Hsp60 Quantification in Human Gastric Mucosa Shows Differences between Pathologies with Various Degrees of Proliferation and Malignancy Grade
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Immunohistochemistry
2.3. Statistical Analysis
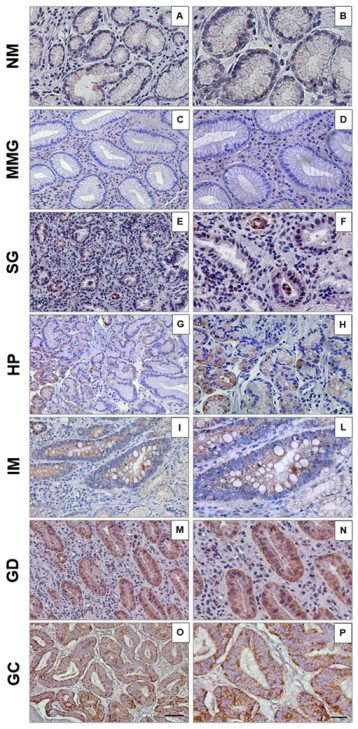
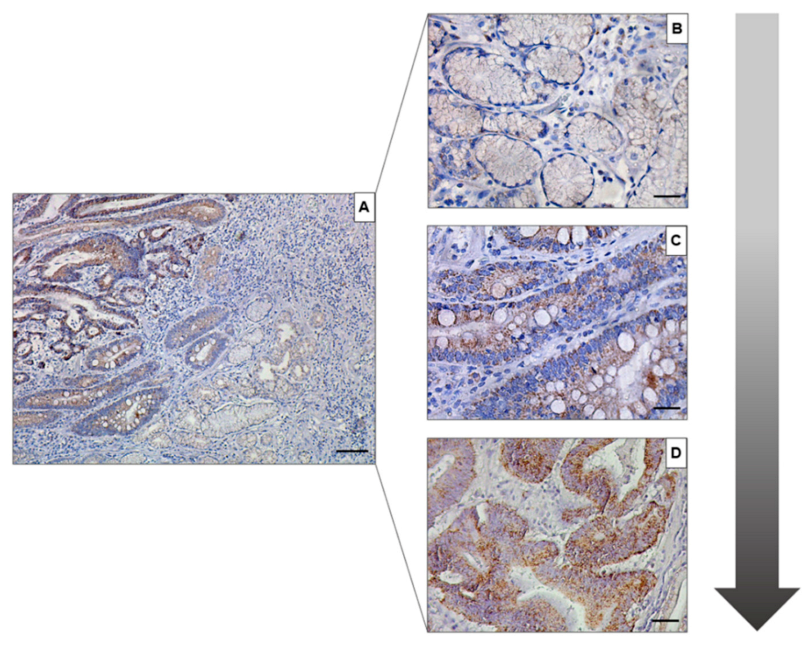
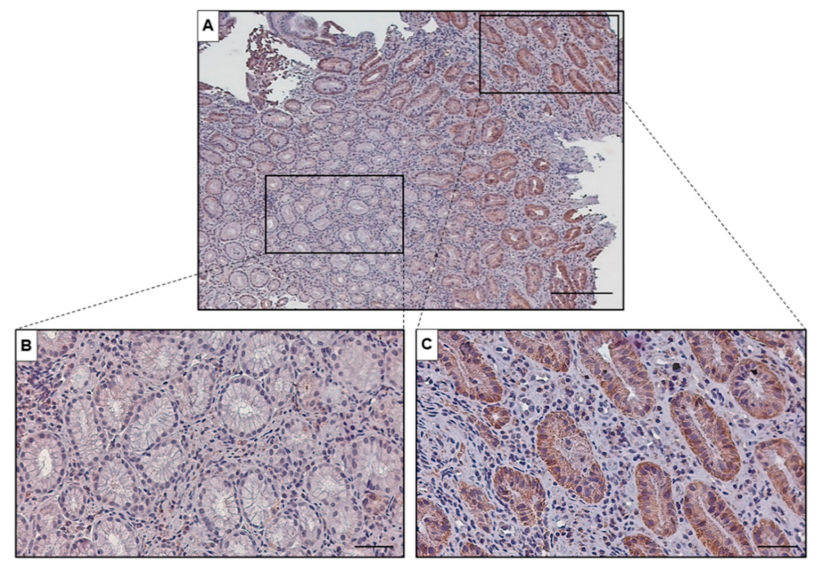
3. Results
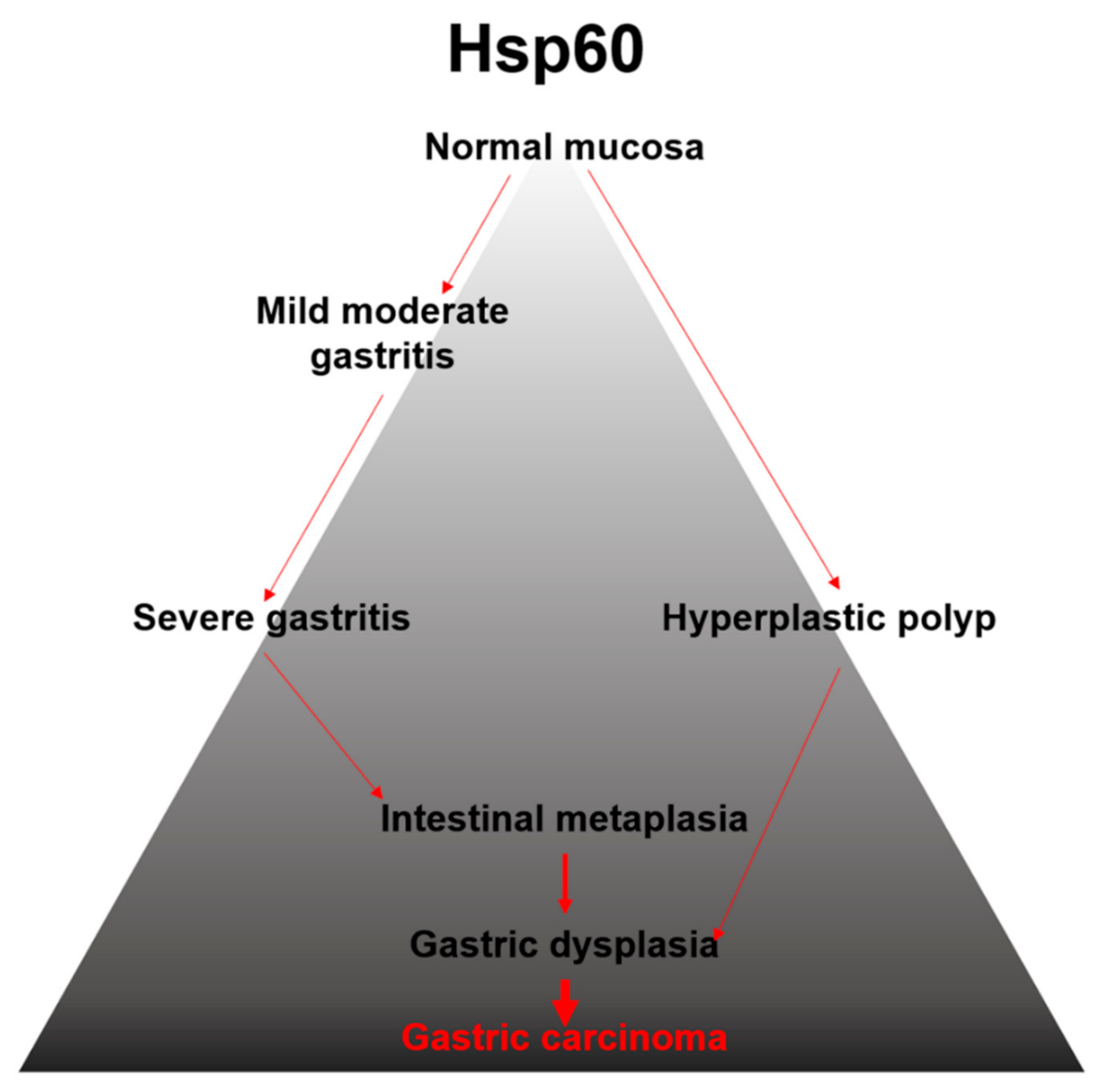
4. Discussion
5. Conclusions and Challenges for the Future
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Luo, B.; Lee, A.S. The critical roles of endoplasmic reticulum chaperones and unfolded protein response in tumorigenesis and anticancer therapies. Oncogene 2013, 32, 805–818. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, K.A.; Britschgi, C.; Schrader, K.; Christinat, Y.; Frischknecht, L.; Krek, W. Colorectal cancer cells display chaperone dependency for the unconventional prefoldin URI1. Oncotarget 2016, 7, 29635–29647. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chatterjee, S.; Burns, T.F. Targeting Heat Shock Proteins in Cancer: A Promising Therapeutic Approach. Int. J. Mol. Sci. 2017, 18, 1978. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Yang, X.; Bu, X.; Hou, N.; Chen, P. Alpha B-crystallin promotes the invasion and metastasis of colorectal cancer via epithelial-mesenchymal transition. Biochem. Biophys. Res. Commun. 2017, 489, 369–374. [Google Scholar] [CrossRef]
- Guo, J.; Li, X.; Zhang, W.; Chen, Y.; Zhu, S.; Chen, L.; Xu, R.; Lv, Y.; Wu, D.; Guo, M.; et al. HSP60-regulated Mitochondrial Proteostasis and Protein Translation Promote Tumor Growth of Ovarian Cancer. Sci. Rep. 2019, 9, 12628. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, C.; Chen, S.; Liu, J.; Fu, Y.; Luo, Y. Extracellular Hsp90α and clusterin synergistically promote breast cancer epithelial-to-mesenchymal transition and metastasis via LRP1. J. Cell Sci. 2019, 132. [Google Scholar] [CrossRef]
- Uretmen Kagiali, Z.C.; Sanal, E.; Karayel, Ö.; Polat, A.N.; Saatci, Ö.; Ersan, P.G.; Trappe, K.; Renard, B.Y.; Önder, T.T.; Tuncbag, N.; et al. Systems-level Analysis Reveals Multiple Modulators of Epithelial-mesenchymal Transition and Identifies DNAJB4 and CD81 as Novel Metastasis Inducers in Breast Cancer. Mol. Cell Proteom. 2019, 18, 1756–1771. [Google Scholar] [CrossRef]
- Xiong, G.; Chen, J.; Zhang, G.; Wang, S.; Kawasaki, K.; Zhu, J.; Zhang, Y.; Nagata, K.; Li, Z.; Zhou, B.P.; et al. Hsp47 promotes cancer metastasis by enhancing collagen-dependent cancer cell-platelet interaction. Proc. Natl. Acad. Sci. USA 2020, 117, 3748–3758. [Google Scholar] [CrossRef]
- Zeng, G.; Wang, J.; Huang, Y.; Lian, Y.; Chen, D.; Wei, H.; Lin, C.; Huang, Y. Overexpressing CCT6A Contributes To Cancer Cell Growth By Affecting The G1-To-S Phase Transition And Predicts A Negative Prognosis In Hepatocellular Carcinoma. OncoTargets Ther. 2019, 12, 10427–10439. [Google Scholar] [CrossRef]
- Harper, A.K.; Fletcher, N.M.; Fan, R.; Morris, R.T.; Saed, G.M. Heat Shock Protein 60 (HSP60) Serves as a Potential Target for the Sensitization of Chemoresistant Ovarian Cancer Cells. Reprod. Sci. 2020, 27, 1030–1036. [Google Scholar] [CrossRef]
- Li, X.S.; Xu, Q.; Fu, X.Y.; Luo, W.S. Heat shock protein 60 overexpression is associated with the progression and prognosis in gastric cancer. PLoS ONE 2014, 9, e107507. [Google Scholar] [CrossRef]
- Qu, H.; Zhu, F.; Dong, H.; Hu, X.; Han, M. Upregulation of CCT-3 Induces Breast Cancer Cell Proliferation Through miR-223 Competition and Wnt/β-Catenin Signaling Pathway Activation. Front. Oncol. 2020, 10. [Google Scholar] [CrossRef]
- Showalter, A.E.; Martini, A.C.; Nierenberg, D.; Hosang, K.; Fahmi, N.A.; Gopalan, P.; Khaled, A.S.; Zhang, W.; Khaled, A.R. Investigating Chaperonin-Containing TCP-1 subunit 2 as an essential component of the chaperonin complex for tumorigenesis. Sci. Rep. 2020, 10, 798. [Google Scholar] [CrossRef]
- Tang, Y.; Yang, Y.; Luo, J.; Liu, S.; Zhan, Y.; Zang, H.; Zheng, H.; Zhang, Y.; Feng, J.; Fan, S.; et al. Overexpression of HSP10 correlates with HSP60 and Mcl-1 levels and predicts poor prognosis in non-small cell lung cancer patients. Cancer Biomark. 2020, 1–10. [Google Scholar] [CrossRef]
- Xiong, H.; Xiao, H.; Luo, C.; Chen, L.; Liu, X.; Hu, Z.; Zou, S.; Guan, J.; Yang, D.; Wang, K. GRP78 activates the Wnt/HOXB9 pathway to promote invasion and metastasis of hepatocellular carcinoma by chaperoning LRP6. Exp. Cell Res. 2019, 383, 111493. [Google Scholar] [CrossRef]
- Cappello, F.; Bellafiore, M.; Palma, A.; David, S.; Marcianò, V.; Bartolotta, T.; Sciumè, C.; Modica, G.; Farina, F.; Zummo, G.; et al. 60KDa chaperonin (HSP60) is over-expressed during colorectal carcinogenesis. Eur. J. Histochem. 2003, 47, 105–110. [Google Scholar] [CrossRef]
- Cappello, F.; Bellafiore, M.; Palma, A.; Marciano, V.; Martorana, G.; Belfiore, P.; Martorana, A.; Farina, F.; Zummo, G.; Bucchieri, F. Expression of 60-kD Heat shock protein increases during carcinogenesis in the uterine exocervix. Pathobiology 2002, 70, 83–88. [Google Scholar] [CrossRef]
- Johansson, B.; Pourian, M.R.; Chuan, Y.-C.; Byman, I.; Bergh, A.; Pang, S.-T.; Norstedt, G.; Bergman, T.; Pousette, A. Proteomic comparison of prostate cancer cell lines LNCaP-FGC and LNCaP-r reveals heatshock protein 60 as a marker for prostate malignancy. Prostate 2006, 66, 1235–1244. [Google Scholar] [CrossRef]
- Glaessgen, A.; Jonmarker, S.; Lindberg, A.; Nilsson, B.; Lewensohn, R.; Ekman, P.; Valdman, A.; Egevad, L. Heat shock proteins 27, 60 and 70 as prognostic markers of prostate cancer. APMIS 2008, 116, 888–895. [Google Scholar] [CrossRef]
- Castilla, C.; Congregado, B.; Conde, J.M.; Medina, R.; Torrubia, F.J.; Japón, M.A.; Sáez, C. Immunohistochemical expression of Hsp60 correlates with tumor progression and hormone resistance in prostate cancer. Urology 2010, 76, 1017.e1–1017.e6. [Google Scholar] [CrossRef]
- Pitruzzella, A.; Paladino, L.; Vitale, A.M.; Martorana, S.; Cipolla, C.; Graceffa, G.; Cabibi, D.; David, S.; Fucarino, A.; Bucchieri, F.; et al. Quantitative immunomorphological analysis of heat shock proteins in thyroid follicular adenoma and carcinoma tissues reveals their potential for differential diagnosis and points to a role in carcinogenesis. Appl. Sci. 2019, 9, 4324. [Google Scholar] [CrossRef]
- Basset, C.A.; Cappello, F.; Rappa, F.; Lentini, V.L.; Jurjus, A.R.; Conway de Macario, E.; Macario, A.J.L.; Leone, A. Molecular chaperones in tumors of salivary glands. J. Mol. Histol. 2020, 51, 109–115. [Google Scholar] [CrossRef]
- Giroux, V.; Rustgi, A.K. Metaplasia: Tissue injury adaptation and a precursor to the dysplasia-cancer sequence. Nat. Rev. Cancer 2017, 17, 594–604. [Google Scholar] [CrossRef]
- Karimi, P.; Islami, F.; Anandasabapathy, S.; Freedman, N.D.; Kamangar, F. Gastric cancer: Descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol. Biomark. Prev. 2014, 23, 700–713. [Google Scholar] [CrossRef]
- Tan, P.; Yeoh, K.G. Genetics and Molecular Pathogenesis of Gastric Adenocarcinoma. Gastroenterology 2015, 149, 1153–1162.e3. [Google Scholar] [CrossRef]
- Tomasello, G.; Rodolico, V.; Zerilli, M.; Martorana, A.; Bucchieri, F.; Pitruzzella, A.; Marino Gammazza, A.; David, S.; Rappa, F.; Zummo, G.; et al. Changes in immunohistochemical levels and subcellular localization after therapy and correlation and colocalization with CD68 suggest a pathogenetic role of Hsp60 in ulcerative colitis. Appl. Immunohistochem. Mol. Morphol. 2011, 19, 552–561. [Google Scholar] [CrossRef]
- Yakirevich, E.; Resnick, M.B. Pathology of gastric cancer and its precursor lesions. Gastroenterol. Clin. N. Am. 2013, 42, 261–284. [Google Scholar] [CrossRef]
- Bosari, S.; Moneghini, L.; Graziani, D.; Lee, A.K.; Murray, J.J.; Coggi, G.; Viale, G. bcl-2 oncoprotein in colorectal hyperplastic polyps, adenomas, and adenocarcinomas. Human Pathol. 1995, 26, 534–540. [Google Scholar] [CrossRef]
- Flohil, C.C.; Janssen, P.A.; Bosman, F.T. Expression of Bcl-2 protein in hyperplastic polyps, adenomas, and carcinomas of the colon. J. Pathol. 1996, 178, 393–397. [Google Scholar] [CrossRef]
- Phelps, R.A.; Chidester, S.; Dehghanizadeh, S.; Phelps, J.; Sandoval, I.T.; Rai, K.; Broadbent, T.; Sarkar, S.; Burt, R.W.; Jones, D.A. A two-step model for colon adenoma initiation and progression caused by APC loss. Cell 2009, 137, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Rappa, F.; Farina, F.; Zummo, G.; David, S.; Campanella, C.; Carini, F.; Tomasello, G.; Damiani, P.; Cappello, F.; Conway de Macario, E.; et al. HSP-molecular chaperones in cancer biogenesis and tumor therapy: An overview. Anticancer Res. 2012, 32, 5139–5150. [Google Scholar] [PubMed]
- Takayama, S.; Reed, J.C.; Homma, S. Heat-shock proteins as regulators of apoptosis. Oncogene 2003, 22, 9041–9047. [Google Scholar] [CrossRef]
- Lianos, G.D.; Alexiou, G.A.; Mangano, A.; Mangano, A.; Rausei, S.; Boni, L.; Dionigi, G.; Roukos, D.H. The role of heat shock proteins in cancer. Cancer Lett. 2015, 360, 114–118. [Google Scholar] [CrossRef]
- Macario, A.J.L.; Conway de Macario, E. Chaperonopathies and chaperonotherapy. FEBS Lett. 2007, 581, 3681–3688. [Google Scholar] [CrossRef]
- Meng, Q.; Li, B.X.; Xiao, X. Toward Developing Chemical Modulators of Hsp60 as Potential Therapeutics. Front. Mol. Biosci. 2018, 5, 35. [Google Scholar] [CrossRef]
- Habich, C.; Burkart, V. Heat shock protein 60: Regulatory role on innate immune cells. Cell. Mol. Life Sci. 2007, 64, 742–751. [Google Scholar] [CrossRef]
- Tsan, M.F.; Gao, B. Heat shock protein and innate immunity. Cell. Mol. Immunol. 2004, 1, 274–279. [Google Scholar]
- Ohashi, K.; Burkart, V.; Flohé, S.; Kolb, H. Cutting edge: Heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J. Immunol. 2000, 164, 558–561. [Google Scholar] [CrossRef]
- Sangiorgi, C.; Vallese, D.; Gnemmi, I.; Bucchieri, F.; Balbi, B.; Brun, P.; Leone, A.; Giordano, A.; Conway de Macario, E.; Macario, A.J.L.; et al. HSP60 activity on human bronchial epithelial cells. Int. J. Immunopathol. Pharmacol. 2017, 30, 333–340. [Google Scholar] [CrossRef]
- Swaroop, S.; Mahadevan, A.; Shankar, S.K.; Adlakha, Y.K.; Basu, A. HSP60 critically regulates endogenous IL-1β production in activated microglia by stimulating NLRP3 inflammasome pathway. J. Neuroinflamm. 2018, 15, 177. [Google Scholar] [CrossRef]
- Tanaka, A.; Kamada, T.; Yokota, K.; Shiotani, A.; Hata, J.; Oguma, K.; Haruma, K. Helicobacter pylori heat shock protein 60 antibodies are associated with gastric cancer. Pathol. Res. Pract. 2009, 205, 690–694. [Google Scholar] [CrossRef] [PubMed]
- Lang, B.J.; Gorrell, R.J.; Tafreshi, M.; Hatakeyama, M.; Kwok, T.; Price, J.T. The Helicobacter pylori cytotoxin CagA is essential for suppressing host heat shock protein expression. Cell Stress Chaperones 2016, 21, 523–533. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pitruzzella, A.; Burgio, S.; Lo Presti, P.; Ingrao, S.; Fucarino, A.; Bucchieri, F.; Cabibi, D.; Cappello, F.; Conway de Macario, E.; Macario, A.J.L.; et al. Hsp60 Quantification in Human Gastric Mucosa Shows Differences between Pathologies with Various Degrees of Proliferation and Malignancy Grade. Appl. Sci. 2021, 11, 3582. https://doi.org/10.3390/app11083582
Pitruzzella A, Burgio S, Lo Presti P, Ingrao S, Fucarino A, Bucchieri F, Cabibi D, Cappello F, Conway de Macario E, Macario AJL, et al. Hsp60 Quantification in Human Gastric Mucosa Shows Differences between Pathologies with Various Degrees of Proliferation and Malignancy Grade. Applied Sciences. 2021; 11(8):3582. https://doi.org/10.3390/app11083582
Chicago/Turabian StylePitruzzella, Alessandro, Stefano Burgio, Pietro Lo Presti, Sabrina Ingrao, Alberto Fucarino, Fabio Bucchieri, Daniela Cabibi, Francesco Cappello, Everly Conway de Macario, Alberto J. L. Macario, and et al. 2021. "Hsp60 Quantification in Human Gastric Mucosa Shows Differences between Pathologies with Various Degrees of Proliferation and Malignancy Grade" Applied Sciences 11, no. 8: 3582. https://doi.org/10.3390/app11083582
APA StylePitruzzella, A., Burgio, S., Lo Presti, P., Ingrao, S., Fucarino, A., Bucchieri, F., Cabibi, D., Cappello, F., Conway de Macario, E., Macario, A. J. L., David, S., & Rappa, F. (2021). Hsp60 Quantification in Human Gastric Mucosa Shows Differences between Pathologies with Various Degrees of Proliferation and Malignancy Grade. Applied Sciences, 11(8), 3582. https://doi.org/10.3390/app11083582









