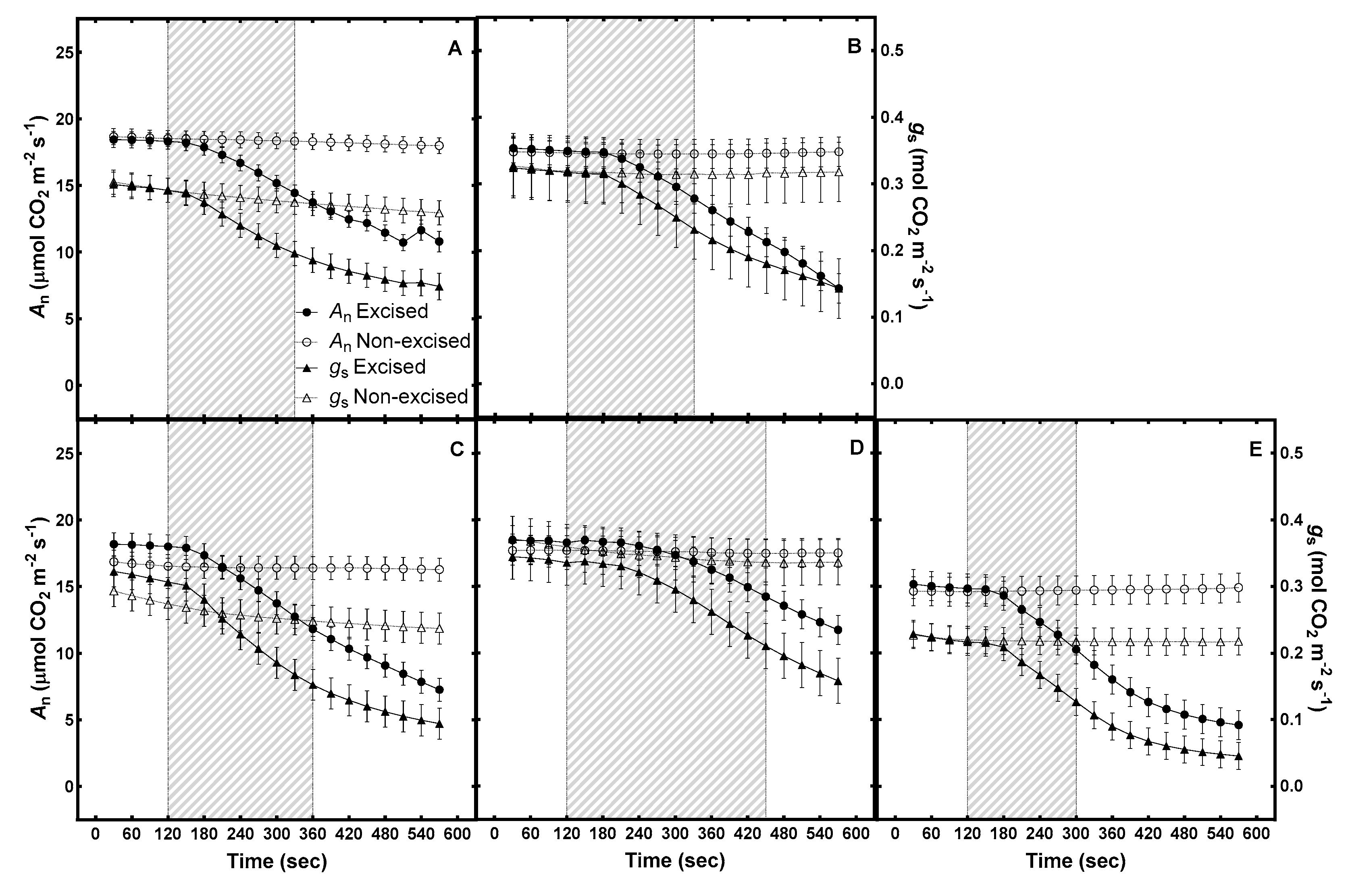
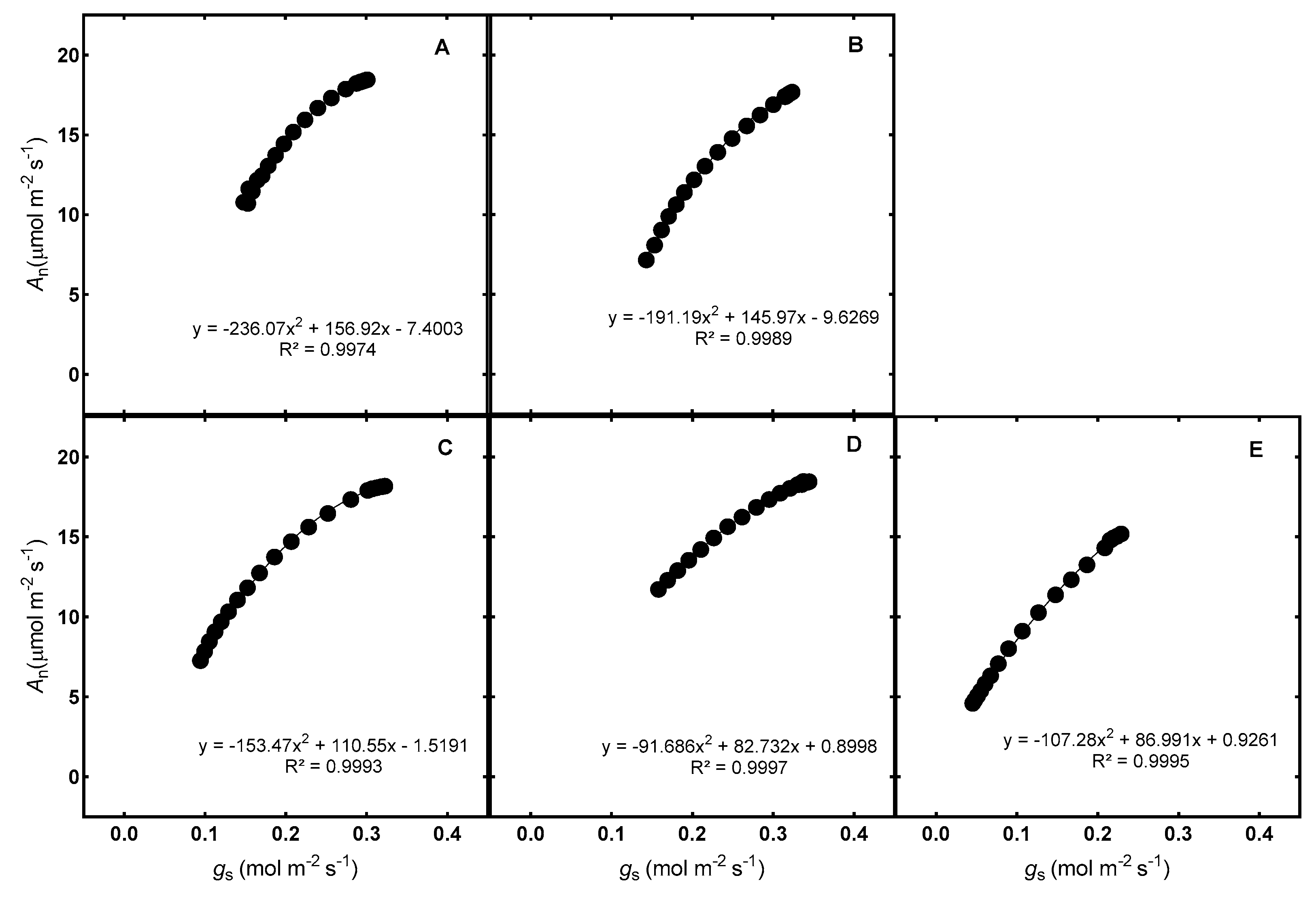
3.1. Accuracy of Gas Exchange Measurements on Excised Leaves
3.2. Duration between Gas Exchange Measurements of In Situ Leaves
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Montague, D.T.; McKenny, C.B. Gas Exchange Response to Leaf Excision for Two Field-grown Quercus Species. HortScience 2016, 51, S23. [Google Scholar]
- Montague, T.; McKenney, C. Gas exchange and growth of landscape tree species in response to drought and post establishment applied organic mulch. Acta Hortic. 2018, 1191, 167–174. [Google Scholar] [CrossRef]
- Kar, S.; Montague, D.T.; Villanueva-Morales, A. Measurement of photosynthesis in excised leaves of ornamental trees: A novel method to estimate leaf level drought tolerance and increase experimental sample size. Trees Struct. Funct. 2021. [Google Scholar] [CrossRef]
- Freiman, J.A.; Chalmers, T.C.; Smith, H.; Kuebler, R.R. The importance of beta, the type II error, and sample size in the design and interpretation of the randomized controlled trial. Med. Uses Stat. 1992, 357–373. [Google Scholar]
- Hernandez, P.A.; Graham, C.H.; Master, L.L.; Albert, D.L. The effect of sample size and species characteristics on performance of different species distribution modeling methods. Ecography 2006, 29, 773–785. [Google Scholar] [CrossRef]
- Garcia, R.L.; Norman, J.M.; McDermitt, D.K. Measurements of canopy gas exchange using an open chamber system. Remote Sens. Rev. 1990, 5, 141–162. [Google Scholar] [CrossRef]
- Rowland, D.L.; Beals, L.; Chaudhry, A.A.; Evans, A.S.; Grodeska, L.S. Physiological, morphological, and environmental variation among geographically isolated cottonwood (Populus deltoides) populations in New Mexico. West. N. Am. Nat. 2001, 61, 452–462. [Google Scholar]
- Kar, S.; Zhang, N.; Nakashima, T.; Villanueva-Morales, A.; Stewart, J.R.; Sacks, E.J.; Terajima, Y.; Yamada, T. Saccharum × Miscanthus intergeneric hybrids (miscanes) exhibit greater chilling tolerance of C4 photosynthesis and postchilling recovery than sugarcane (Saccharum spp. hybrids). GCB Bioenergy 2019, 11, 1318–1333. [Google Scholar] [CrossRef]
- Pearcy, R.W. Photosynthetic Gas Exchange Responses of Australian Tropical Forest Trees in Canopy, Gap and Understory Micro-Environments. Funct. Ecol. 1987, 1, 169–178. [Google Scholar] [CrossRef]
- Zotz, G.; Winter, K. Diel Patterns of CO2 Exchange in Rainforest Canopy Plants. Trop. For. Plant Ecophysiol. 1996, 89–113. [Google Scholar] [CrossRef]
- Dang, Q.L.; Margolis, H.A.; Coyea, M.R.; Sy, M.; Collatz, G.J. Regulation of branch-level gas exchange of boreal trees: Roles of shoot water potential and vapor pressure difference. Tree Physiol. 1997, 17, 521–535. [Google Scholar] [CrossRef]
- Clarke, J.M.; McCaig, T.N. Evaluation of Techniques for Screening for Drought Resistance in Wheat. Crop Sci. 1982, 22, 503. [Google Scholar] [CrossRef]
- Quisenberry, J.E.; Roark, B.; McMichael, B.L. Use of Transpiration Decline Curves to Identify Drought-Tolerant Cotton Germplasm1. Crop Sci. 1982, 22, 918. [Google Scholar] [CrossRef]
- Xu, H.; Wang, X.; Wang, J. Modeling Photosynthesis Decline of Excised Leaves of Sweet Corn Plants Grown with Organic and Chemical Fertilization. J. Crop Prod. 2001, 3, 157–171. [Google Scholar] [CrossRef]
- Santesteban, L.G.; Miranda, C.; Royo, J.B. Effect of water deficit and rewatering on leaf gas exchange and transpiration decline of excised leaves of four grapevine (Vitis vinifera L.) cultivars. Sci. Hortic. 2009, 121, 434–439. [Google Scholar] [CrossRef]
- Jacobs, W.P.; Suthers, H.B. Effects of Leaf Excision on Flowering of Xanthium Apical Buds in Culture Under Inductive and Noninductive Photoperiods. Am. J. Bot. 1974, 61, 1016–1020. [Google Scholar] [CrossRef]
- Lee, M.; Huang, Y.; Yao, H.; Thomson, S.J.; Bruce, L.M. Effects of Sample Storage on Spectral Reflectance Changes in Corn Leaves Excised From the Field. J. Agric. Sci. 2014, 6, 214–220. [Google Scholar] [CrossRef][Green Version]
- Menz, K.M.; Moss, D.N.; Cannell, R.Q.; Brun, W.A. Screening for Photosynthetic Efficiency. Crop Sci. 1969, 9, 692. [Google Scholar] [CrossRef]
- Pasternak, D.; Wilson, G.L. Differing Effects of Water Deficit on Net Photosynthesis of Intact and Excised Sorghum Leaves. New Phytol. 1974, 73, 847–850. [Google Scholar] [CrossRef]
- Parys, E.; Romanowska, E.; Siedlecka, M.; Poskuta, J.W. The effect of lead on photosynthesis and respiration in detached leaves and in mesophyll protoplasts of Pisum sativum. Acta Physiol. Plant. 1998, 20, 313–322. [Google Scholar] [CrossRef]
- McAusland, L.; Atkinson, J.A.; Lawson, T.; Murchie, E.H. High throughput procedure utilising chlorophyll fluorescence imaging to phenotype dynamic photosynthesis and photoprotection in leaves under controlled gaseous conditions. Plant Methods 2019, 15, 1–15. [Google Scholar] [CrossRef]
- Gerzon, E.; Biton, I.; Yaniv, Y.; Zemach, H.; Netzer, Y.; Schwartz, A.; Fait, A.; Ben-Ari, G. Grapevine anatomy as a possible determinant of isohydric or anisohydric behavior. Am. J. Enol. Vitic. 2015, 66, 340–347. [Google Scholar] [CrossRef]
- Bowen, P.; Bogdanoff, C.; Poojari, S.; Usher, K.; Lowery, T.; Úrbez-Torres, J.R. Effects of Grapevine Red Blotch Disease on Cabernet franc Vine Physiology, Bud Hardiness, and Fruit and Wine Quality. Am. J. Enol. Vitic. 2020, 71, 308–318. [Google Scholar] [CrossRef]
- Plank, C.M.; Hellman, E.W.; Montague, T. Light-Emitting diodes as supplemental lighting in viticulture field research. Am. J. Enol. Vitic. 2016, 67, 251–256. [Google Scholar] [CrossRef]
- Lipe, W.N.; Perry, R.L. Effects of rootstocks on wine grape scion vigor, yield, and juice quality. HortScience 1988, 23, 317–321. [Google Scholar]
- Kamas, J. Growing Grapes in Texas: From the Commercial Vineyard to the Backyard Vine; Texas A&M University Press: College Station, TX, USA, 2014. [Google Scholar]
- Merli, M.C.; Gatti, M.; Galbignani, M.; Bernizzoni, F.; Magnanini, E.; Poni, S. Comparison of whole-canopy water use efficiency and vine performance of cv. Sangiovese (Vitis vinifera L.) vines subjected to a post-veraison water deficit. Sci. Hortic. 2015, 185, 113–120. [Google Scholar] [CrossRef]
- Soar, C.J.; Collins, M.J.; Sadras, V.O. Irrigated Shiraz vines (Vitis vinifera) upregulate gas exchange and maintain berry growth in response to short spells of high maximum temperature in the field. Funct. Plant Biol. 2009, 36, 801–814. [Google Scholar] [CrossRef] [PubMed]
- Padgett-Johnson, M.; Williams, L.E.; Walker, M.A. Vine water relations, gas exchange, and vegetative growth of seventeen Vitis species grown under irrigated and nonirrigated conditions in California. J. Am. Soc. Hortic. Sci. 2003, 128, 269–276. [Google Scholar] [CrossRef]
- Hurvich, C.M.; Tsai, C.L. Regression and time series model selection in small samples. Biometrika 1989, 76, 297–307. [Google Scholar] [CrossRef]
- Buckley, T.N. How do stomata respond to water status? New Phytol. 2019, 224, 21–36. [Google Scholar] [CrossRef]
- Morellato, L.P.C.; Camargo, M.G.G.; Neves, F.F.D.; Luize, B.G.; Mantovani, A.; Hudson, I.L. The Influence of Sampling Method, Sample Size, and Frequency of Observations on Plant Phenological Patterns and Interpretation in Tropical Forest Trees. In Phenological Research: Methods for Environmental and Climate Change Analysis; Hudson, I.L., Keatley, M.R., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 99–121. ISBN 9789048133352. [Google Scholar]
- Wisz, M.S.; Hijmans, R.J.; Li, J.; Peterson, A.T.; Graham, C.H.; Guisan, A.; Elith, J.; Dudík, M.; Ferrier, S.; Huettmann, F.; et al. Effects of sample size on the performance of species distribution models. Divers. Distrib. 2008, 14, 763–773. [Google Scholar] [CrossRef]
- Barden, J.A.; Love, J.M.; Porpiglia, P.J.; Marini, R.P.; Caldwell, J.D. Net photosynthesis and dark respiration of apple leaves are not affected by shoot detachment. HortScience 1980, 15, 595–597. [Google Scholar]
- Lakso, A.N. Precautions on the use of excised shoots for photosynthesis and water relations measurements of apple and grape leaves. HortScience 1982, 17, 368–370. [Google Scholar]
| Treatment | Year | Rootstock | Scion | Location | Collection Date |
|---|---|---|---|---|---|
| In situ | 2006 | Harmony | Carignon, Grenache | Ropesville, TX (33°24′55″ N; 102°9′33″ W) | 25 August and 7 September |
| 2008 | 5BB, 44-53, 110R, 1103 P | Merlot | Texas A&M AgriLife Research and Extension Center vineyard in Lubbock, TX (33°41′33″ N; 101°49′17″ W) | 3 June, 10 June, 16 June, 19 June, 26 June, 5 August, 7 August, 14 August, and 21 August | |
| 110R | Grenache, Cabernet sauvignon, Pinot noir, Mourvedre | ||||
| 2009 | 5BB, 44-53, 110R, 1103 P | Merlot, | Texas A&M AgriLife Research and Extension Center vineyard in Lubbock, TX (33°41′33″ N; 101°49′17″ W) | 21 May, 26 May, 9 June, 16 June, 1 July, 8 July, 15 July, 21 July, 28 July, 29 July, 6 August, 11 August, 21 August, 27 August, 31 August, and 21 September | |
| 110R | Grenache, Cabernet sauvignon, Pinot noir, Mourvedre | ||||
| 2010 | Own Rooted, Riparia, 1103 P, SO4, Freedom, Harmony, 3309C, 420A, 5BB | Cabernet sauvignon | Meadow, TX (33°20′16″ N; 102°12′33″ W) | 27 July, 3 August, 17 August, and 24 August | |
| Excised | 2016 | 110R | Cabernet sauvignon | Texas A&M AgriLife Research and Extension Center vineyard in Lubbock, TX (33°41′33″ N; 101°49′17″ W) | 13 June and 25 July |
| 110R, Freedom, 1103 P | Grenache | ||||
| 2017 | 110R | Cabernet sauvignon | Texas A&M AgriLife Research and Extension Center vineyard in Lubbock, TX (33°41′33″ N; 101°49′17″ W) | 6 June, 20 July, and 7 August | |
| 110R, Freedom, 1103 P | Grenache | ||||
| 2018 | Own rooted | Malbec, Pinot gris | Brownfield, TX (33°10′37″ N; 102°16′3″ W) | 17 May, 1 June, and 18 June |
| Treatment | Sample Size (n) | Time between Measurements (s) |
|---|---|---|
| In situ leaves a | 2388 | 86.96 ± 0.41 c |
| Excised leaves b | 710 | 57.52 ± 0.39 |
| 95% confidence interval for mean difference between In situ and Excised treatments | (−30.56, −28.33) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kar, S.; Montague, T.; Villanueva-Morales, A.; Hellman, E. Measurement of Gas Exchange on Excised Grapevine Leaves Does Not Differ from In Situ Leaves, and Potentially Shortens Sampling Time. Appl. Sci. 2021, 11, 3644. https://doi.org/10.3390/app11083644
Kar S, Montague T, Villanueva-Morales A, Hellman E. Measurement of Gas Exchange on Excised Grapevine Leaves Does Not Differ from In Situ Leaves, and Potentially Shortens Sampling Time. Applied Sciences. 2021; 11(8):3644. https://doi.org/10.3390/app11083644
Chicago/Turabian StyleKar, Suraj, Thayne Montague, Antonio Villanueva-Morales, and Edward Hellman. 2021. "Measurement of Gas Exchange on Excised Grapevine Leaves Does Not Differ from In Situ Leaves, and Potentially Shortens Sampling Time" Applied Sciences 11, no. 8: 3644. https://doi.org/10.3390/app11083644
APA StyleKar, S., Montague, T., Villanueva-Morales, A., & Hellman, E. (2021). Measurement of Gas Exchange on Excised Grapevine Leaves Does Not Differ from In Situ Leaves, and Potentially Shortens Sampling Time. Applied Sciences, 11(8), 3644. https://doi.org/10.3390/app11083644






