Important Roles of Oligo- and Polysaccharides against SARS-CoV-2: Recent Advances
Abstract
:1. Introduction
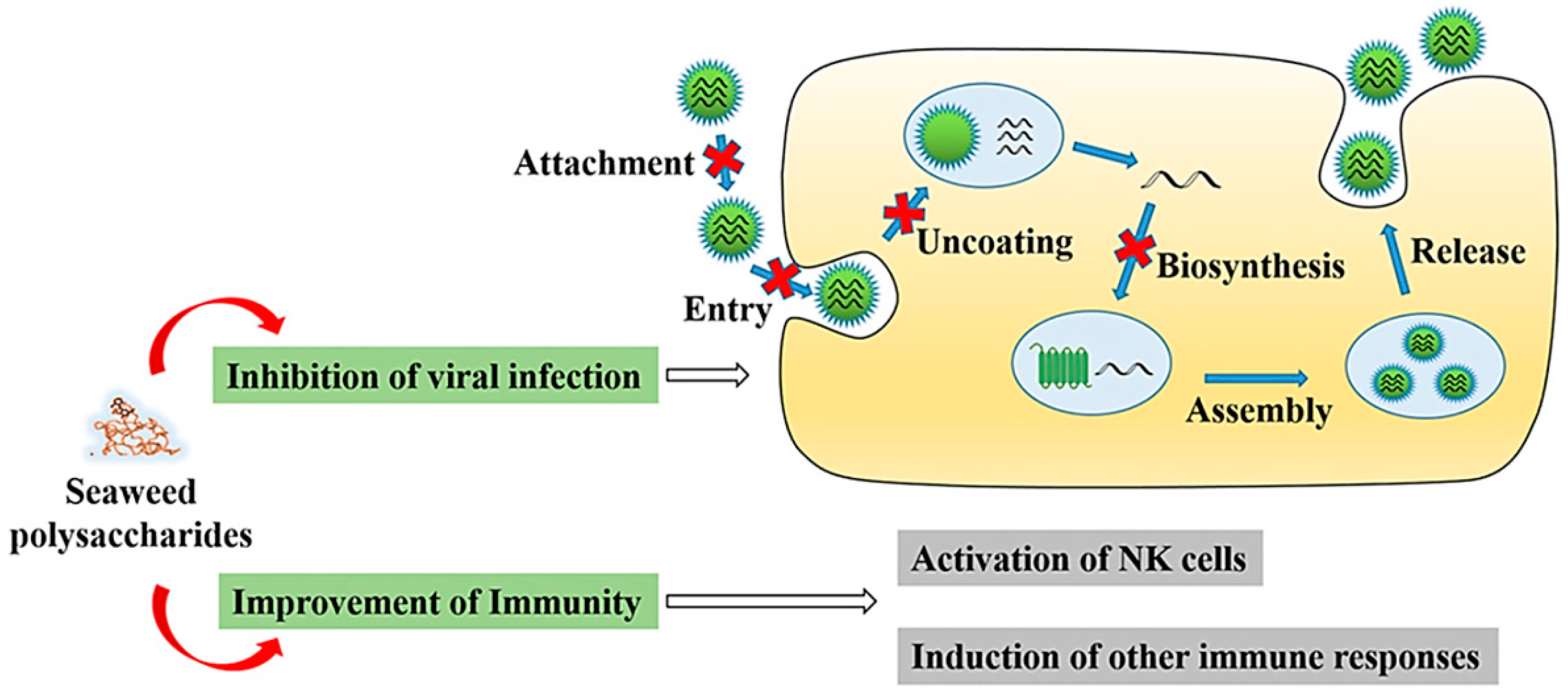
2. Oligo- and Polysaccharides against SARS-CoV-2
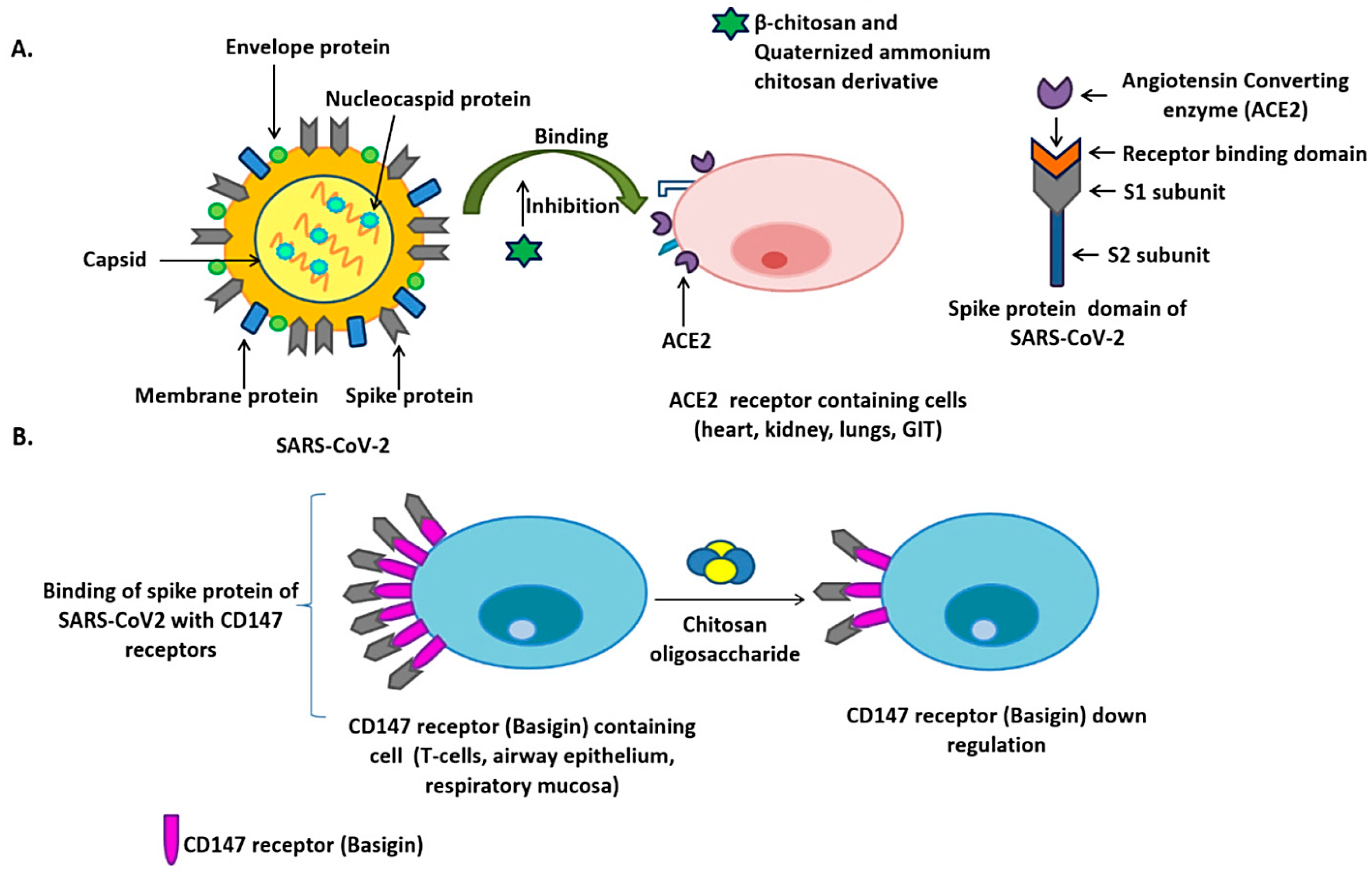
2.1. Chitosan
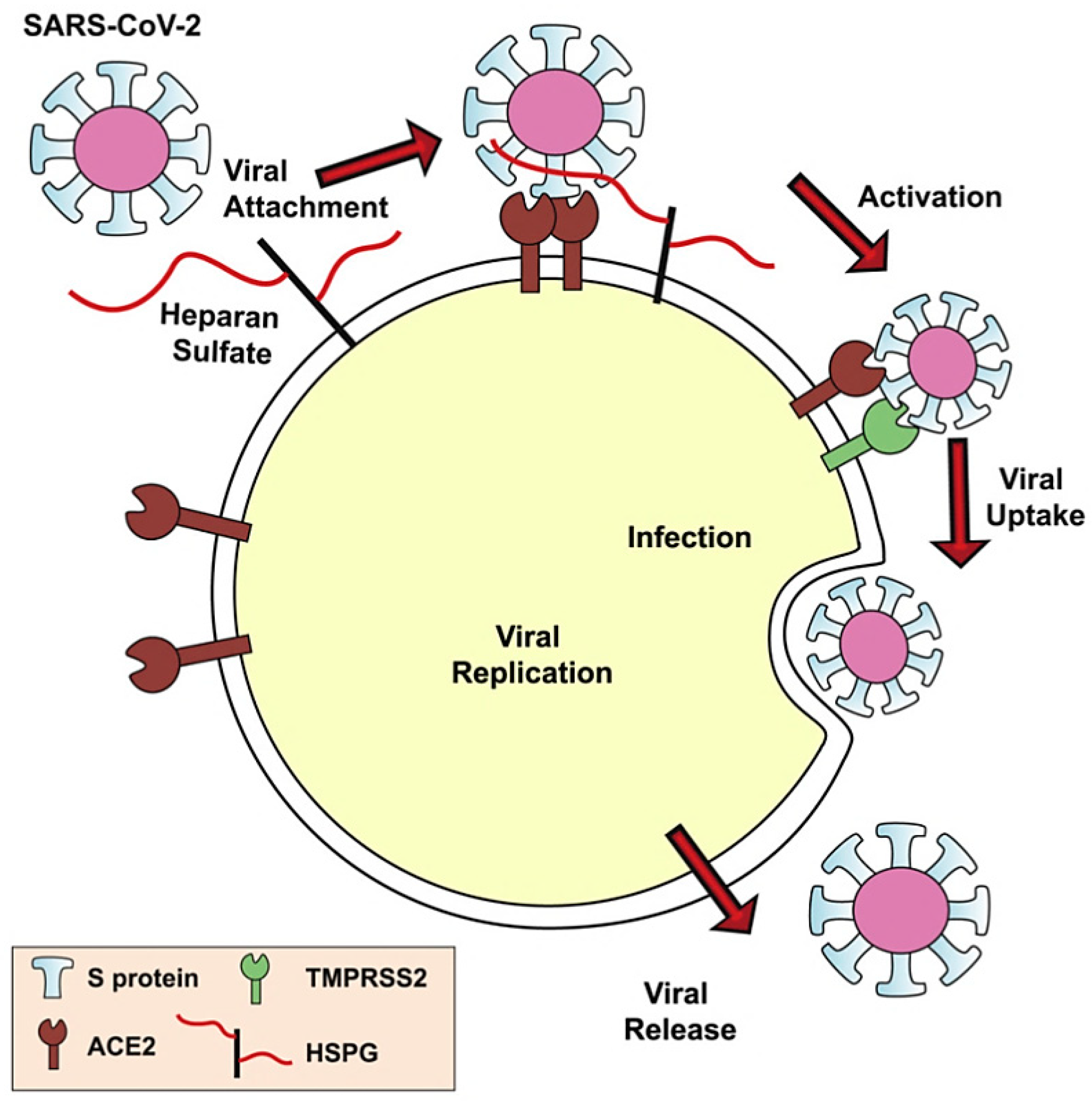
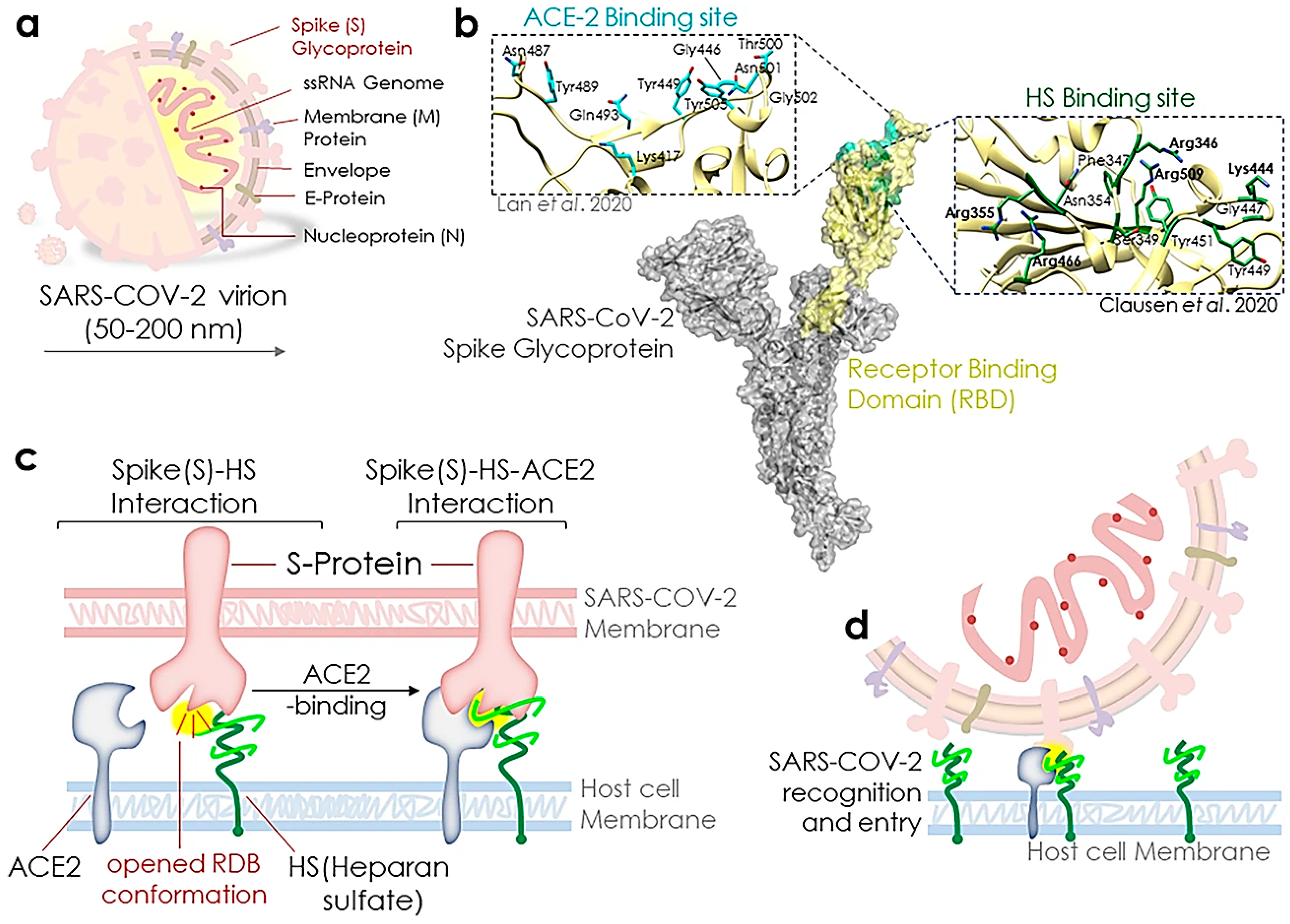
2.2. Heparin or Heparin-Like Materials
2.3. Carrageenan
2.4. Cyclodextrins
2.5. Cellulosic Materials
3. Conclusions and Future Perspectives
- (1)
- The identification of innovative formulations with antiviral properties and new delivery systems using polysaccharides with improved efficacy and low toxicity.
- (2)
- Research on antiviral oligosaccharides/polysaccharides and derivatives to find the relationship between the structure and related bioactivities; research on the utilization of these natural materials as suitable platforms for producing vaccines, as well as their application as co-adjuvants because of their capabilities for the simple binding to different receptor of the cells.
- (3)
- Understanding the effect and underlying mechanism implicated in the antiviral effects of oligosaccharides/polysaccharides and derivates; structure–activity relationship analyses and mechanisms involved in the anti-CoVs activity of oligo- and polysaccharides can provide insights into future research direction.
- (4)
- The clinical efficacy needs to be specifically probed to provide solutions; clinical trials regarding the application of oligo- and polysaccharides (e.g., in a formulation containing drugs) should be planned, comprehensively.
- (5)
- The functionalization of natural-based nanostructures and polymers should be analytically explored, as well as their therapeutic effects and biological activities; modern and innovative strategies/technologies for the effective extraction/isolation of native oligosaccharides/polysaccharides and related active ingredients from natural resources (e.g., biowastes, plants, and microorganisms) should be recognized.
- (6)
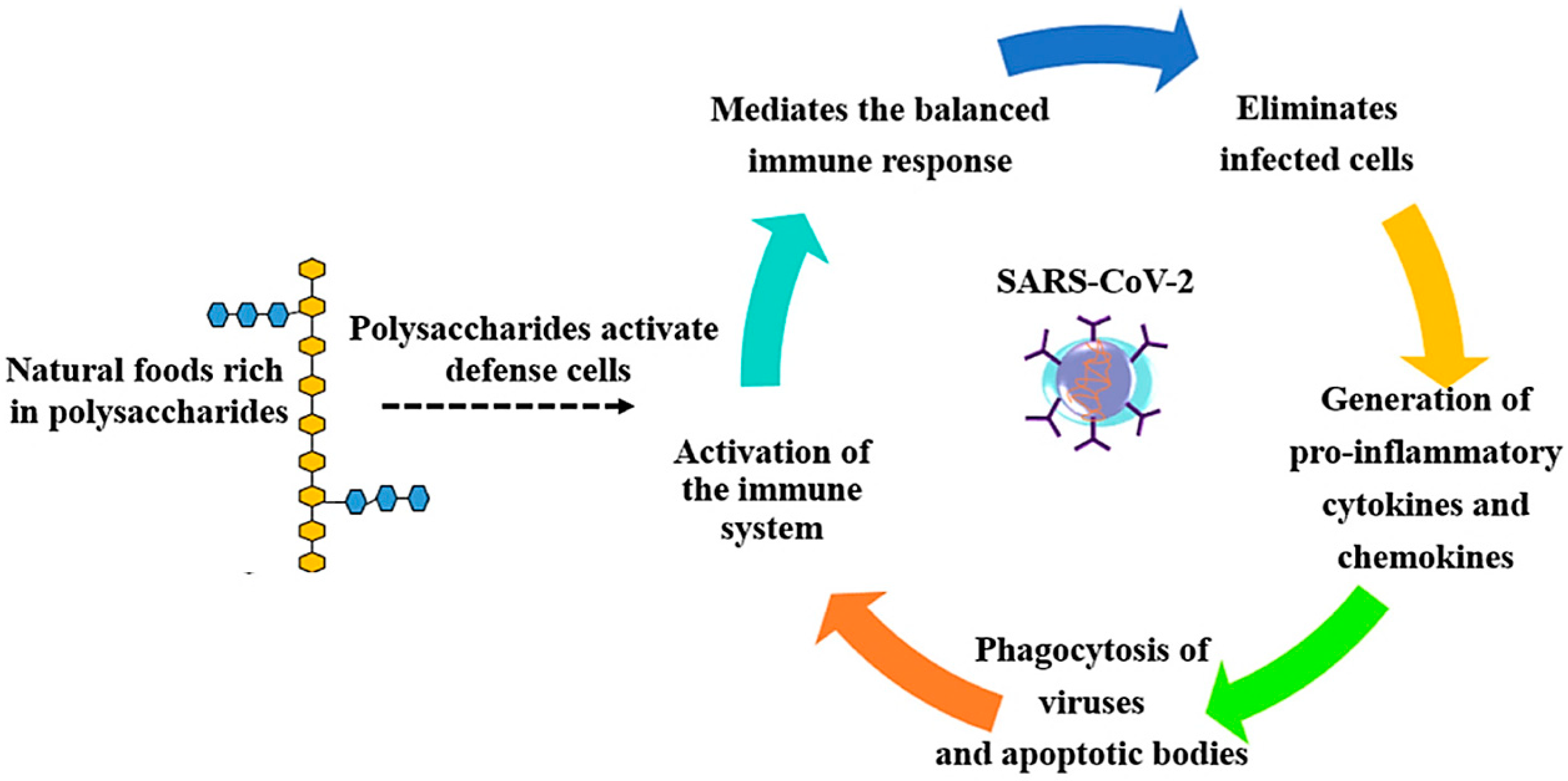
- Uncovering the related biochemical pathways of immune response modulation/activation by oligo- and polysaccharides, and the investigation to find effective ways to further stimulate/activate the immune systems, as the effective roles of polysaccharides for balancing the cytokines production and maintaining the hemostasis of cells have been illustrated. These studies should be focused on the effects of polysaccharides for modulating the immune systems, especially via cytokines (TNF-α and IL-6) release, increased phagocytosis of macrophages, production of nitrous oxide (NO), reactive oxygen species (ROS) formation, and signaling pathways activation (e.g., toll-like 4, type A hijacker receptor, NF-κB, and glucan receptor) [28,92].
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, S.; Hillyer, C.; Du, L. Neutralizing antibodies against SARS-CoV-2 and other human coronaviruses. Trends Immunol. 2020, 41, 355–359. [Google Scholar] [CrossRef]
- Jamalipour Soufi, G.; Hekmatnia, A.; Nasrollahzadeh, M.; Shafiei, N.; Sajjadi, M.; Iravani, P.; Fallah, S.; Iravani, S.; Varma, R.S. SARS-CoV-2 (COVID-19): New Discoveries and Current Challenges. Appl. Sci. 2020, 10, 3641. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajjadi, M.; Jamalipour Soufi, G.; Iravani, S.; Varma, R.S. Nanomaterials and Nanotechnology-Associated Innovations against Viral Infections with a Focus on Coronaviruses. Nanomaterials 2020, 10, 1072. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, S.-X.; Guan, H.-S. The Antiviral Activities and Mechanisms of Marine Polysaccharides: An Overview. Mar. Drugs 2012, 10, 2795–2816. [Google Scholar] [CrossRef] [PubMed]
- Kwon, P.S.; Oh, H.; Kwon, S.-J.; Jin, W.; Zhang, F.; Fraser, K.; Hong, J.J.; Linhardt, R.J.; Dordick, J.S. Sulfated polysaccharides effectively inhibit SARS-CoV-2 in vitro. Cell Discov. 2020, 6, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Tahir Khan, M.; Ali, A.; Wang, Q.; Irfan, M.; Khan, A.; Tariq Zeb, M.; Zhang, Y.-J.; Chinnasamy, S.; Wei, D.-Q. Marine natural compounds as potents inhibitors against the main protease of SARS-CoV-2—a molecular dynamic study. J. Biomol. Struct. Dyn. 2020. [Google Scholar] [CrossRef]
- de Gomes Sousa Cardozo, F.T.; Camelini, C.M.; Mascarello, A.; Rossi, M.J.; Nunes, R.J.; Barardi, C.R.M.; Mendonça, M.M.d.; Simões, C.M.O. Antiherpetic activity of a sulfated polysaccharide from Agaricus brasiliensis mycelia. Antivir. Res. 2011, 92, 108–114. [Google Scholar] [CrossRef] [Green Version]
- Shi, Q.; Wang, A.; Lu, Z.; Qin, C.; Hu, J.; Yin, J. Overview on the antiviral activities and mechanisms of marine polysaccharides from seaweeds. Carbohydr. Res. 2017, 453–454, 1–9. [Google Scholar] [CrossRef]
- Chen, L.; Huang, G. The antiviral activity of polysaccharides and their derivatives. Int. J. Biol. Macromol. 2018, 115, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Jinno, A.; Park, P.W. Role of Glycosaminoglycans in Infectious Disease. Methods Mol. Biol. 2015, 1229, 567–585. [Google Scholar]
- Mulloy, B. The specificity of interactions between proteins and sulfated polysaccharides. An. Acad. Bras. Cienc. 2005, 77, 651. [Google Scholar] [CrossRef] [Green Version]
- Talarico, L.B.; Pujol, C.A.; Zibetti, R.G.M.; Faría, P.C.S.; Noseda, M.D.; Duarte, M.E.R.; Damonte, E.B. The antiviral activity of sulfated polysaccharides against dengue virus is dependent on virus serotype and host cell. Antivir. Res. 2005, 66, 103–110. [Google Scholar] [CrossRef]
- Rahman, O.u.; Shi, S.; Ding, J.; Donglin, W.; Ahmad, S.; Yu, H. Lignin nanoparticles: Synthesis, characterization and their corrosion protection performance. New J. Chem. 2018, 42, 3415–3425. [Google Scholar] [CrossRef]
- Lievonen, M.; Valle-Delgado, J.J.; Mattinen, M.-L.; Hult, E.-L.; Lintinen, K.; Kostiainen, M.A.; Paananen, A.; Szilvay, G.R.; Setälä, H.; Österberg, M. A simple process for lignin nanoparticle preparation. Green Chem. 2016, 18, 1416–1422. [Google Scholar] [CrossRef] [Green Version]
- Myint, A.A.; Lee, H.W.; Seo, B.; Son, W.-S.; Yoon, J.; Yoon, T.J.; Park, H.J.; Yu, J.; Yoon, J.; Lee, Y.-W. One pot synthesis of environmentally friendly lignin nanoparticles with compressed liquid carbon dioxide as an antisolvent. Green Chem. 2016, 18, 2129–2146. [Google Scholar] [CrossRef]
- Chen, X.; Han, W.; Wang, G.; Zhao, X. Application prospect of polysaccharides in the development of anti-novel coronavirus drugs and vaccines. Int. J. Biol. Macromol. 2020, 164, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Pujol, C.A.; Ray, S.; Ray, B.; Damonte, E.B. Antiviral activity against dengue virus of diverse classes of algal sulfated polysaccharides. Int. J. Biol. Macromol. 2012, 51, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Ciejka, J.; Botwina, P.; Nowakowska, M.; Szczubialka, K.; Pyrc, K. Synthetic sulfonated derivatives of poly (allylamine hydrochloride) as inhibitors of human metapneumovirus. PLoS ONE 2019, 14, e0214646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagle, V.; Gaikwad, M.; Pawar, Y.; Dasgupta, S. Marine Red Alga Porphyridium sp. as a Source of Sulfated Polysaccharides (SPs) for Combating Against COVID-19. Preprints 2020, 2020040168, 1–18. [Google Scholar]
- Zhao, P.; Praissman, J.L.; Grant, O.C.; Cai, Y.; Xiao, T.; Rosenbalm, K.E.; Aoki, K.; Kellman, B.P.; Bridger, R.; Barouch, D.H.; et al. Virus-Receptor Interactions of Glycosylated SARS-CoV-2 Spike and Human ACE2 Receptor. Cell Host Microbe 2020, 28, 586–601. [Google Scholar] [CrossRef]
- Gupta, R.K.; Apte, G.R.; Lokhande, K.B.; Mishra, S.; Pal, J.K. Carbohydrate-Binding Agents: Potential of Repurposing for COVID-19 Therapy. Curr. Protein Pept. Sci. 2020, 21, 1085–1096. [Google Scholar] [CrossRef] [PubMed]
- Vankadari, N.; Wilce, J.A. Emerging WuHan (COVID-19) coronavirus: Glycan shield and structure prediction of spike glycoprotein and its interaction with human CD26. Emerg. Microbes Infect. 2020, 9, 601–604. [Google Scholar] [CrossRef]
- Raman, R.; Tharakaraman, K.; Sasisekharan, V.; Sasisekharan, R. Glycan–protein interactions in viral pathogenesis. Curr. Opin. Struct. Biol. 2016, 40, 153–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinha, V.R.; Kumria, R. Polysaccharides in colon-specific drug delivery. Int. J. Pharm. 2001, 224, 19–38. [Google Scholar] [CrossRef]
- Baran, T.; Nasrollahzadeh, M. Cyanation of aryl halides and Suzuki-Miyaura coupling reaction using palladium nanoparticles anchored on developed biodegradable microbeads. Int. J. Biol. Macromol. 2020, 148, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Hebbalalu, D.; Lalley, J.; Nadagouda, M.N.; Varma, R.S. Greener techniques for the synthesis of silver nanoparticles using plant extracts, enzymes, bacteria, biodegradable polymers, and microwaves. ACS Sustain. Chem. Eng. 2013, 1, 703–712. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Shafiei, N.; Nezafat, Z.; Bidgoli, N.S.S.; Soleimani, F. Recent progresses in the application of cellulose, starch, alginate, gum, pectin, chitin and chitosan based (nano) catalysts in sustainable and selective oxidation reactions: A review. Carbohydr. Polym. 2020, 241, 116353. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, J.R.; Junior, R.N.d.C. Polysaccharides obtained from natural edible sources and their role in modulating the immune system: Biologically active potential that can be exploited against COVID-19. Trends Food Sci. Technol. 2021, 108, 223–235. [Google Scholar] [CrossRef]
- Bemiller, J.N. Carbohydrates. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J. Adv. Res. 2016, 7, 17–28. [Google Scholar] [CrossRef] [Green Version]
- Kaliaraj, G.S.; Subramaniyan, B.; Manivasagan, P.; Kim, S.-K. Green synthesis of metal nanoparticles using seaweed polysaccharides. In Seaweed Polysaccharides; Elsevier: Amsterdam, The Netherlands, 2017; pp. 101–109. [Google Scholar]
- Baglivo, M.; Baronio, M.; Natalini, G.; Beccari, T.; Chiurazzi, P.; Fulcheri, E.; Petralia, P.P.; Michelini, S.; Fiorentini, G.; Miggiano, G.A.; et al. Natural small molecules as inhibitors of coronavirus lipid-dependent attachment to host cells: A possible strategy for reducing SARS-COV-2 infectivity? Acta Biomed. 2020, 91, 161–164. [Google Scholar]
- Garrido, P.F.; Calvelo, M.; Blanco-González, A.; Veleiro, U.; Suárez, F.; Conde, D.; Cabezón, A.; Piñeiro, Á.; Garcia-Fandino, R. The Lord of the NanoRings: Cyclodextrins and the battle against SARS-CoV-2. Int. J. Pharm. 2020, 588, 119689. [Google Scholar] [CrossRef] [PubMed]
- Das, G.; Ghosh, S.; Garg, S.; Ghosh, S.; Jana, A.; Samat, R.; Mukherjee, N.; Roya, R.; Ghosh, S. An overview of key potential therapeutic strategies for combat in the COVID-19 battle. RSC Adv. 2020, 10, 28243–28266. [Google Scholar] [CrossRef]
- Song, S.; Peng, H.; Wang, Q.; Liu, Z.; Dong, X.; Wen, C.; Ai, C.; Zhang, Y.; Wang, Z.; Zhu, B. Inhibitory activities of marine sulfated polysaccharides against SARS-CoV-2. Food Funct. 2020, 11, 7415–7420. [Google Scholar] [CrossRef] [PubMed]
- Kalinina, T.S.; Zlenko, D.V.; Kiselev, A.V.; Litvin, A.A.; Stovbun, S.V. Antiviral activity of the high-molecular-weight plant polysaccharides (Panavir®). Int. J. Biol. Macromol. 2020, 161, 936–938. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Pei, R.; Li, M.; Sun, H.; Su, M.; Ding, Y.; Chen, X.; Du, Z.; Jin, C.; Huang, C.; et al. Structural characterization of cocktail-like targeting polysaccharides from Ecklonia kurome Okam and their anti-SARS-CoV-2 activities in vitro. bioRxiv 2021. [Google Scholar] [CrossRef]
- Jin, W.; Zhang, W.; Mitra, D.; McCandless, M.G.; Sharma, P.; Tandon, R.; Zhang, F.; Linhardt, R.J. The structure-activity relationship of the interactions of SARS-CoV-2 spike glycoproteins with glucuronomannan and sulfated galactofucan from Saccharina japonica. Int. J. Biol. Macromol. 2020, 163, 1649–1658. [Google Scholar] [CrossRef]
- Xu, C.; Nasrollahzadeh, M.; Sajjadi, M.; Maham, M.; Luque, R.; Puente-Santiago, A.R. Benign-by-design nature-inspired nanosystems in biofuels production and catalytic applications. Renew. Sustain. Energy Rev. 2019, 112, 195–252. [Google Scholar] [CrossRef]
- Xu, C.; Nasrollahzadeh, M.; Selva, M.; Issaabadi, Z.; Luque, R. Waste-to-wealth: Biowaste valorization into valuable bio (nano) materials. Chem. Soc. Rev. 2019, 48, 4791–4822. [Google Scholar] [CrossRef]
- Peniche, H.; Peniche, C. Chitosan nanoparticles: A contribution to nanomedicine. Polym. Int. 2011, 60, 883–889. [Google Scholar] [CrossRef]
- Alarcón, B.; Lacal, J.C.; Fernández-Sousa, J.; Carrasco, L. Screening for new compounds with antiherpes activity. Antivir. Res. 1984, 4, 231–244. [Google Scholar] [CrossRef]
- Qin, C.; Li, H.; Xiao, Q.; Liu, Y.; Zhu, J.; Du, Y. Water-solubility of chitosan and its antimicrobial activity. Carbohydr. Polym. 2006, 63, 367–374. [Google Scholar] [CrossRef]
- Doostmohammadi, M.; Ameri, A.; Mohammadinejad, R.; Dehghannoudeh, N.; Banat, I.M.; Ohadi, M.; Dehghannoudeh, G. Hydrogels for peptide hormones delivery: Therapeutic and tissue engineering applications. Drug Des. Devel. Ther. 2019, 13, 3405–3418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marón, L.B.; Covas, C.P.; Da Silveira, N.P.; Pohlmann, A.; Mertins, O.; Tatsuo, L.N.; Sant’ Anna, O.A.B.; Moro, A.M.; Takata, C.S.; De Araujo, P.S. LUVs recovered with chitosan: A new preparation for vaccine delivery. J. Liposome Res. 2007, 17, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Alinejad, Y.; Adoungotchodo, A.; Hui, E.; Zehtabi, F.; Lerouge, S. An injectable chitosan/chondroitin sulfate hydrogel with tunable mechanical properties for cell therapy/tissue engineering. Int. J. Biol. Macromol. 2018, 113, 132–141. [Google Scholar] [CrossRef]
- Intini, C.; Elviri, L.; Cabral, J.; Mros, S.; Bergonzi, C.; Bianchera, A.; Flammini, L.; Govoni, P.; Barocelli, E.; Bettini, R. 3D-printed chitosan-based scaffolds: An in vitro study of human skin cell growth and an in-vivo wound healing evaluation in experimental diabetes in rats. Carbohydr. Polym. 2018, 199, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Wieckiewicz, M.; W Boening, K.; Grychowska, N.; Paradowska-Stolarz, A. Clinical application of chitosan in dental specialities. Mini Rev. Med. Chem. 2017, 17, 401–409. [Google Scholar] [CrossRef]
- Abasi, S.; Aggas, J.R.; Guiseppi-Elie, A. Physiochemical and morphological dependent growth of NIH/3T3 and PC-12 on polyaniline-chloride/chitosan bionanocomposites. Mater. Sci. Eng. C 2019, 99, 1304–1312. [Google Scholar] [CrossRef] [PubMed]
- Nadimi, A.E.; Ebrahimipour, S.Y.; Afshar, E.G.; Falahati-Pour, S.K.; Ahmadi, Z.; Mohammadinejad, R.; Mohamadi, M. Nano-scale drug delivery systems for antiarrhythmic agents. Eur. J. Med. Chem. 2018, 157, 1153–1163. [Google Scholar] [CrossRef] [PubMed]
- Mu, M.; Li, X.; Tong, A.; Guo, G. Multi-functional chitosan-based smart hydrogels mediated biomedical application. Expert Opin. Drug Deliv. 2019, 16, 239–250. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Ahmadi, Z.; Mohamadi, N.; Zarrabi, A.; Abasi, S.; Dehghannoudeh, G.; Tamaddondoust, R.N.; Khanbabaei, H.; Mohammadinejad, R.; Thakur, V.K. Chitosan-based advanced materials for docetaxel and paclitaxel delivery: Recent advances and future directions in cancer theranostics. Int. J. Biol. Macromol. 2020, 145, 282–300. [Google Scholar] [CrossRef] [PubMed]
- Yhee, J.Y.; Son, S.; Kim, S.H.; Park, K.; Choi, K.; Kwon, I.C. Self-assembled glycol chitosan nanoparticles for disease-specific theranostics. J. Control. Release 2014, 193, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Swierczewska, M.; Han, H.S.; Kim, K.; Park, J.H.; Lee, S. Polysaccharide-based nanoparticles for theranostic nanomedicine. Adv. Drug Deliv. Rev. 2016, 99, 70–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loutfy, S.A.; Elberry, M.H.; Farroh, K.Y.; Mohamed, H.T.; Mohamed, A.A.; Mohamed, E.B.; Faraag, A.H.I.; Mousa, S.A. Antiviral Activity of Chitosan Nanoparticles Encapsulating Curcumin Against Hepatitis C Virus Genotype 4a in Human Hepatoma Cell Lines. Int. J. Nanomed. 2020, 15, 2699–2715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, M.; Qu, D.; Wang, H.; Sun, Z.; Liu, X.; Chen, J.; Li, C.; Li, X.; Chen, Z. Intranasal Administration of Chitosan Against Influenza A (H7N9) Virus Infection in a Mouse Model. Sci. Rep. 2016, 6, 28729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Ghananeem, A.M.; Saeed, H.; Florence, R.; Yokel, R.A.; Malkawi, A.H. Intranasal drug delivery of didanosine-loaded chitosan nanoparticles for brain targeting; an attractive route against infections caused by aids viruses. J. Drug Target. 2010, 18, 381–388. [Google Scholar] [CrossRef]
- Alitongbieke, G.; Li, X.-m.; Wu, Q.-C.; Lin, Z.-C.; Huang, J.-F.; Xue, Y.; Liu, J.-N.; Lin, J.-M.; Pan, T.; Chen, Y.-X.; et al. Study on β-Chitosan against the binding of SARS-CoV-2S-RBD/ACE2. bioRxiv 2020. [Google Scholar] [CrossRef]
- Sharma, N.; Modak, C.; Singh, P.K.; Kumar, R.; Khatri, D.; Singh, S.B. Underscoring the immense potential of chitosan in fighting a wide spectrum of viruses: A plausible molecule against SARS-CoV-2? Int. J. Biol. Macromol. 2021, 179, 33–44. [Google Scholar] [CrossRef]
- van Haren, F.M.P.; Page, C.; Laffey, J.G.; Artigas, A.; Camprubi-Rimblas, M.; Nunes, Q.; Smith, R.; Shute, J.; Carroll, M.; Tree, J.; et al. Nebulised heparin as a treatment for COVID-19: Scientific rationale and a call for randomised evidence. Crit. Care 2020, 24, 454. [Google Scholar] [CrossRef]
- Tandon, R.; Sharp, J.S.; Zhang, F.; Pomin, V.H.; Ashpole, N.M.; Mitra, D.; McCandless, M.G.; Jin, W.; Liu, H.; Sharma, P.; et al. Effective Inhibition of SARS-CoV-2 Entry by Heparin and Enoxaparin Derivatives. J. Virol. 2021, 95, e01987-20. [Google Scholar]
- Kim, S.Y.; Jin, W.; Sood, A.; Montgomery, D.W.; Grant, O.C.; Fuster, M.M.; Fu, L.; Dordick, J.S.; Woods, R.J.; Zhang, F.; et al. Characterization of heparin and severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) spike glycoprotein binding interactions. Antivir. Res. 2020, 181, 104873. [Google Scholar] [CrossRef]
- Mycroft-West, C.J.; Su, D.; Pagani, I.; Rudd, T.R.; Elli, S.; Guimond, S.E.; Miller, G.; Meneghetti, M.C.Z.; Nader, H.B.; Li, Y.; et al. Heparin inhibits cellular invasion by SARS-CoV-2: Structural dependence of the interaction of the surface protein (spike) S1 receptor binding domain with heparin. Thromb. Haemost. 2020, 120, 1700–1715. [Google Scholar] [PubMed]
- Cagno, V.; Andreozzi, P.; D’Alicarnasso, M.; Jacob Silva, P.; Mueller, M.; Galloux, M.; Goffic, R.L.; Jones, S.T.; Vallino, M.; Hodek, J.; et al. Broad-spectrum non-toxic antiviral nanoparticles with a virucidal inhibition mechanism. Nat. Mater. 2017, 17, 195–203. [Google Scholar] [CrossRef]
- Clausen, T.M.; Sandoval, D.R.; Spliid, C.B.; Pihl, J.; Perrett, H.R.; Painter, C.D.; Narayanan, A.; Majowicz, S.A.; Kwong, E.M.; McVicar, R.N.; et al. SARS-CoV-2 Infection Depends on Cellular Heparan Sulfate and ACE2. Cell 2020, 183, 1043–1057.e1015. [Google Scholar] [CrossRef] [PubMed]
- Kalra, R.S.; Kandimalla, R. Engaging the spikes: Heparan sulfate facilitates SARS-CoV-2 spike protein binding to ACE2 and potentiates viral infection. Signal Transduct. Target. Ther. 2021, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Coviello, T.; Matricardi, P.; Marianecci, C.; Alhaique, F. Polysaccharide hydrogels for modified release formulations. J. Control. Release 2007, 119, 5–24. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhan, X.; Wan, J.; Wang, Y.; Wang, C. Review for carrageenan-based pharmaceutical biomaterials: Favourable physical features versus adverse biological effects. Carbohydr. Polym. 2015, 121, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Bonferoni, M.C.; Chetoni, P.; Giunchedi, P.; Rossi, S.; Ferrari, F.; Burgalassi, S.; Caramella, C. Carrageenan-gelatin mucoadhesive systems for ion-exchange based ophthalmic delivery: In vitro and preliminary in vivo studies. Eur. J. Pharm. Biopharm. 2004, 57, 465–472. [Google Scholar] [CrossRef]
- Zhang, Y.-Q.; Tsai, Y.-C.; Monie, A.; Hung, C.-F.; Wu, T.C. Carrageenan as an adjuvant to enhance peptide-based vaccine potency. Vaccine 2010, 28, 5212–5219. [Google Scholar] [CrossRef] [Green Version]
- Kalitnik, A.A.; Byankina Barabanova, A.O.; Nagorskaya, V.P.; Reunov, A.V.; Glazunov, V.P.; Solov’eva, T.F.; Yermak, I.M. Low molecular weight derivatives of different carrageenan types and their antiviral activity. J. Appl. Phycol. 2013, 25, 65–72. [Google Scholar] [CrossRef]
- Morokutti-Kurz, M.; Graf, C.; Prieschl-Grassauer, E. Amylmetacresol/2,4-dichlorobenzyl alcohol, hexylresorcinol, or carrageenan lozenges as active treatments for sore throat. Int. J. Gen. Med. 2017, 10, 53–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morokutti-Kurz, M.; Graf, P.; Grassauer, A.; Prieschl-Grassauer, E. SARS-CoV-2 in-vitro neutralization assay reveals inhibition of virus entry by iota-carrageenan. bioRxiv 2020. [Google Scholar] [CrossRef]
- Iravani, S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011, 13, 2638–2650. [Google Scholar] [CrossRef]
- Iravani, S.; Jamalipour Soufi, G. Gold Nanostructures in Medicine and Biology. In Nanoparticles in Medicine; Shukla, A.K., Ed.; Springer Nature: Singapore, 2019; pp. 175–183. [Google Scholar]
- Fan, Q. Polyolefin nanocomposite fibers and films. In Polyolefin Fibres, Industrial and Medical Applications; Series in Textiles; Woodhead Publishing: Cambridge, UK, 2009; pp. 341–362. [Google Scholar]
- Saokham, P.; Muankaew, C.; Jansook, P.; Loftsson, T. Solubility of Cyclodextrins and Drug/Cyclodextrin Complexes. Molecules 2018, 23, 1161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jambhekar, S.S.; Breen, P. Cyclodextrins in pharmaceutical formulations II: Solubilization, binding constant, and complexation efficiency. Drug Discov. Today 2016, 21, 1161. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, G.; Tiwari, R.; Rai, A.K. Cyclodextrins in delivery systems: Applications. J. Pharm. Bioallied Sci. 2010, 2, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Jarho, P.; Másson, M.; Järvinen, T. Cyclodextrins in drug delivery. Expert Opin. Drug Deliv. 2005, 2, 335–351. [Google Scholar] [CrossRef] [PubMed]
- Carrouel, F.; Conte, M.P.; Fisher, J.; Gonçalves, L.S.; Dussart, C.; Llodra, J.C.; Bourgeois, D. COVID-19: A Recommendation to Examine the Effect of Mouthrinses with β-Cyclodextrin Combined with Citrox in Preventing Infection and Progression. J. Clin. Med. 2020, 9, 1126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braga, S.S. Cyclodextrins: Emerging Medicines of the New Millennium. Biomolecules 2019, 9, 801. [Google Scholar] [CrossRef] [Green Version]
- Jones, S.T.; Cagno, V.; Janecek, M.; Ortiz, D.; Gasilova, N.; Piret, J.; Gasbarri, M.; Constant, D.A.; Han, Y.; Vukovi, L.; et al. Modified cyclodextrins as broad-spectrum antivirals. Sci. Adv. 2020, 6, eaax9318. [Google Scholar] [CrossRef] [Green Version]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsenf, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef]
- Mokhena, T.C.; John, M.J. Cellulose nanomaterials: New generation materials for solving global issues. Cellulose 2020, 27, 1149–1194. [Google Scholar] [CrossRef]
- Junter, G.-A.; Lebrun, L. Cellulose-based virus-retentive filters: A review. Rev. Environ. Sci. Biotechnol. 2017, 16, 455–489. [Google Scholar] [CrossRef] [PubMed]
- Trache, D.; Hussin, M.H.; Haafiz, M.M.; Thakur, V.K. Recent progress in cellulose nanocrystals: Sources and production. Nanoscale 2017, 9, 1763–1786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin-Martinez, F.J.; Jin, K.; López Barreiro, D.; Buehler, M.J. The Rise of Hierarchical Nanostructured Materials from Renewable Sources: Learning from Nature. ACS Nano 2018, 12, 7425–7433. [Google Scholar] [CrossRef]
- Sun, B.; Zhang, M.; Shen, J.; He, Z.; Fatehi, P.; Ni, Y. Applications of cellulose-based materials in sustained drug delivery systems. Curr. Med. Chem. 2019, 26, 2485–2501. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Manukyan, L.; Mantas, A.; Mihranyan, A. Nanocellulose-Based Nanoporous Filter Paper for Virus Removal Filtration of Human Intravenous Immunoglobulin. ACS Appl. Nano Mater. 2019, 2, 6352–6359. [Google Scholar] [CrossRef]
- Jorfi, M.; Foster, E.J. Recent advances in nanocellulose for biomedical applications. J. Appl. Polym. Sci. 2015, 132, 41719. [Google Scholar] [CrossRef]
- Ray, B.; Schütz, M.; Mukherjee, S.; Jana, S.; Ray, S.; Marschall, M. Exploiting the Amazing Diversity of Natural Source-Derived Polysaccharides: Modern Procedures of Isolation, Engineering, and Optimization of Antiviral Activities. Polymers 2021, 13, 136. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iravani, S.; Varma, R.S. Important Roles of Oligo- and Polysaccharides against SARS-CoV-2: Recent Advances. Appl. Sci. 2021, 11, 3512. https://doi.org/10.3390/app11083512
Iravani S, Varma RS. Important Roles of Oligo- and Polysaccharides against SARS-CoV-2: Recent Advances. Applied Sciences. 2021; 11(8):3512. https://doi.org/10.3390/app11083512
Chicago/Turabian StyleIravani, Siavash, and Rajender S. Varma. 2021. "Important Roles of Oligo- and Polysaccharides against SARS-CoV-2: Recent Advances" Applied Sciences 11, no. 8: 3512. https://doi.org/10.3390/app11083512
APA StyleIravani, S., & Varma, R. S. (2021). Important Roles of Oligo- and Polysaccharides against SARS-CoV-2: Recent Advances. Applied Sciences, 11(8), 3512. https://doi.org/10.3390/app11083512






