Antioxidant and Understanding the Anticancer Properties in Human Prostate and Breast Cancer Cell Lines of Chemically Characterized Methanol Extract from Berberis hispanica Boiss. & Reut
Abstract
1. Introduction
2. Material and Methods
2.1. Preparation of Plant Extract
2.2. Cell Cultures
2.3. In Vitro Antiproliferative Activity Assay
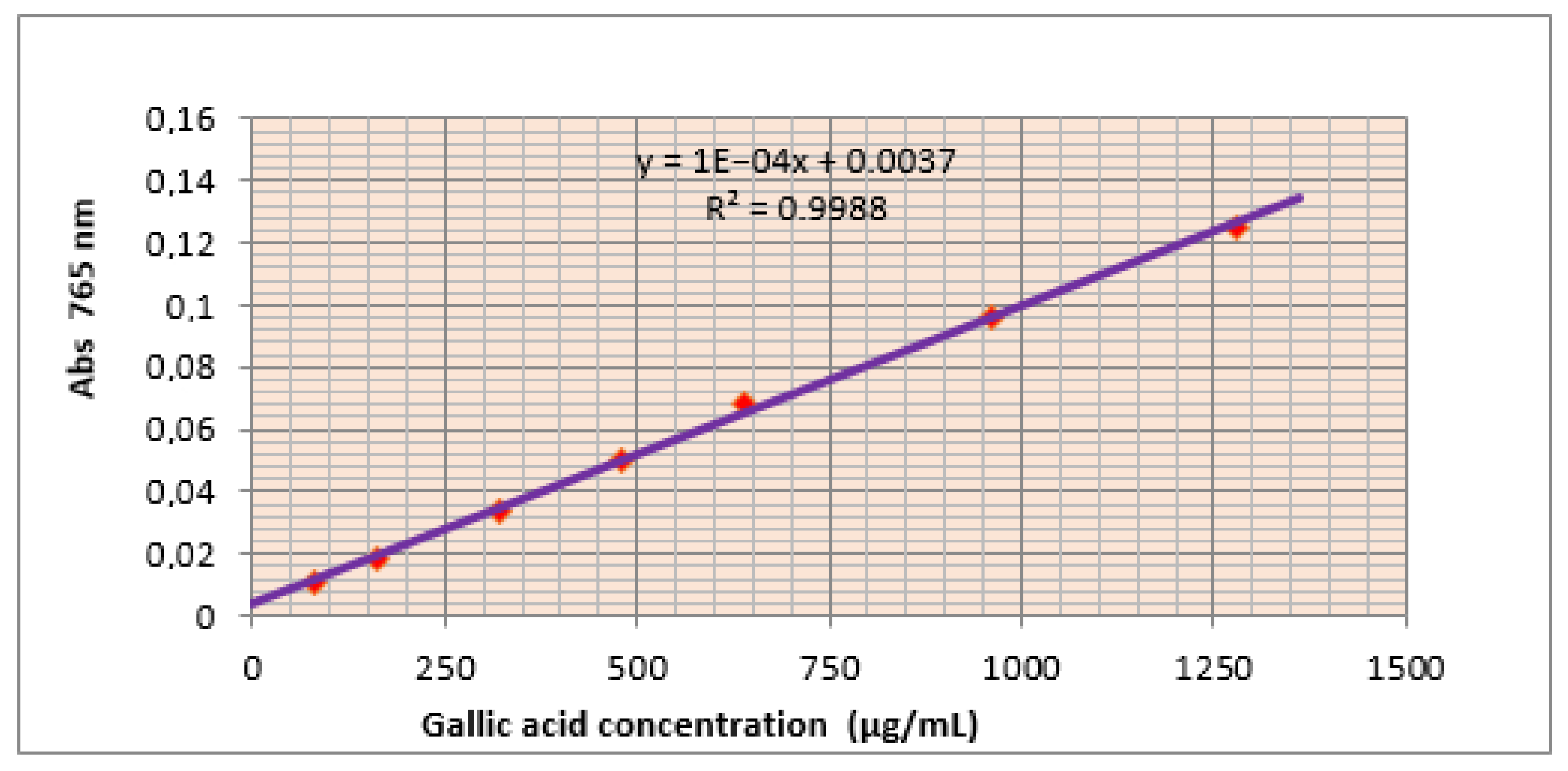
2.4. Determination of Phenolic Contents
2.5. Determination of Total Flavonoid Content
2.6. Evaluation of Antioxidant Activity
2.6.1. DPPH Radical Scavenging Assay
2.6.2. Ferric Reducing Antioxidant Power (FRAP) Assay
2.6.3. Trolox Equivalent Antioxidant Capacity (TEAC) Assay
2.7. Gas Chromatography-Mass Spectrometry Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Total Phenolic and Flavonoids Contents
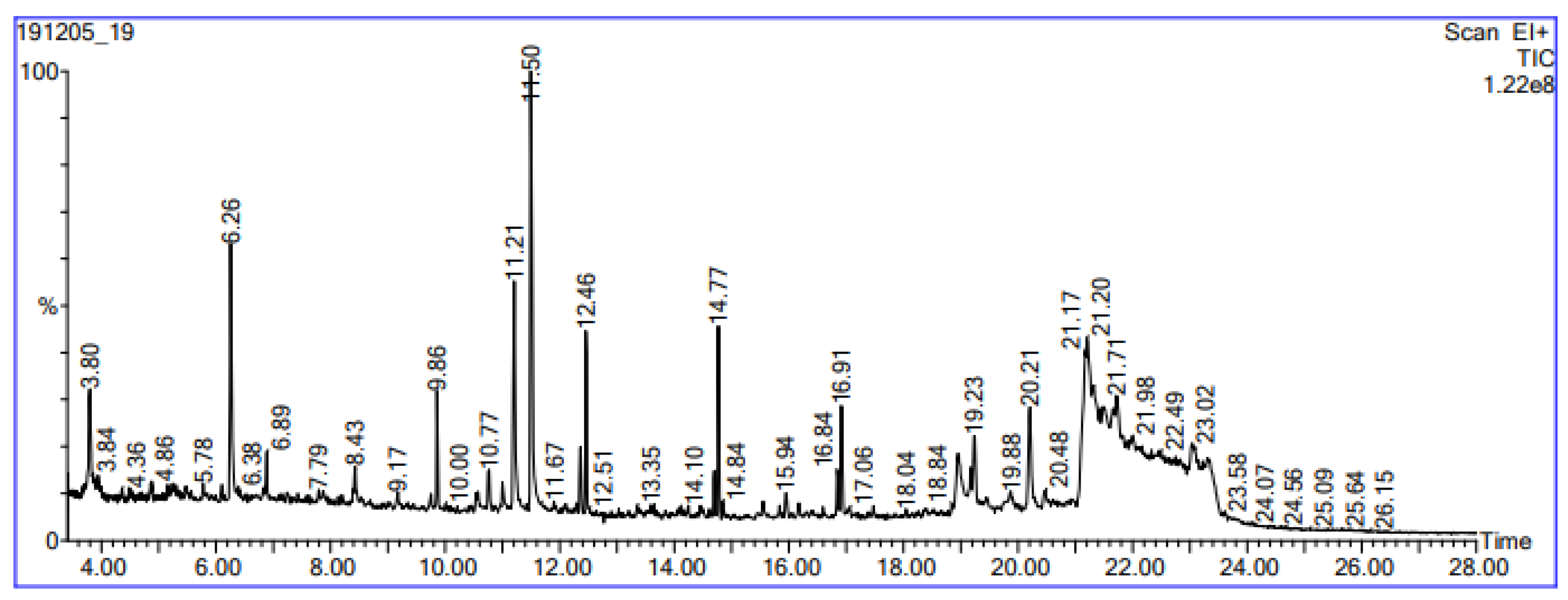
3.2. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
3.3. Antioxidant Activity
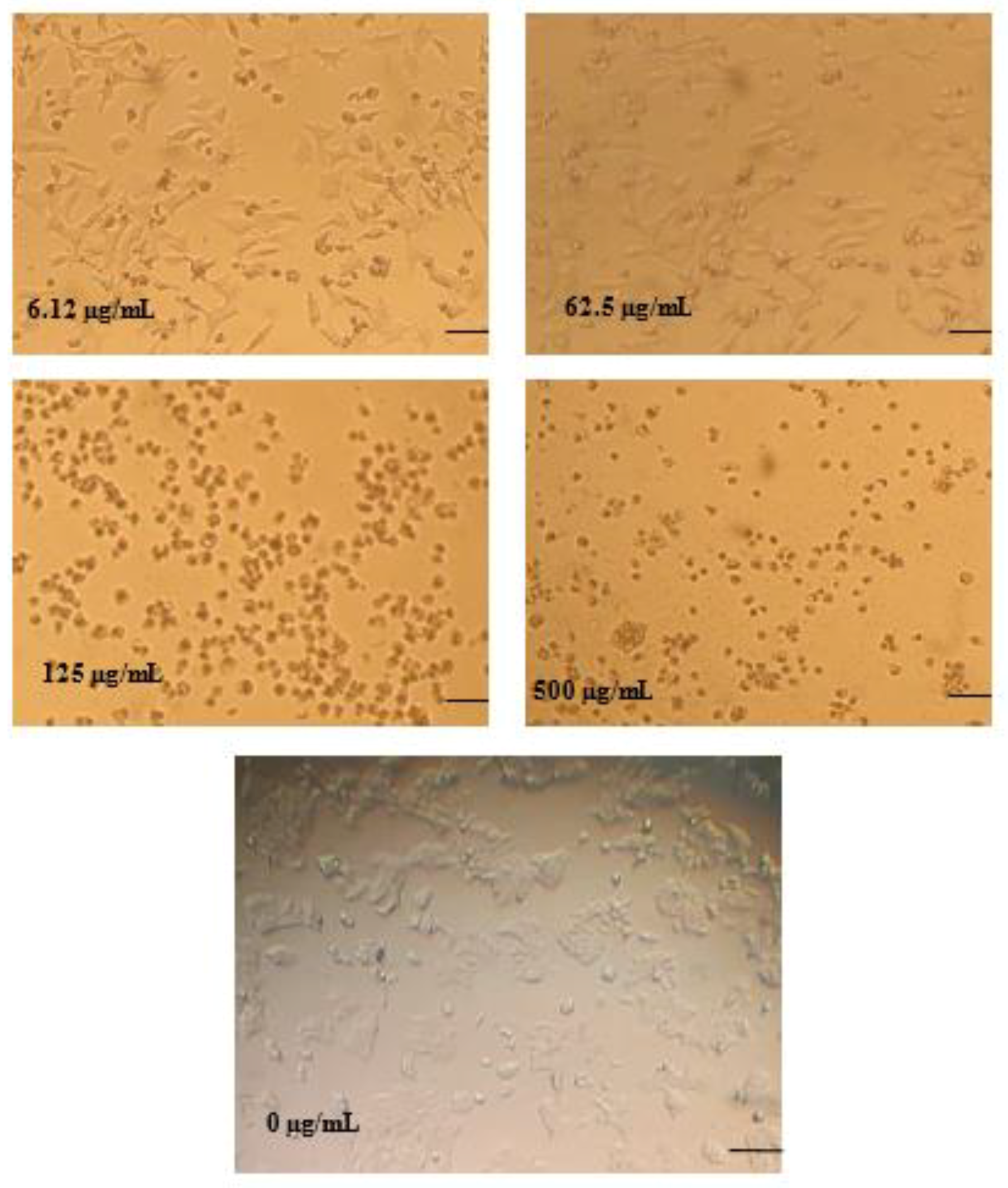
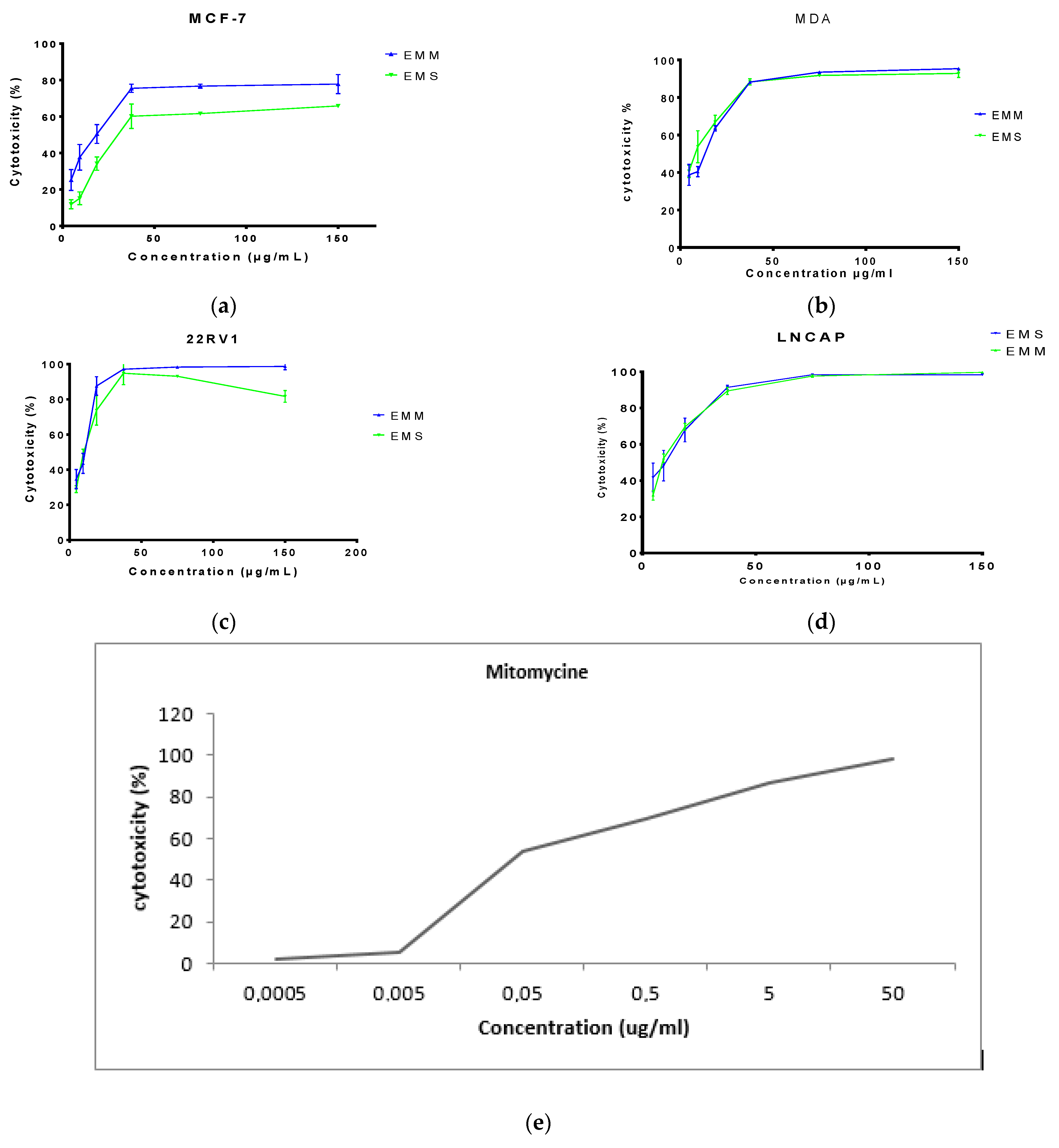
3.4. In Vitro Antiproliferative Activity Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, S.B.; Gupta, R. Drug Development from Natural Resource: A Systematic Approach. Mini-Rev. Med. Chem. 2015, 15, 52–57. [Google Scholar] [CrossRef]
- Bourhia, M.; Amrati, F.E.-Z.; Ullah, R.; Alqahtani, A.S.; Bousta, D.; Ibenmoussa, S.; Khlil, N. Coronavirus Treatments: What Drugs Might Work Against COVID-19? Nat. Prod. Commun. 2020, 15. [Google Scholar] [CrossRef]
- Ullah, R.; Alqahtani, A.S.; Noman, O.M.A.; Alqahtani, A.M.; Ibenmoussa, S.; Bourhia, M. A review on ethno-medicinal plants used in traditional medicine in the Kingdom of Saudi Arabia. Saudi Biol. Sci. 2020, 27, 2706–2718. [Google Scholar] [CrossRef] [PubMed]
- Bourhia, M.; Bari, A.; Ali, S.S.; Benbacer, L.; Khlil, N. Phytochemistry and toxicological assessment of Bryonia dioica roots used in north-African alternative medicine. Open Chem. 2019, 17, 1403–1411. [Google Scholar] [CrossRef]
- Amrati, F.E.-Z.; Bourhia, M.; Saghrouchni, H.; Slighoua, M.; Grafov, A.; Ullah, R.; Ezzeldin, E.; Mostafa, G.A.; Bari, A.; Ibenmoussa, S.; et al. Caralluma europaea (Guss.) N.E.Br.: Anti-Inflammatory, Antifungal, and Antibacterial Activities against Nosocomial Antibiotic-Resistant Microbes of Chemically Characterized Fractions. Molecules 2021, 26, 636. [Google Scholar] [CrossRef] [PubMed]
- Fatehi-Hassanabad, Z.; Jafarzadeh, M.; Tarhini, A.; Fatehi, M. The antihypertensive and vasodilator effects of aqueous extract from Berberis vulgaris fruit on hypertensive rats. Phytother. Res. 2005, 19, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, A.F.; Assenov, Y.; Martin-Subero, J.I.; Balint, B.; Siebert, R.; Taniguchi, H.; Yamamoto, H.; Hidalgo, M.; Tan, A.-C.; Galm, O.; et al. A DNA methylation fingerprint of 1628 human samples. Genome Res. 2012, 22, 407–419. [Google Scholar] [CrossRef]
- Mokhber-Dezfuli, N.; Saeidnia, S.; Gohari, A.R.; Kurepaz-Mahmoodabadi, M. Phytochemistry and pharmacology of berberis species. Pharmacogn. Rev. 2014, 8, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Bober, Z.; Stępień, A.; Aebisher, D.; Ożóg, L.; Bartusik-Aebisher, D. Fundamentals of the use of Berberis as a medicinal plant. Eur. J. Clin. Exp. Med. 2018, 16, 41–46. [Google Scholar] [CrossRef]
- Lemoui, R.; Benyahia, S.; Noman, L.; Bencherchar, I.; Oke-Altuntas, F.; Rebbas, K.; Benayache, S.; Benayache, F.; Demirtas, I. Isolation of phytoconstituents and evaluation of biological potentials of Berberis hispanica from Algeria. Bangladesh J. Pharm. 2018, 13, 179. [Google Scholar] [CrossRef]
- Youbi, A.E.H.E.; Bousta, D.; Ouahidi, I.; Aarab, L. Effets antidépresseurs, antinociceptifs et immunomodulateurs des extraits aqueux et protéique de Berberishispanica Boiss. & Reut. du Maroc. Phytothérapie 2011, 9, 25–32. [Google Scholar] [CrossRef]
- del Pilar Fernández-Poyatos, M.; Ruiz-Medina, A.; Salazar-Mendías, C.; Llorent-Martínez, E.J. Spectrophotometric determination of the antioxidant properties and characterization of the phenolic content by high-performance liquid chromatography–diode array detection–tandem mass spectrometry (HPLC–DAD–MS/MS) of Berberis hispanica Boiss. & Reut. leaves. Anal. Lett. 2020, 54, 646–657. [Google Scholar] [CrossRef]
- El Youbi, A. El H.; Bousta, D.; Jamoussi, B.; Greche, H.; El Mansouri, L.; Benjilali, J.; Soidrou, S.H. Activités antioxydante, apoptotique et antiproliférative de Tetraena gaetula (Emb. & Maire) Beier & Thulin et de Berberis hispanica Boiss. & Reut. originaires du Maroc. Phytothérapie 2012, 10, 151–160. [Google Scholar] [CrossRef]
- Sammar, M.; Abu‑Farich, B.; Rayan, I.; Falah, M.; Rayan, A. Correlation between cytotoxicity in cancer cells and free radical‑scavenging activity: In Vitro evaluation of 57 medicinal and edible plant extracts. Oncol. Lett. 2019, 18, 6563–6571. [Google Scholar] [CrossRef] [PubMed]
- Spanos, G.A.; Wrolstad, R.E. Influence of processing and storage on the phenolic composition of Thompson Seedless grape juice. J. Agric. Food Chem. 1990, 38, 1565–1571. [Google Scholar] [CrossRef]
- Dewanto, V.; Wu, X.; Liu, R.H. Processed Sweet Corn Has Higher Antioxidant Activity. J. Agric. Food Chem. 2002, 50, 4959–4964. [Google Scholar] [CrossRef] [PubMed]
- Sayah, K.; Marmouzi, I.; Naceiri Mrabti, H.; Cherrah, Y.; Faouzi, M.E.A. Antioxidant Activity and Inhibitory Potential of Cistus salviifolius (L.) and Cistus monspeliensis (L.) Aerial Parts Extracts against Key Enzymes Linked to Hyperglycemia. BioMed Res. Int. 2017, 2017, 2789482. [Google Scholar] [CrossRef]
- Tuberoso, C.I.G.; Boban, M.; Bifulco, E.; Budimir, D.; Pirisi, F.M. Antioxidant capacity and vasodilatory properties of Mediterranean food: The case of Cannonau wine, myrtle berries liqueur and strawberry-tree honey. Food Chem. 2013, 140, 686–691. [Google Scholar] [CrossRef]
- Boudjlida, A.; Kaci, S.; Karaki, S.; Benayad, T.; Rocchi, P.; Smati, D.; Bouguerra Aouichat, S. Berberis hispanica alkaloids extract induced cell death and apoptosis in human laryngeal cancer cells Hep-2. S. Afr. J. Bot. 2019, 125, 134–141. [Google Scholar] [CrossRef]
- Alonso-Castro, A.J.; Domínguez, F.; García-Carrancá, A. Rutin Exerts Antitumor Effects on Nude Mice Bearing SW480 Tumor. Arch. Med. Res. 2013, 44, 346–351. [Google Scholar] [CrossRef]
- Ben Sghaier, M.; Pagano, A.; Mousslim, M.; Ammari, Y.; Kovacic, H.; Luis, J. Rutin inhibits proliferation, attenuates superoxide production and decreases adhesion and migration of human cancerous cells. Biomed. Pharmacother. 2016, 84, 1972–1978. [Google Scholar] [CrossRef]
- Srinivasan, R.; Natarajan, D.; Shivakumar, M.S. In Vitro evaluation of antioxidant, antiproliferative potentials of bioactive extract-cum-rutin compound Isolated from Memecylon edule leaves and its molecular docking study. J. Biol. Act. Prod. Nat. 2016, 6, 43–58. [Google Scholar] [CrossRef]
- Kalinowska, M.; Sienkiewicz-Gromiuk, J.; Świderski, G.; Pietryczuk, A.; Cudowski, A.; Lewandowski, W. Zn(II) Complex of Plant Phenolic Chlorogenic Acid: Antioxidant, Antimicrobial and Structural Studies. Materials 2020, 13, 3745. [Google Scholar] [CrossRef]
- Liu, W.; Li, J.; Zhang, X.; Zu, Y.; Yang, Y.; Liu, W.; Xu, Z.; Gao, H.; Sun, X.; Jiang, X.; et al. Current Advances in Naturally Occurring Caffeoylquinic Acids: Structure, Bioactivity, and Synthesis. J. Agric. Food Chem. 2020, 68, 10489–10516. [Google Scholar] [CrossRef] [PubMed]
- Santana-Gálvez, J.; Villela Castrejón, J.; Serna-Saldívar, S.O.; Jacobo-Velázquez, D.A. Anticancer potential of dihydrocaffeic acid: A chlorogenic acid metabolite. CyTA J. Food 2020, 18, 245–248. [Google Scholar] [CrossRef]

| Methanol Extract | ||||
|---|---|---|---|---|
| Berberis Hispanica | TPC (mg GAE/g Extract) | TFC (mg RE/g Extract) | ||
| EMM | EMS | EMM | EMS | |
| 321.56 ± 3.05 | 289.02 ± 2.32 | 118.4 ± 2.24 | 98.4 ± 2.56 | |
| RT | Molecular Weight (g/mol) | Formula | Compound Name | Area (%) | |
|---|---|---|---|---|---|
| 1 | 3.80 | 100.16 | C6H12O | Hexanal | 4.542 |
| 2 | 3.84 | 100.16 | C6H12O | 2-Hexen-1-ol, (E) | 4.542 |
| 3 | 4.36 | 130.23 | C8H18O | 1-Heptanol, 3-methyl | 1.062 |
| 4 | 4.86 | 394.5 | C23H26N2O4 | N-(1-Hydroxy-4-oxo-1-phenylperhydroquinolizin-3-yl) carbamic acid, benzyl ester | 1.075 |
| 5 | 5.78 | 130.23 | C8H18O | 3,4-Dimethyl-2-hexano | 0.683 |
| 6 | 6.26 | 112.17 | C7H12O | 2-Heptenal, (Z) | 2.608 |
| 7 | 6.38 | 414.4 | C19H14F4O4S | Benzoic acid 3-methyl-4-(1,3,3,3-tetrafluoro-2-methoxycarbonyl-propenylsulfanyl)-phenyl ester | 0.250 |
| 8 | 6.89 | 184.36 | C13H28 | 2,5,6-Trimethyldecane | 0.344 |
| 9 | 7.79 | 142.2 | C7H15ClN2O | N-Methyl-3-piperidinecarboxamide | 0.250 |
| 10 | 8.43 | 210.4 | C15H30 | 2,4,6,8-Tetramethyl-1-undecene | 0.552 |
| 11 | 9.17 | 186.33 | C12H26O | 1-Octanol, 2-butyl- | 0.552 |
| 12 | 9.86 | 170.33 | C12H26 | Dodecane | 0.698 |
| 13 | 10.0 | 166.26 | C11H18O | 1-Oxaspiro [2.2]pentane,5-isopropylidene-2,2,4,4-tetramethyl | 0.344 |
| 14 | 10.77 | 154.25 | C10H18O | 2-Decenal, (Z) | 0.438 |
| 15 | 11.21 | 152.23 | C10H16O | 2,4-Decadiena | 2.340 |
| 16 | 11.50 | 152.23 | C10H16O | 2,4-Decadienal | 3.786 |
| 17 | 11.67 | 138.21 | C9H14O | 2,4-Nonadienal | 3.786 |
| 18 | 12.46 | 296.6 | C21H44 | Heptadecane,2,6,10,14-tetramethyl- | 1.290 |
| 19 | 12.51 | 268.5 | C19H40 | 2,3-Dimethylheptadecane | 1.290 |
| 20 | 13.35 | 198.39 | C14H30 | Tetradecane | 1.290 |
| 21 | 14.10 | 224.42 | C16H32 | 7-Hexadecene, (Z) | 0.276 |
| 22 | 14.77 | 226.41 | C16H34 | Hexadecane | 1.266 |
| 23 | 14.84 | 184.37 | C13H28 | Tridecane | 1.266 |
| 24 | 15.94 | 201.22 | C12H11NO2 | trans-Ethylalpha cyanocinnamate | 0.313 |
| 25 | 16.84 | 186.33 | C12H26O | 2-Dodecanol | 0.435 |
| 26 | 16.91 | 212.42 | C15H32 | Pentadecane | 1.075 |
| 27 | 17.06 | 184.36 | C13H28 | 2-Methyldodecane | 1.075 |
| 28 | 18.04 | 256.42 | C16H32O2 | n-Hexadecanoicacid | 1.993 |
| 29 | 18.84 | 280.5 | C20H40 | 9-Eicosene, (E) | 0.643 |
| 30 | 19.23 | 212.41 | C15H32 | Dodecane, 2,6,10-trimethyl | 1.325 |
| 31 | 19.88 | 378.6 | C21H34O4Si | Decan-2-yl trimethylsilyl phthalate 1,2 Benzenedicarboxylic acid | 0.702 |
| 32 | 20.21 | 292.25 | C16H11F3O2 | 1-fluorenecarboxylic acid, 2,2,2-trifluoroethyl ester | 1.662 |
| 33 | 20.48 | 324.6 | C23H48 | Heptadecane, 9-hexyl | 0.320 |
| 34 | 21.17 | 322.5 | C21H38O2 | 11,14-Eicosadienoic acid, methyl ester | 14.287 |
| 35 | 21.20 | 280.4 | C18H32O2 | Linoelaidic acid | 14.287 |
| 36 | 21.71 | 254.5 | C18H38 | 2,6,10-trimethyl-pentadecane | 7.151 |
| 37 | 21.98 | 212.42 | C15H32 | Pentadecane | 7.151 |
| 38 | 22.49 | 166.26 | C11H18O | 2,6-Nonadienal, 3,7-dimethyl | 15.390 |
| 39 | 23.02 | 240.5 | C17H36 | Tetradecane, 2,6,10-trimethyl | 3.445 |
| 40 | 23.58 | 272.9 | C17H33 | 7-Heptadecene | 15.390 |
| 41 | 24.07 | 100.16 | C6H12O | 2-Hexen-1-ol, (E) | 0.514 |
| 42 | 24.56 | 118.17 | C6H14O2 | meso-3,4-Hexanediol | 0.529 |
| 43 | 25.09 | 130.23 | C8H18O | 2-Hexanol, 3,4-dimethyl | 0.683 |
| 44 | 25.64 | 112.17 | C7H12O | 2-Heptenal, (E) | 0.324 |
| 45 | 26.15 | 170.33 | C12H26 | Dodecane | 0.698 |
| DPPH (IC50 in mg/mL) | ABTS (mg TE/g Extract) | FRAP (mg AAE/g Extract) | |
|---|---|---|---|
| EMS | 0.210 ± 0.017 | 56.564 ± 1.63 | 79.4 ± 0.45 |
| EMM | 0.180 ± 0.020 | 60.203 ± 0.76 | 80.066 ± 3.28 |
| BHT | 0.029 ± 0.006 | ||
| Ascorbic acid | 0.007 ± 0.001 | ||
| Trolox | 1.93 ± 0.05 |
| MDA-MB-231 | MCF-7 | 22 RV-1 | LnCap | |
|---|---|---|---|---|
| EMM (µg/mL) | 16.55 ± 0.58 | 17.95 ± 0.58 | 11.75 ± 0.35 | 11.91 ± 0.54 |
| EMS (µg/mL) | 19.93 ± 0.74 | 20.22 ± 0.89 | 13.47 ± 0.52 | 19.64 ± 1.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Fakir, L.; Bouothmany, K.; Alotaibi, A.; Bourhia, M.; Ullah, R.; Zahoor, S.; El Mzibri, M.; Gmouh, S.; Alaoui, T.; Zaid, A.; et al. Antioxidant and Understanding the Anticancer Properties in Human Prostate and Breast Cancer Cell Lines of Chemically Characterized Methanol Extract from Berberis hispanica Boiss. & Reut. Appl. Sci. 2021, 11, 3510. https://doi.org/10.3390/app11083510
El Fakir L, Bouothmany K, Alotaibi A, Bourhia M, Ullah R, Zahoor S, El Mzibri M, Gmouh S, Alaoui T, Zaid A, et al. Antioxidant and Understanding the Anticancer Properties in Human Prostate and Breast Cancer Cell Lines of Chemically Characterized Methanol Extract from Berberis hispanica Boiss. & Reut. Applied Sciences. 2021; 11(8):3510. https://doi.org/10.3390/app11083510
Chicago/Turabian StyleEl Fakir, Loubna, Kaoutar Bouothmany, Amal Alotaibi, Mohammed Bourhia, Riaz Ullah, Saira Zahoor, Mohamed El Mzibri, Said Gmouh, Tajelmolk Alaoui, Abdelhamid Zaid, and et al. 2021. "Antioxidant and Understanding the Anticancer Properties in Human Prostate and Breast Cancer Cell Lines of Chemically Characterized Methanol Extract from Berberis hispanica Boiss. & Reut" Applied Sciences 11, no. 8: 3510. https://doi.org/10.3390/app11083510
APA StyleEl Fakir, L., Bouothmany, K., Alotaibi, A., Bourhia, M., Ullah, R., Zahoor, S., El Mzibri, M., Gmouh, S., Alaoui, T., Zaid, A., & Benbacer, L. (2021). Antioxidant and Understanding the Anticancer Properties in Human Prostate and Breast Cancer Cell Lines of Chemically Characterized Methanol Extract from Berberis hispanica Boiss. & Reut. Applied Sciences, 11(8), 3510. https://doi.org/10.3390/app11083510







