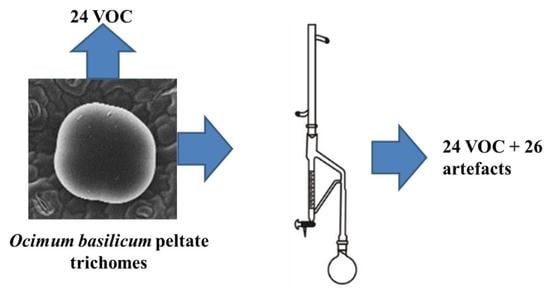
Volatile Organic Compounds of the Glandular Trichomes of Ocimum basilicum and Artifacts during the Distillation of the Leaves
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Sampling from Capitate and Peltate Trichomes
2.3. Isolation of the Essential Oil
2.4. GC-FID e GC-MS Analysis
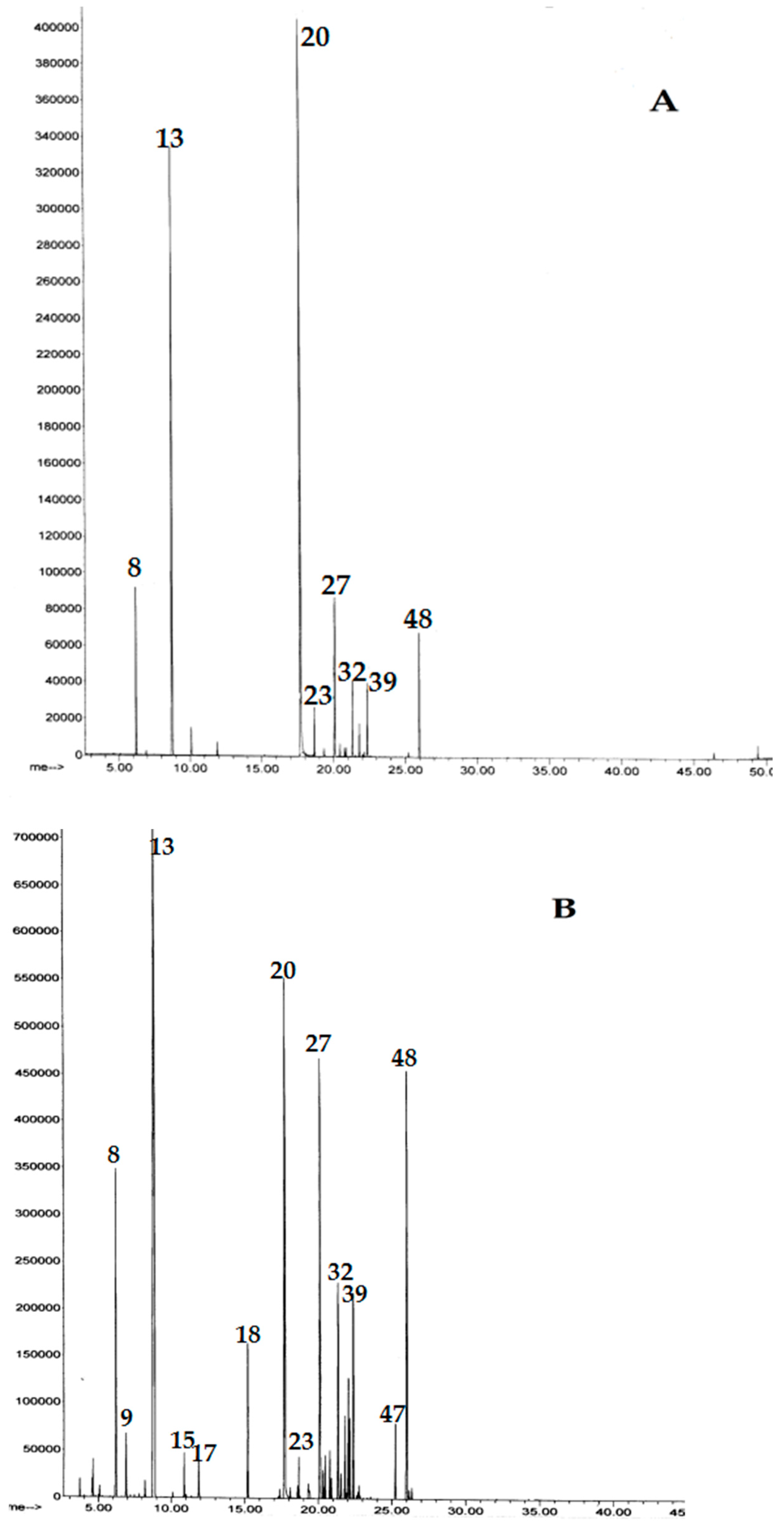
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Chikkaswamy, B.K.; Paramanik, R.C.; Varadaraj, N.; Paramanik, A.; Ramesh, H.L.; Shivashankar, M.; Sivaram, V.R. Determination of genetic variation in Piper species using 4C nuclear DNA and RAPD marker. Int. J. Res. Pharm. Sci. 2013, 4, 58–64. [Google Scholar] [CrossRef]
- Simon, J.E.; Morales, M.R.; Phippen, W.B.; Vieira, R.F.; Hao, Z. Basil: A Source of Aroma Compounds and a Popular Culinary and Ornamental Herb. In Perspectives on New Crops and New Uses; Janick, J., Ed.; ASHS Press: Alexandria, VA, USA, 1999; pp. 499–505. [Google Scholar]
- Carović-Stanko, K.; Liber, Z.; Besendorfer, V.; Javornik, B.; Bohanec, B.; Kolak, I.; Satovic, Z. Genetic relations among basil taxa (Ocimum L.) based on molecular markers, nuclear DNA content, and chromosome number. Plant Syst. Evol. 2010, 285, 13–22. [Google Scholar] [CrossRef]
- Meyers, M. Basil: An Herb Society of America Guide; The Herb Society of America: Kirtland, OH, USA, 2003. [Google Scholar]
- Purushothaman, B.; Prasannasrinivasan, R.; Suganthi, P.; Ranganathan, B.; Gimbun, J.; Shanmugam, K. A comprehensive review on Ocimum basilicum. J. Nat. Remedies 2018, 18, 71–85. [Google Scholar] [CrossRef]
- Dhama, K.; Sharun, K.; Gugjoo, M.B.; Tiwari, R.; Alagawany, M.; Iqbal Yatoo, M.; Thakur, P.; Iqbal, H.M.N.; Chaicumpa, W.; Michalak, I.; et al. A Comprehensive Review on Chemical Profile and Pharmacological Activities of Ocimum basilicum. Food Rev. Int. 2021, 37, 1–29. [Google Scholar] [CrossRef]
- Jayasinghe, C.; Gotoh, N.; Aoki, T.; Wada, S. Phenolics composition and antioxidant activity of sweet basil (Ocimum basilicum L.). J. Agric. Food Chem. 2003, 51, 4442–4449. [Google Scholar] [CrossRef]
- Chiang, L.C.; Ng, L.T.; Cheng, P.W.; Chiang, W.; Lin, C.C. Antiviral activities of extracts and selected pure constituents of Ocimum basilicum. Clin. Exp. Pharmacol. Physiol. 2005, 32, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Manosroi, J.; Dhumtanom, P.; Manosroi, A. Anti-proliferative activity of essential oil extracted from Thai medicinal plants on KB and P388 cell lines. Cancer Lett. 2006, 235, 114–120. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Chemical components and pharmacological benefits of Basil (Ocimum basilicum): A review. Int. J. Food Prop. 2020, 23, 1961–1970. [Google Scholar] [CrossRef]
- Hegnauer, R. (Ed.) Chemotaxonomie der Pflanzen; Birkhiuser: Basel, Switzerland, 1989; Volume 8. [Google Scholar]
- Grayer, R.J.; Kite, G.C.; Goldstone, F.J.; Bryan, S.E.; Paton, A.; Putievsky, E. Infraspecific taxonomy and essential oil chemotypes in sweet basil, Ocimum basilicum. Phytochemistry 1996, 43, 1033–1039. [Google Scholar] [CrossRef]
- Ravid, U.; Putievsky, E.; Katzir, I.; Lewinsohn, E. Enantiomeric composition of linalol in the essential oils of Ocimum species and in commercial basil oils. Flavour Fragr. J. 1997, 12, 293–296. [Google Scholar] [CrossRef]
- Fahn, A. (Ed.) Secretory Tissues in Plants; Academic Press: London, UK, 1979. [Google Scholar]
- Antunes, T.; Sevinate-Pinto, I. Glandular Trichomes of Teucrium scorodonia L. Morphology and Histochemistry. Flora 1991, 185, 65–70. [Google Scholar] [CrossRef]
- Ascensao, L.; Marques, N.; Pais, M.S. Peltate glandular trichomes of Leonotis leonurus leaves: Ultrastructure and histochemical characterization of secretions. Int. J. Plant Sci. 1997, 158, 249–258. [Google Scholar] [CrossRef]
- Ascensão, L.; Marques, N.; Pais, M.S. Glandular trichomes on vegetative and reproductive organs of Leonotis leonurus (Lamiaceæ). Ann. Bot. 1995, 75, 619–626. [Google Scholar] [CrossRef]
- Ascensão, L.; Pais, M.S. The leaf capitate trichomes of Leonotis leonurus: Histochemistry, ultrastructure and secretion. Ann. Bot. 1998, 81, 263–271. [Google Scholar] [CrossRef]
- Bouret, T.M.; Howard, R.J.; O’Keefe, D.P.; Hallahan, D.L. Gland Development on Leaf Surfaces of Nepeta racemosa. Int. J. Plant Sci. 1994, 155, 623–632. [Google Scholar] [CrossRef]
- Voirin, B.; Bayet, C. Developmental changes in the monoterpene composition of Mentha x piperita leaves from individual peltate trichomes. Phytochemistry 1996, 43, 573–580. [Google Scholar] [CrossRef]
- McCaskill, D.; Croteau, R. Monoterpene and sesquiterpene biosynthesis in glandular trichomes of peppermint (Mentha x piperita) rely exclusively on plastid-derived isopentenyl diphosphate. Planta 1995, 197, 49–56. [Google Scholar] [CrossRef]
- Tirillini, B.; Ricci, A.; Pellegrino, R. Secretion constituents of leaf glandular trichome of Salvia officinalis L. J. Essent. Oil Res. 1999, 11, 565–569. [Google Scholar] [CrossRef]
- Falk, K.L.; Gershenzon, J.; Croteau, R. Metabolism of monoterpenes in cell cultures of common sage (Salvia officinalis): Biochemical rationale for the lack of monoterpene accumulation. Plant Physiol. 1990, 93, 1559–1567. [Google Scholar] [CrossRef]
- Venkatachalam, K.V.; Kionaas, R.; Croteau, R. Development and essential oil content of secretory glands of sage (Salvia officinalis). Plant Physiol. 1984, 76, 148–150. [Google Scholar] [CrossRef]
- Yamaura, T.; Tanaka, S.; Tabata, M. Localization of the biosynthesis and accumulation of monoterpenoids in glandular trichomes of thyme. Planta Med. 1992, 58, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Boix, Y.F.; Victório, C.P.; Defaveri, A.C.A.; do Carmo de Oliveira Arruda, R.; Sato, A.; Lage, C.L.S. Glandular trichomes of Rosmarinus officinalis L.: Anatomical and phytochemical analyses of leaf volatiles. Plant Biosyst. 2011, 145, 848–856. [Google Scholar] [CrossRef]
- Sagawa, T.; Ikeda, H.; Hiraoka, T.; Hayakawa, K. Study of rosemary peltate glandular trichomes using combined morphological and chemical approach. Food Sci. Technol. Res. 2013, 19, 491–495. [Google Scholar] [CrossRef]
- Maleci Bini, L.; Gentili, L.; Tirillini, B.; Pellegrino, R. Secretion Constituents of Leaf Glandular Trichomes of Ocìmum basilicum L. In Flavour and Fragrance Chemistry; Lanzotti, V., Taglialatela-Scafati, O., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000; pp. 143–150. [Google Scholar]
- Tozin, L.R.S.; Marques, M.O.M.; Rodrigues, T.M. Herbivory by leaf-cutter ants changes the glandular trichomes density and the volatile components in an aromatic plant model. AoB Plants 2017, 9, plx057. [Google Scholar] [CrossRef]
- Manan, A.A.; Taha, R.M.; Mubarak, E.E.; Elias, H. In vitro flowering, glandular trichomes ultrastructure, and essential oil accumulation in micropropagated Ocimum basilicum L. Vitr. Cell. Dev. Biol. Plant 2016, 52, 303–314. [Google Scholar] [CrossRef]
- Werker, E.; Putievsky, E.; Ravid, U.; Dudai, N.; Katzir, I. Glandular hairs and essential oil in developing leaves of Ocimum basilicum L. (Lamiaceae). Ann. Bot. 1993, 71, 43–50. [Google Scholar] [CrossRef]
- Schnepf, E. Glands Cells. In Dynamic Ultrastructure; Robards, A.W., Ed.; McGraw-Hill: New York, NY, USA, 1974. [Google Scholar]
- Dong, F.; Fu, X.; Watanabe, N.; Su, X.; Yang, Z. Recent advances in the emission and functions of plant vegetative volatiles. Molecules 2016, 21, 124. [Google Scholar] [CrossRef] [PubMed]
- Boncan, D.A.T.; Tsang, S.S.K.; Li, C.; Lee, I.H.T.; Lam, H.-M.; Chan, T.-F.; Hui, J.H.L. Terpenes and terpenoids in plants: Interactions with environment and insects. Int. J. Mol. Sci. 2020, 21, 7382. [Google Scholar] [CrossRef]
- Abbas, F.; Ke, Y.; Yu, R.; Yue, Y.; Amanullah, S.; Jahangir, M.M.; Fan, Y. Volatile terpenoids: Multiple functions, biosynthesis, modulation and manipulation by genetic engineering. Planta Med. 2017, 246, 803–816. [Google Scholar] [CrossRef]
- Yang, C.; Wang, J.; Li, D. Microextraction techniques for the determination of volatile and semivolatile organic compounds from plants: A review. Anal. Chim. Acta 2013, 799, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Xu, J.; Ke, Y.; Huang, S.; Zeng, F.; Luan, T.; Ouyang, G. Applications of in vivo and in vitro solid-phase microextraction techniques in plant analysis: A review. Anal. Chim. Acta 2013, 794, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Keene, C.K.; Wagner, G.J. Direct demonstration of duvatrienediol biosynthesis in glandular heads of tobacco trichomes. Plant Physiol. 1985, 79, 1026–1032. [Google Scholar] [CrossRef]
- Gershenzon, J.; Duffy, M.A.; Karp, F.; Croteau, R. Mechanized techniques for the selective extraction of enzymes from plant epidermal glands. Anal. Biochem. 1987, 163, 159–164. [Google Scholar] [CrossRef]
- Slone, J.H.; Kelsey, R.G. Isolation and purification of glandular secretory cells from Artemisia tridentata (ssp. vaseyana) by percoll density gradient centrifugation. Amer. J. Bot. 1985, 72, 1445–1451. [Google Scholar] [CrossRef]
- Tirillini, B.; Stoppini, A.M. Injection of a sample by means of microneedles followed by capillary gas chromatography. J. Chromatogr. Sci. 1995, 33, 139–142. [Google Scholar] [CrossRef]
- Mastelic, J.; Jerkovic, I. Application of co-distillation with superheated pentane vapour to the isolation of unstable essential oils. Flavour Fragr. J. 2003, 18, 521–526. [Google Scholar] [CrossRef]
- Baines, D.A.; Jones, R.A.; Webb, T.C.; Campion-Smith, I.H. The chemistry of terpenes-I. The effect of hydrogen ion concentration and oxygen upon the acid catalysed cyclization of citral. Tetrahedron 1970, 26, 4901–4913. [Google Scholar] [CrossRef]
- Banthorpe, D.V.; Charlwood, V.; Francis, M.J.O. The biosynthesis of monoterpenes. Chem. Rev. 1972, 72, 115–155. [Google Scholar] [CrossRef]
- Schratz, E.; Wahlig, T. Gaschromatographische analyse aetherischer ole aus pflanzenextrakten. Planta Med. 1965, 13, 218–225. [Google Scholar] [CrossRef]
- Minteguiaga, M.A.; Frizzo, C.D.; Dellacassa, E.S. Odour-active compounds of Citrus deliciosa Tenore var. Caí essential oils detected by gas chromatography-mass spectrometry and gas chromatography-olfactometry. J. Pharm. Pharmacogn. Res. 2017, 5, 345–353. [Google Scholar]
- Hofmann, L. Einfluss Von Genotyp, Ontogenese Und Äusseren Faktoren Auf Pflanzenbauliche Merkmale Sowie Ätherische Öle und Flavonoide von Klonen der Schafgarbe (Achillea millefolium Aggregat); Technische Universität: München, Germany, 1993. [Google Scholar]
- Tressl, R.; Engel, K.H.; Kossa, M.; Koppler, H. Characterization of tricyclic sesquiterpenes in hop (Humulus lupulus, var Hersbrucker Spat). J. Agric. Food Chem. 1983, 31, 892–897. [Google Scholar] [CrossRef]
- Koedam, A.; Scheffer, J.J.C.; Svendsen, A.B. Monoterpenes in the volatile leaf oil of Abies X Arnoldiana Nitz. J. Agric. Food Chem. 1980, 28, 862–866. [Google Scholar] [CrossRef]
- Toyota, M.; Koyama, H.; Mizljtani, M.; Asakawa, Y. (−)-Ent-spathulenol isolated from liverworts is an artefact. Phytochemistry 1996, 41, 1347–1350. [Google Scholar] [CrossRef]
- Njoroge, S.M.; Ukeda, H.; Sawamura, M. Changes of the volatile profile and artifact formation in daidai (Citrus aurantium) cold-pressed peel oil on storage. J. Agric. Food Chem. 2003, 51, 4029–4035. [Google Scholar] [CrossRef]
- Kashiwagi, T.; Phi, N.T.L.; Sawamura, M. Compositional changes in yuzu (Citrus junos) steam-distilled oil and effects of antioxidants on oil quality during storage. Food Sci. Technol. Res. 2010, 16, 51–58. [Google Scholar] [CrossRef][Green Version]
- Bryant, R. The Sesquiterpenoids. In Rodd’s Chemistry of Carbon Compounds; Coffey, S., Ed.; Elsevier: Amsterdam, The Netherlands, 1969; pp. 256–366. [Google Scholar]
| Peak | a Compounds | LEO% | VOC% | RI | RI * |
|---|---|---|---|---|---|
| 1 | alpha-pinene | 0.19 ± 0.02 | 0.02 ± 0.01 | 938 | 939 |
| 2 | camphene | 0.02 ± 0.01 | ------ | 954 | 954 |
| 3 | sabinene | 0.23 ± 0.02 | 0.02 ± 0.01 | 976 | 975 |
| 4 | beta-pinene | 0.54 ± 0.05 | 0.04 ± 0.01 | 979 | 979 |
| 5 | octan-3-one | 0.03 ± 0.01 | ------ | 983 | 984 |
| 6 | myrcene | 0.17 ± 0.02 | 0.03 ± 0.01 | 990 | 991 |
| 7 | delta-2-carene | 0.01 ± 0.01 | ------ | 1003 | 1002 |
| 8 | 1.8-cineole | 5.84 ± 0.54 | 5.71 ± 0.53 | 1031 | 1031 |
| 9 | (E)-beta-ocimene | 1.06 ± 0.12 | 0.16 ± 0.02 | 1050 | 1050 |
| 10 | gamma-terpinene | 0.03 ± 0.01 | ------ | 1060 | 1060 |
| 11 | n-octanol | 0.04 ± 0.01 | ------ | 1067 | 1068 |
| 12 | terpinolene | 0.27 ± 0.02 | ------ | 1087 | 1089 |
| 13 | linalool | 32.05 ± 2.57 | 28.99 ± 2.32 | 1097 | 1097 |
| 14 | camphor | 0.07 ± 0.01 | 0.93 ± 0.09 | 1144 | 1146 |
| 15 | borneol | 0.76 ± 0.06 | ------ | 1169 | 1169 |
| 16 | terpinen-4-ol | 0.03 ± 0.01 | ------ | 1179 | 1177 |
| 17 | alpha-terpineol | 0.67 ± 0.07 | 0.55 ± 0.05 | 1187 | 1189 |
| 18 | bornyl acetate | 2.77 ± 0.22 | ------ | 1289 | 1289 |
| 19 | alpha-terpinyl acetate | 0.14 ± 0.01 | ------ | 1349 | 1349 |
| 20 | eugenol | 15.47 ± 1.05 | 41.89 ± 2.83 | 1359 | 1359 |
| 21 | alpha-copaene | 0.18 ± 0.02 | 0.17 ± 0.02 | 1378 | 1377 |
| 22 | beta-cubebene | 0.21 ± 0.02 | ------ | 1388 | 1388 |
| 23 | beta-elemene | 0.69 ± 0.06 | 1.7 ± 0.15 | 1391 | 1391 |
| 24 | beta-longipinene | 0.04 ± 0.01 | ------ | 1401 | 1401 |
| 25 | methyl eugenol | 0.3 ± 0.01 | 0.45 ± 0.05 | 1404 | 1404 |
| 26 | (Z)-caryophyllene | 0.22 ± 0.02 | ------ | 1409 | 1409 |
| 27 | cis-alpha-bergamotene | 9.35 ± 0.79 | 6.34 ± 0.54 | 1414 | 1413 |
| 28 | cadina-3.5-diene | 0.47 ± 0.04 | ------ | 1452 | 1452 |
| 29 | alpha-humulene | 0.72 ± 0.07 | 0.41 ± 0.04 | 1454 | 1455 |
| 30 | cis-muurola-4(14).5-diene | 0.86 ± 0.08 | 0.32 ± 0.03 | 1467 | 1467 |
| 31 | (Z)-beta-bergamotene | 0.35 ± 0.03 | 0.28 ± 0.02 | 1482 | 1483 |
| 32 | alpha-amorphene | 4.24 ± 0.36 | 2.91 ± 0.24 | 1491 | 1490 |
| 33 | cis-beta-guaiene | 0.02 ± 0.01 | ------ | 1494 | 1493 |
| 34 | gamma-amorphene | 0.47 ± 0.04 | ------ | 1496 | 1496 |
| 35 | bicyclogermacrene | 1.51 ± 0.13 | 1.15 ± 0.14 | 1500 | 1500 |
| 36 | gamma-patchoulene | 0.11 ± 0.01 | ------ | 1506 | 1506 |
| 37 | germacrene A | 2.26 ± 0.22 | 0.07 ± 0.01 | 1508 | 1509 |
| 38 | alpha-bulnesene | 1.41 ± 0.09 | 0.14 ± 0.01 | 1510 | 1510 |
| 39 | gamma-cadinene | 3.88 ± 0.35 | 2.64 ± 0.24 | 1514 | 1514 |
| 40 | trans-cycloisolongifol-5-ol | 0.06 ± 0.01 | ------ | 1516 | 1515 |
| 41 | delta-cadinene | 0.11 ± 0.01 | ------ | 1523 | 1523 |
| 42 | beta-sesquiphellandrene | 0.24 ± 0.02 | ------ | 1525 | 1524 |
| 43 | 10-epi-cubebol | 0.06 ± 0.01 | ------ | 1535 | 1535 |
| 44 | cis-muurol-5-en-4-beta-ol | 0.02 ± 0.01 | ------ | 1552 | 1552 |
| 45 | cis-muurol-5-en-4-alpha-ol | 0.03 ± 0.01 | ------ | 1561 | 1561 |
| 46 | spathulenol | 0.02 ± 0.01 | ------ | 1579 | 1578 |
| 47 | 1.10-di-epi-cubenol | 1.29 ± 0.14 | 0.18 ± 0.02 | 1620 | 1619 |
| 48 | epi-alpha-cadinol | 9.97 ± 0.85 | 4.9 ± 0.41 | 1640 | 1640 |
| 49 | alpha-cadinol | 0.14 ± 0.01 | ------ | 1654 | 1654 |
| 50 | 7-epi-alpha-eudesmol | 0.19 ± 0.02 | ------ | 1665 | 1664 |
| Class of compounds | |||||
| Oxygenated monoterpenes | 39.42 ± 3.02 | 36.18 ± 2.77 | |||
| Sesquiterpenes | 27.34 ± 2.12 | 16.13 ± 1.25 | |||
| Allylbenzenes | 15.77 ± 1.34 | 42.34 ± 3.34 | |||
| Oxygenated sesquiterpenes | 11.78 ± 0.96 | 5.08 ± 0.41 | |||
| Monoterpenes | 2.52 ± 0.23 | 0.27 ± 0.02 | |||
| Esters | 2.91 ± 0.27 | ------ | |||
| Ketones | 0.03 ± 0.01 | ------ | |||
| Alcohol | 0.04 ± 0.01 | ------ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tirillini, B.; Maggi, F. Volatile Organic Compounds of the Glandular Trichomes of Ocimum basilicum and Artifacts during the Distillation of the Leaves. Appl. Sci. 2021, 11, 7312. https://doi.org/10.3390/app11167312
Tirillini B, Maggi F. Volatile Organic Compounds of the Glandular Trichomes of Ocimum basilicum and Artifacts during the Distillation of the Leaves. Applied Sciences. 2021; 11(16):7312. https://doi.org/10.3390/app11167312
Chicago/Turabian StyleTirillini, Bruno, and Filippo Maggi. 2021. "Volatile Organic Compounds of the Glandular Trichomes of Ocimum basilicum and Artifacts during the Distillation of the Leaves" Applied Sciences 11, no. 16: 7312. https://doi.org/10.3390/app11167312
APA StyleTirillini, B., & Maggi, F. (2021). Volatile Organic Compounds of the Glandular Trichomes of Ocimum basilicum and Artifacts during the Distillation of the Leaves. Applied Sciences, 11(16), 7312. https://doi.org/10.3390/app11167312