Abstract
Dialyzable leukocyte extracts are clinically used under different commercial names (IMMUNEPOTENT CRP®, IMMODIN®, Transferon®) to modulate the immune response altered by pathological conditions such as cancer, inflammation, and viral infections. The purpose of this study is to improve the production process of bovine dialyzable leukocyte extract without decreasing its biological activities (antioxidant, anti-inflammatory, and antitumoral). Our product modification consists of adding a dry heating step in the production process. In this study, we evaluated and compared the chemical composition (bromatological analysis), physical structure (infrared spectroscopy, X-ray diffraction, SEM, and zeta potential) and biological function of the dialyzable leukocyte extracts obtained from fresh and dry bovine spleens. Our results showed that the use of a drying step in the production process of the bovine dialyzable leukocyte extract (bDLE) did not affect its antioxidant and anti-inflammatory effects and it improved its antitumor properties. We suggest that this process modification could be applied to other biological products, such as dialyzable leukocyte extracts derived from other sources, in order to improve its functionality and formulation.
1. Introduction
The dialyzable leukocyte extract is a biocompound described for the first time in 1949 by Sherwood Lawrence [1]. The extract is obtained from fresh human or animal blood or lymphoid tissues [2]. In order to preserve the properties of the dialyzable leukocyte extract, it is necessary to keep it vacuum-sealed and refrigerated until use.
Our research team worked with the dialyzable leukocyte extract from bovine spleen (bDLE). The spleen was chosen because it is one of the largest lymphoid organs, rich in nutrients, and it was obtained from an animal already certified as safe for human consumption. Furthermore, the consumption of high amounts of bovine meat guarantees the availability of our raw material, even in the case that the production volume needs to be increased. Finally, the bovine spleen is commonly discarded by slaughterhouses, and repurposing it decreases waste.
To produce the extract, bovine spleens are mechanically disaggregated, dialyzed against a 10–12 kDa cut-off dialysis membrane, and tested for the presence of lipopolysaccharide (LPS) to ensure a pyrogen free product. The resulting product is a bDLE commercialized under the name IMMUNEPOTENT CRP®, and it is used as complement for the treatment of various pathological conditions including cancer, inflammation, immunity disorders, and viral infections [2,3].
IMMUNEPOTENT CRP® contains bioactive peptides smaller than 12 kDa that possesses antioxidant, anti-inflammatory, and antitumoral properties [4]. Several techniques are employed to preserve the bioactive properties of the natural compounds during the production process [5].
Dehydration is one of the oldest preservation techniques. The main objective of dehydration is to extend the shelf-life of the product without the need to refrigerate it; additionally, dehydration reduces volume and mass and, therefore, the cost of transport and storage. Dehydrated goods can be reconstituted to their original form by adding water, making this technique exceptionally convenient [6]. Dehydration also augments nutrient concentration by increasing the amounts of proteins, fats, and carbohydrates present per unit of weight [7].
The purpose of this study is to improve the production process of the dialyzable leukocyte extract derived from bovine spleen without decreasing its antioxidant, anti-inflammatory, and antitumoral properties.
We propose that including dry heating to the IMMUNEPOTENT CRP® production process will be beneficial for our product quality and safety. Furthermore, dry weight formulations are more accurate and practical for standardization of the final product. To test this hypothesis, dialyzable spleen extract was obtained from fresh or dry spleens and characterized by infrared analysis, zeta potential, scanning electron microscope, X-ray diffraction and bromatology, and its antioxidant, anti-inflammatory and antitumor biological activities were evaluated.
Our study is original because many studies have focused on the biological properties of dialyzable leukocyte extracts [2,4,8,9], but to our knowledge, there are no previous studies reporting substantial modifications to their production process.
2. Materials and Methods
2.1. Bovine Spleen Dialyzable Leukocyte Extract
Five bovine spleens were weighed and cut in half to form two 500 g portions. To prepare the wet extract, one half of each spleen was mechanically homogenized. For the dry bDLE, the remaining halves of the spleens were thinly sliced and dehydrated in an oven at 90 °C for 30 h, and ground into a powder. Both extracts were dialyzed with a 10–12 kDa cut-off membrane (SpectraPor) against Milli-Q water, keeping a 1:1 spleen/water ratio for 48 h at 4 °C. The resulting dialysates were filtered against a 0.2 μm-pore size membrane (Merck), lyophilized (Telstar), and stored at −20 °C until use. The resulting lots were analyzed by Fourier Transform Infrared Spectroscopy as a means to evaluate the homogeneity of both the wet and dry extracts (Supplementary Figure S1A,B). The animal study was reviewed and approved by Comité de Ética Animal (CEIBA39-17) of the Laboratorio de Inmunología y Virología, Facultad de Ciencias Biológicas, Universidad Autónoma de Nuevo León, México. Written informed consent was obtained from the owners for the participation of their animals in this study.
2.2. Bromatological Analysis
The wet and dry bDLE dialysates were sent to the Facultad de Ciencias Químicas, Unidad de Alimentos for crude protein, ash, and ethereal extract determination, following the standard methods approved by the association of official analytical chemists (928.08) for protein, and the Mexican regulations NMX-F-607-NORMEX-2013 for ash, and NOM-086-SSA-11994 for lipid determinations. For further characterization and experiments, the concentrations of the wet and dry bDLE were normalized using the protein quantification method described by Lowry, using the PierceTM Modified Lowry Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA) and following the manufacturer guidelines.
2.3. Characterization by Fourier Transform Infrared Spectroscopy (FT-IR)
Attenuated total reflection (ATR) was used in conjunction with the Fourier Transform Infrared Spectroscopy (FT-IR) technique to observe changes in the structure of the dry bDLE with respect to the wet bDLE. The spectra of the dry bDLE and wet bDLE were recorded by a Universal ATR Sampling Accessory Perkin Elmer by the accumulation of 16 scan at 4 cm−1 at the wavenumber range 4000 to 500 cm−1. Data were analyzed with the PERKIN ELMER spectrum software (Waltam, MA, USA).
2.4. Characterization by X-ray Diffraction (XRD)
The wet and dry bDLEs were characterized by XRD in a Dmax2100 diffractometer (Rigaku, Tokyo, Japan) at 30 kV and 20 mA with CuKα (λ = 1.5406 Å) radiation. The data were collected over the 2 θ range from 5° to 80° with a step size of 0.02° intervals with a counting time of 0.6 s at each step. The data were analyzed with the MDI Jade software ver. 6.0.
2.5. Scanning Electron Microscopy (SEM)
Scanning electron microscopy was used to reveal the morphology of the wet bDLE and dry bDLE, and the elemental contents were determined by energy dispersive X-ray spectroscopy (EDS). The samples were examined on a NOVA NANOSEM 200 (FEI®).
2.6. Determination of Zeta Potential (ζ)
The ζ of the wet and dry bDLE was determined by dynamic light scattering (DLS) in a Zetasizer ZS90-Nano (Malvern Instruments, Malvern, UK). The samples were analyzed in the 4 to 10 pH range with different diluents: biphthalate buffer, de-ionized water, phosphate buffer, and borate buffer at 25 °C.
2.7. Cell Viability
For the cell viability assays, B16F10, A427, HT-29, MCF-7, and HEPG-2 cancer cell lines, peritoneal macrophages and bone marrow cells from healthy Balb/C mice, and human peripheral blood mononuclear cells (PBMC) were used. The cells used for the study are adherent cells, except for the murine bone marrow cells and human PBMC, which include adherent monocytes and non-adherent lymphocytes. All cells were adjusted to a 5 × 103 cells/mL concentration in DMEM F-12 medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% (v/v) fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA, USA) and incubated with different concentrations of wet and dry bDLE, in the range of 0.1 to 2.55 mg/mL, in polystyrene tissue culture treated 96 well plates for 72 h at 37 °C and 5% CO2 atmosphere. After incubation, the supernatant was removed by centrifugation at 250× g for 10 min. Then, cells were washed twice with a DMEM F-12 medium supplemented with 10% FBS. Cell viability was determined by the colorimetric MTT reduction assay, and the optical densities resulting from formazan production were determined at 570 nm. Cell viability was expressed as the percentage of viable cells. The results are given as the mean ± standard deviation (SD) of three independent experiments.
2.8. Isolation of Human PBMC
Peripheral blood mononuclear cells (PBMC) were isolated by loading diluted blood (1:1 with saline) on Histopaque 1077 (Sigma, USA) and centrifuging at 400× g for 30 min at 25 °C. The PBMC layer was collected from the interface and then washed twice with Hank’s Balanced Salt Solution (HBSS) by centrifugation at 100× g for 10 min at 25 °C. After centrifugation, the cells were re-suspended in DMEM F-12 supplemented with 10% FBS. All the steps were carried out under sterile conditions. The viability of the isolated blood cells was above 90%, as assessed by the Trypan blue exclusion test.
2.9. Cytokines
Cytokines were determined by analyzing treated murine peritoneal macrophages or human PBMC supernatants. The murine IL-12p70, TNF-α, IFN-γ, MCP-1, IL-10, and IL-6 cytokines were determined by flow cytometry (Accuri C6, BD Bioscience, San Jose, CA, USA), using the BD Cytometric Bead Array (CBA) Mouse Inflammation Kit (BD Bioscience, Bedford, MA, USA). The human IL-2, IL-4, IL-6, IL-10, IFN-γ, TNF-α, and IL-17A cytokines were determined by flow cytometry (Accuri C6, BD Bioscience, San Jose, CA, USA), using the CBA Th1/Th2/Th17 Cytokine Kit BD (BD Bioscience, Bedford, MA, USA). CBA Flex Set analysis was performed using FCAP array v1.0 software (Soft Flow Inc., Minneapolis, MN, USA).
2.10. ROS
Human PBMC were isolated as previously described and cultured in tissue culture treated 24 well plates. After 24 h, the non-adherent cell fraction (lymphocytes) was dis-carded by the cell culture medium removal, and the adherent cell fraction (human macrophages) was kept. Murine macrophages were isolated from mouse peritoneal cavity. Briefly, after cervical dislocation, 5 mL of ice cold DMEM-F12 culture media was injected into the peritoneal cavity. After this, the peritoneal liquid was aspirated with a 5 mL sterile syringe. The recovered liquid was centrifuged at 400× g for 10 min. Peritoneal cells present in the pellet were counted and their viability was evaluated by the Tryphan blue exclusion technique. Then, human macrophages and murine peritoneal macrophages were grown in 24-well plates (1 × 105 cells/well) for 24 h in DMEM F-12 supplemented with 10% FBS. After this, the cell culture medium was replaced with serum-free DMEM F-12, and the cells were incubated for 6 h. Then, cells were treated simultaneously with the wet or dry bDLE and LPS, 100 ng/mL for human macrophages, and 2 mg/mL for murine peritoneal macrophages, and incubated for 6 h. Next, cells were stained with 100 μL of ROS/Superoxide detection reagent for 1 h (ROS/superoxide detection kit ENZ-51010, Enzo Life Sciences, Lausen, Switzerland). Fluorescence was measured in a multi-detection reader (Bio-Tek Instruments Inc., Winooski, VT, USA) at the excitation and emission wavelengths of 488 nm and 520 nm, respectively, according to manufacturer instructions. For the assay, negative control cells were treated with the antioxidant N-acetylcysteine (NAC) at 5 mM for 60 min; positive control cells were treated with pyocyanin at 400 μM for 20 min.
2.11. Nitrate/Nitrite
Human macrophages derived from PBMC were cultured in 24-well plates (1 × 105 cells/well) for 24 h in DMEM F-12 supplemented with 10% FBS. After this, the cell culture medium was replaced with serum-free DMEM-F12 for 6 h. Then cells were treated with wet or dry bDLE, with or without LPS, (100 ng/mL) and incubated for 6 h. Nitrate and nitrite, stable products derived from nitric oxide (NO), were determined with a Cayman Chemical Nitrate/Nitrite Assay Kit (Ann Arbor, MI, USA) according to the manufacturer’s instructions. Absorbance was measured in a multi-detection reader (Bio-Tek Instruments Inc., Winooski, VT, USA) at the 540 nm wavelength.
2.12. MTT Antioxidant Assay
Stock solutions of the wet and dry bDLE were prepared with Milli-Q water and normalized to an 8 mg/mL protein concentration. The MTT, (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) was dissolved with Milli-Q water to a concentration of 1 mg/mL and filtered against a 0.2 µm pore diameter filter. Then, 190 μL of MTT and 10 μL of wet and dry bDLE were mixed by vortex in a capped glass vial for 1 min, after which 200 μL of Dimethyl sulfoxide (DMSO) was added, and the vial was mixed again. The reaction mixture was then incubated at 37 °C for 10 min, and 200 μL of the reaction mixture was pipetted to a 96-well cell culture plate; finally, the absorbance was measured at 570 nm wavelength in a multi-detection reader (Bio-Tek Instruments Inc., Winooski, VT, USA). Each sample was assayed in triplicate, and the experiment was repeated 10 times. The absorbance of the MTT reduced by ascorbic acid is considered to be 100% [10].
2.13. ABTS Assay
The radical scavenging activity of the wet and dry bDLE were determined by the ABTS˙+ method, which is based on the reduction of the green ABTS˙+ radical cation. First, the wet and dry bDLE stock solutions and serial dilutions in a concentration range from 30 to 350 µg/mL were prepared with Milli-Q water. Then, 25 μL of each solution was added to 1 mL of ABTS˙+ methanol solution (7 mM). After 7 min, the absorbances were measured at 734 nm (Epoch Microplate Spectrophotometer, Biotek, Winooski, VT, USA). The percentage inhibition of free radicals (I %) was calculated using the equation: I % = [(ABSo − ABS sample)/ABSo] × 100, where ABSo is the absorbance of the ABTS˙+ radicals in methanol and ABS sample is the absorbance of the ABTS radical solution–sample mix. Each concentration was assayed in triplicate and the mean values were calculated. Finally, the IC50 (mean ± standard deviation, n = 3) was determined using the Probit analysis. The ABTS˙+ IC50 value is the concentration of the wet or dry bDLE required to inhibit 50% of the free radicals [11].
2.14. Statistical Analysis
All experiments were performed in triplicate. A statistical analysis was performed using SPSS (IBM, New York, NY, USA). The results are expressed as mean ± standard deviation (SD). The statistical evaluations used one-way analysis of variance (ANOVA) with multiple comparisons, followed by Dunnett’s t-tests. Statistical significance was determined at * p < 0.05.
3. Results
3.1. Bromatological Analysis of bDLE
Dry bDLE contains a higher amount of protein (2.11%) and ash (0.31%), as compared to the wet bDLE (0.41 and 0.13%, respectively), as was expected. The total lipid content found was equal (0.14%) for both bDLEs. The results of the analysis can be seen in Table 1.

Table 1.
Bromatological composition of wet and dry bovine dialyzable leukocyte extract (bDLE).
3.2. FT-IR Spectra of Wet and Dry bDLE
The IR spectra of wet and dry bDLE (Figure 1) shows absorption bands at 3450 and 2400 cm−1 that correspond to the stretching vibration of water (H2O); bands at 3000 and 1470 cm−1, characteristic of the stretching and bending of alkane groups (CH); a band at 1582 cm−1 attributed to the antisymmetric bending modes of carboxyl groups (COO); and a band at 1516 cm−1 that corresponds to amide II (NH) groups. Moreover, we observed in the spectra of wet bDLE the presence of CH groups at 1400, 1350 and 1330 cm−1, a CN group at 1330 cm−1, and CC and COC stretching of glycosidic bonds at 1260 cm−1 and 1220 cm−1.
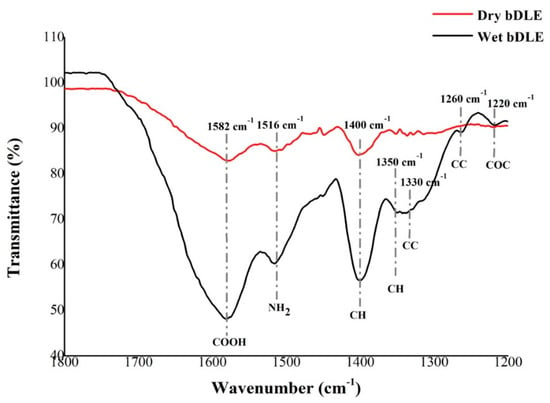
Figure 1.
IR spectrum of wet and dry bDLE. Dry bDLE (red) showed three characteristic peaks at 1582 cm−1 (COOH), 1516 cm−1 (NH2) and 1400 cm−1 (CH), whereas wet bDLE (black) showed seven characteristic peaks at 1582 cm−1 (COOH), 1516 cm−1 (NH2) and 1400 cm−1 and 1350 cm−1 (CH), 1330 cm−1 (CN), 1260 cm−1 (CC), and 1220 cm−1 (COC).
3.3. Characterization by X-ray Diffraction (XRD)
The X-ray diffraction pattern of wet and dry bDLE is shown in Figure 2. We observed diffraction peaks at 2 θ of 7°and 20° in the XRD patterns of both samples. However, the intensity of the diffraction peaks was higher for dry bDLE, indicating a more crystalline structure.
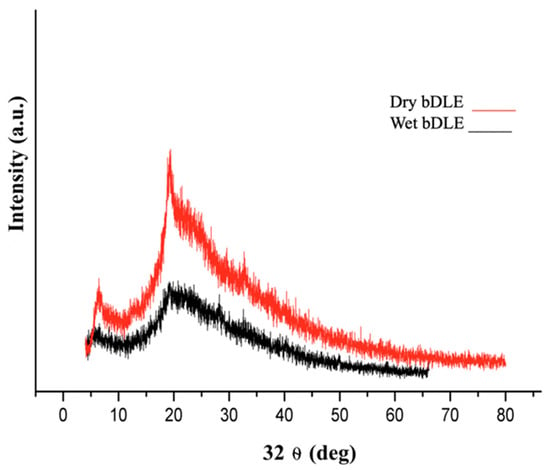
Figure 2.
X-ray diffraction pattern of wet and dry bDLE. X-ray diffractogram of wet (black) and dry (red) bDLE.
3.4. Scanning Electron Microscopy (SEM)
Wet and dry bDLE present an irregular shape (Figure 3A,B); however, dry bDLE has higher organic material density (Figure 3B). An elemental chemical analysis confirmed the presence of C, N, O, Na, Al, and P in both samples (Figure 4A). Additionally, we observed the presence of Mg, and Cl in wet bDLE (Figure 4B).
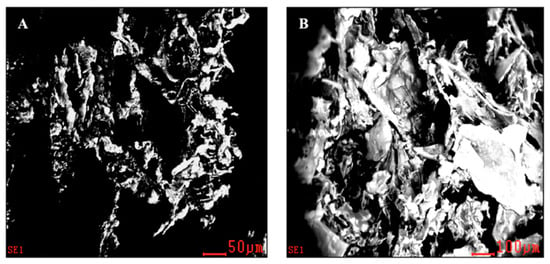
Figure 3.
Scanning electron microscopy (SEM) of dry and wet bDLE. (A) Topography of the wet bDLE in which a lower density is observed at 50 μm. (B) Topography of dry bDLE, showing a higher density, presumably due to an agglomeration of the components at a scale of 100 μm. Micrographs taken in a scanning electron microscope (SEM).
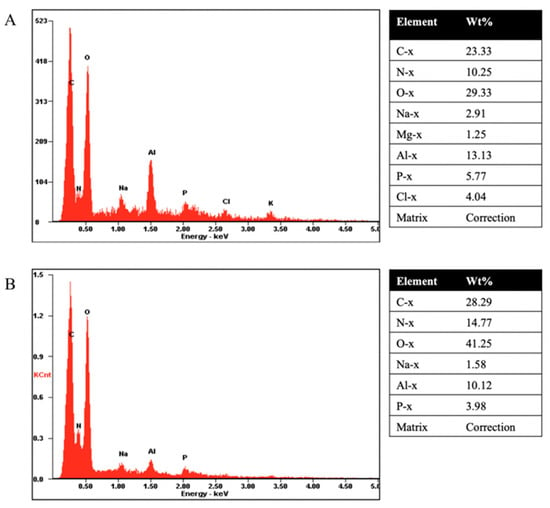
Figure 4.
Chemical–elemental analysis obtained by SEM. (A) X-ray analysis of each element pre-sent in the wet bDLE; the respective table shows the containing weight percentage for each element. (B) X-ray analysis of each element present in the dry bDLE; the respective table shows the containing weight percentage for each element.
3.5. Zeta Potential (ζ)
The zeta potential (ζ) of the wet and dry bDLE was determined at various physiologically relevant pH values (Figure 5). Increasingly positive ζ values were determined for both extracts with respect to pH (Table 2).
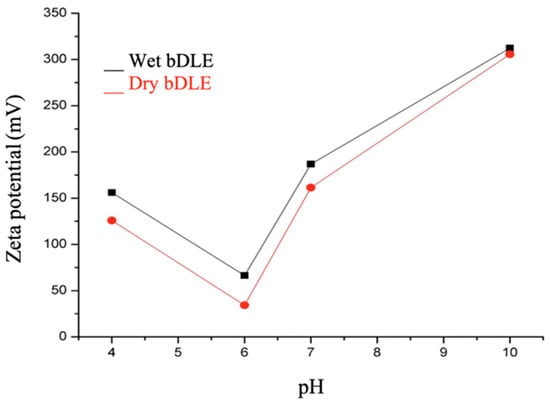
Figure 5.
Zeta potential values. Zeta potential values of wet (black) and dry (red) bDLE at 4, 6, 7, and 10 pH values as determined by dynamic light scattering (DLS) in a Zetasizer ZS90-Nano (Malvern Instruments, Malvern, UK).

Table 2.
Zeta potential values of wet and dry bDLE.
3.6. Effect of Wet and Dry bDLE over Cell Viability of Cancer Cell Lines
Wet and dry bDLE significantly decreased (* p < 0.05) the viability of B16F10, A427, HT-29, MCF-7, and HEPG-2 cell lines in a concentration- and cell line-dependent manner; HT-29 cell line was the least susceptible (Table 3). Furthermore, dry bDLE exerts a higher cytotoxic effect over cancer cell lines when compared to wet bDLE. However, none of the tested concentrations of wet and dry bDLE affected the viability of human PBMC, peritoneal murine macrophages, and murine bone marrow cells (Figure 6 and Figure 7).

Table 3.
Wet and dry bDLE IC50 for B16F10, A427, HT-29, MCF-7 and HEPG-2 cancer cell lines.
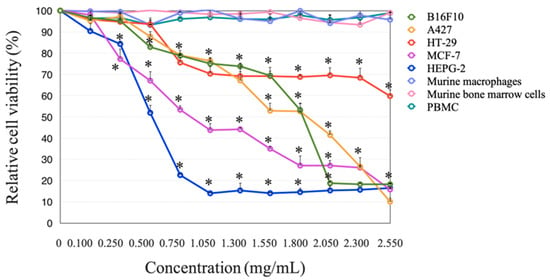
Figure 6.
Wet bDLE effect over the viability of B16F10, A427, HT-29, MCF-7, HEPG-2, human PBMC, murine macrophages, and murine bone marrow cells. Cells (5 × 103) were exposed to wet bDLE in a concentration range from 0.1 to 2.55 mg/mL for 24 h. Cell viability was determined by the MTT assay comparing each cell line or primary culture with the respective untreated control. Statistical significance was determined by one-way ANOVA followed by the Dunnett’s post hoc test (* p < 0.05).
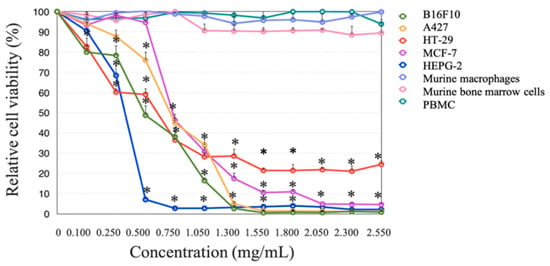
Figure 7.
Dry bDLE effect over the viability of B16F10, A427, HT-29, MCF-7, HEPG-2, human peripheral blood mononuclear cells (PBMC), murine macrophages and murine bone marrow cells. Cells (5 × 103) were exposed to wet bDLE in a concentration range from 0.1 to 2.55 mg/mL for 24 h. Cell viability was determined by the MTT assay comparing each cell line or primary culture with the respective untreated control. Statistical significance was determined by one-way ANOVA followed by the Dunnett’s post hoc test (* p < 0.05).
3.7. ROS Production
Wet and dry bDLE significantly (* p < 0.05) increased ROS levels in unstimulated human PBMC, as compared to the control. A similar effect was observed with the antioxidant positive control N-acetyl-L-cysteine (NAC) treatment. In contrast, wet and dry bDLE significantly decreased (* p < 0.05) ROS levels in LPS-stimulated PBMC (Figure 8).
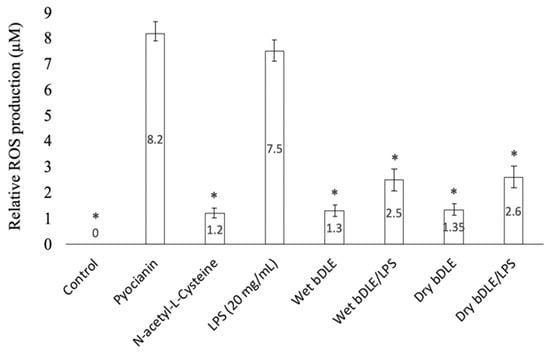
Figure 8.
Effect of wet and dry bDLE on ROS production by LPS stimulated PBMC. PBMC (5 × 105) unstimulated or stimulated with LPS (100 ng/mL) were treated with wet and dry bDLE for 5 h. ROS levels were determined by fluorescence as measured in a multi detection reader (Bio-Tek Instruments Inc. Winooski, VT, USA). N-acetylcysteine (NAC) at a 5 mM was used as a ROS inhibition control and pyocyanin at a 400 μM was used as a ROS positive inducer control. Statistical significance was determined by one-way ANOVA followed by the Dunnett’s post hoc test (* p < 0.05).
3.8. Antioxidant Capacity
Antioxidant capacity was assessed by MTT assay. At the median relative antioxidant capacity, the dry bDLE had more activity as compared to the wet bDLE; however, no difference was found for the IC100 doses (Figure 9). This result was corroborated by the ABTS (2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt) assay method, which yielded an IC50 of 180 ± 17.6 µg/mL and 156.4 ± 22.1, for wet and dry bDLE, respectively (Table 4).
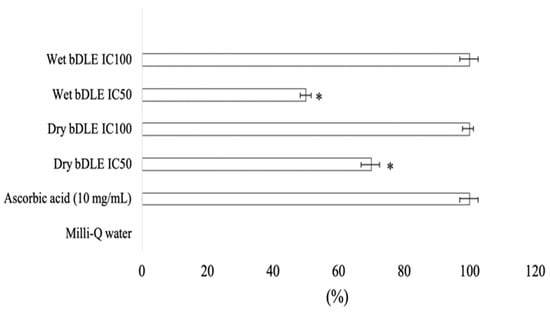
Figure 9.
Antioxidant capacity of wet and dry bDLE by MTT method. Wet and dry bDLE (8 mg/mL) were incubated with MTT (1 mg/mL) for 10 min at 37 °C. Absorbance was measured at 570 nm wavelength to detect formazan, the MTT salt-reduced form. Antioxidant capacity (%) was determined by comparison against ascorbic acid reduced MTT absorbance (100%). All values shown are the means of ten determinations (mean ± SD); SD: standard deviation. Statistical significance was determined by one-way ANOVA followed by the Dunnett’s post hoc test (* p < 0.05).

Table 4.
Antioxidant capacity of wet and dry bDLE.
3.9. Nitric Oxide Determination
On their own, LPS and wet and dry bDLE significantly increased (* p < 0.05) nitric oxide (NO) production in human monocytes, with LPS inducing the highest level in comparison with the unstimulated cells. On the other hand, the combined treatment of LPS and wet or dry bDLE significantly decreased (* p < 0.05) NO production to levels similar to those of monocytes treated with wet or dry bDLE alone (Figure 10).
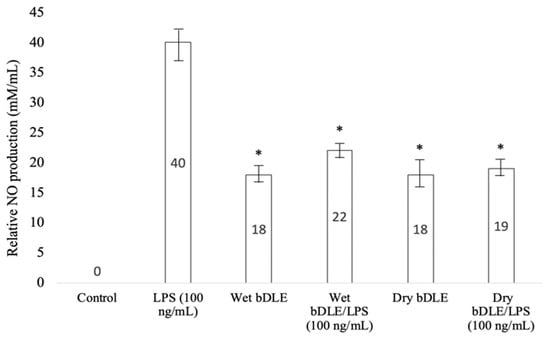
Figure 10.
Effect of wet and dry bDLE on NO production by lipopolysaccharide (LPS) stimulated human monocytes. Human macrophages (1 × 105) unstimulated or stimulated with 100 ng/mL of LPS were treated with wet and dry bDLE for 24 h. NO (nitric oxide) levels were determined with the Griess method. Statistical significance was determined by one-way ANOVA followed by the Dunnett’s post hoc test (* p < 0.05).
3.10. Cytokine Production
Wet and dry bDLE significantly decreased (* p < 0.05) the production of IL-12p70, MCP-1, TNF-α, and IL-6, but not IFN-γ production in LPS-stimulated murine macrophages. Dry bDLE significantly decreased (* p < 0.05) IL-6 in LPS-stimulated murine macrophages, when compared to wet bDLE. Dry bDLE significantly decreased (* p < 0.05) IL-10 when compared to wet bDLE. No difference was found in IL-12p70, TNF-α, IFN-γ, MCP-1 and IL-6 production when comparing wet and dry bDLE treated murine macrophages (Table 5). Wet and dry bDLE significantly decreased (* p < 0.05) the production of IL-4, TNF-α, IFN-γ, IL-17a, and IL-10 production in LPS stimulated human PBMC. Dry bDLE significantly decreased (* p < 0.05) TNF-α, IFN-γ, IL-17A and IL-10 in LPS-stimulated human PBMC, when compared to wet bDLE + LPS. Dry bDLE significantly decreased (* p < 0.05) IFN-γ in human PBMC, when compared to wet bDLE. No difference was found in IL-2, IL-4, TNF-α, IL-17a, and IL-10 production when comparing wet and dry bDLE treated human PBMC (Table 6).

Table 5.
Cytokine levels in LPS stimulated murine macrophages treated with wet and dry bDLE.

Table 6.
Cytokine levels in LPS stimulated human PBMC treated with wet and dry bDLE.
4. Discussion
IMMUNEPOTENT CRP® is a bovine dialyzable leukocyte extract. Our research group has published extensive research about IMMUNEPOTENT CRP’s antioxidant, antitumor and anti-inflammatory properties at in vitro, preclinical and clinical levels [12,13,14,15]. However, the classical preparation process of the bDLE IMMUNEPOTENT CRP® presents some challenges for the establishment of a production line.
Our main challenge is that fresh bovine spleen is difficult to formulate; therefore, we added a drying step in the production process of the bDLE. Dried spleen can be turned into powder and this powder is our active ingredient.
This is the first study to use dry, powdered bovine spleen as a functional compound to elaborate IMMUNEPOTENT CRP®. We designed the present study to evaluate if this modification in the preparation method would affect the physical, chemical and biological properties of the final product.
For wet bDLE, we observed vibration modes related to radical groups CC at 1330 cm−1, CH at 1350, and 1400 cm−1 and NH2+ at 1516 cm−1. These wavelengths were not observed in dry bDLE, showing that the structures of wet and dry bDLE present clear differences in the interaction levels of the aforementioned groups. This result could be related to the percentage of humidity, given that the bandwidth observed at 1300 and 1350 cm−1 that corresponds to CH and CC bonds was greater for wet bDLE [16].
We noted a difference in the diffraction pattern of the bDLEs, with the dry bDLE being more crystalline. The higher quantity of radical groups of wet bDLE correlates with a tendency to generate hydrogen bonds with its environment [17], which produces a modification of crystallinity in the structure, which was confirmed by the X-ray diffraction.
Wet bDLE possibly presents an α-helix structure due to the presence of a greater number of CH bonds. Dry bDLE, on the other hand, does not present these bands and thus we presume a β-folded structure. The positions of the hydrogens are important for the structural determination of the molecules present in both extracts, since these structures are determined by the intramolecular and intermolecular hydrogen bonds.
The zeta potential values were slightly higher for wet bDLE; however, the values were positive at pH 4, 6, 7 and 10 for both extracts, which indicates high stability in solution and that the particles are dispersed (there is repulsion between the charged molecules and the ions found in the solution) [18].
Once we determined that there are structural differences between the wet and dry bDLE, we proceeded to evaluate whether there were any differences in their biological properties.
Both extracts exhibited a MTT and ABTS radical cation scavenging ability, which indicates that the antioxidant components have not been inactivated by the heating process, and therefore maintain their therapeutic potential for the prevention and treatment of disorders where the oxidative free radicals are involved.
The anti-inflammatory activity was evidenced by the decrease in free radicals (NO and ROS) and modulation of cytokines in cells stimulated with LPS and wet or dry bDLE. Previous results have demonstrated that wet bDLE induces cytokine expression in murine macrophages [19] and PBMC [20] and, when challenged with LPS, has the capacity to modulate inflammatory cytokines and mediators, including TNF-α through a NF-κB dependent mechanism [20]. bDLE also has an antioxidant capacity that has been previously reported [20], which can affect the inflammation process, as can be seen when comparing TNF-α levels of wet bDLE and wet bDLE + LPS, against LPS alone.
Complementary to this, our results show that the dry bDLE contains higher amounts of proteins, and it has been reported that the bioactive peptides present in food from vegetable and animal origin, including bovine milk, possess antioxidant and anti-inflammatory properties [21]. Furthermore, it has been proven that the processing method can significantly alter the biological activity, and that the activity of some peptides can be increased by heat through denaturation, while others could completely lose their activities after heating [22].
The anti-inflammatory mechanism for wet bDLE is seen for occurs through the regulation of Nrf2 and NF-κB transcription factors [4,19]. It remains to be elucidated whether dry bDLE shares the same action mechanism.
We found selective cytotoxic activity against cancer cells for both wet and dry bDLE. Dry bDLE had a higher cytotoxic activity against cancer cell lines; however, none of the extracts affected human PBMC, murine bone marrow or macrophage cells at the tested concentrations. This is a highly desirable property, because our studies show that the in vitro cytotoxic activity correlates with a tumor decrease in preclinical models.
Our results show that the drying step in the production process of the bDLE did not affect our product’s antioxidant and anti-inflammatory properties, and it improved the antitumor activity. For this reason, we conclude that the inclusion of a drying step in the spleen preparation to elaborate the bDLE represents an efficient modification that preserves its antioxidant, anti-inflammatory and antitumor properties.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/app11083505/s1, Figure S1: IR spectrum of wet and dry bDLB. (A) IR spectrum of dry bDLE showed three characteristics peaks in all lots produced at 1579 cm−1 (COOH), 1515 cm−1 (NH2) and 1402 cm−1 (CH). (B) IR spectrum of wet bDLE showed seven characteristics peaks in all lots produced at 1582 cm−1 (COOH), 1516 cm−1 (NH2), 1400 cm−1 and 1350 cm−1 (CH), 1330 cm−1 (CN), 1260 cm−1 (CC), and 1220 cm−1 (COC).
Author Contributions
Conceptualization, M.A.F.-M.; Formal analysis, D.G.Z.-T.; Funding acquisition, C.R.-P. and M.A.F.-M.; Investigation, M.A.F.-M. and F.C.-W.; Methodology, M.A.F.-M.; Project administration, C.R.-P.; Supervision, P.Z.-B.; Validation, F.C.-W.; Writing—original draft, S.E.S.-K. and S.P.H.-M.; Writing—review and editing, M.A.F.-M., S.E.S.-K. and S.P.H.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Laboratorio de Inmunología y Virología de la Universidad Nacional Autónoma de Nuevo León, Mexico; by “Fondo Sectorial de Investigación para la Educación”, grant A1-S-35951, CONACYT, México; and by LONGEVEDEN S.A de C.V.
Institutional Review Board Statement
The study was approved by Comité de ética animal (CEIBA-39-17 approval august 20-2017) del Laboratorio de Inmunología y Virología, Facultad de Ciencias Biológicas, Universidad Autónoma de Nuevo, Mexico.
Informed Consent Statement
Not applicable.
Acknowledgments
We thank Alejandra Elizabeth Arreola Triana for the manuscript revision.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lawrence, H.S. The cellular transfer of cutaneous hypersensitivity to tuberculin in man. Proc. Soc. Exp. Biol. Med. 1949, 71, 516–522. [Google Scholar] [CrossRef]
- Arnaudov, A.; Kostova, Z. Dialysable leukocyte extracts in immunotherapy. Biotechnol. Biotechnol. Equip. 2015, 29, 1017–1023. [Google Scholar] [CrossRef]
- Lara, H.H.; Ixtepan-Turrent, L.; Garza-Treviño, E.N.; Badillo-Almaraz, J.I.; Rodriguez-Padilla, C. Antiviral mode of action of bovine dialyzable leukocyte extract against human immunodeficiency virus type 1 infection. BMC Res. Notes 2011, 4, 474. [Google Scholar] [CrossRef] [PubMed]
- Coronado-Cerda, E.E.; Franco-Molina, M.A.; Mendoza-Gamboa, E.; Prado-García, H.; Rivera-Morales, L.G.; Zapata-Benavides, P.; Rodríguez-Salazar, M.d.C.; Caballero-Hernandez, D.; Tamez-Guerra, R.S.; Rodríguez-Padilla, C. In vivo chemoprotective activity of bovine dialyzable leukocyte extract in mouse bone marrow cells against damage induced by 5-fluorouracil. J. Immunol. Res. 2016, 2016, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Joana Gil-Chávez, G.; Villa, J.A.; Fernando Ayala-Zavala, J.; Basilio Heredia, J.; Sepulveda, D.; Yahia, E.M.; González-Aguilar, G.A. Technologies for extraction and production of bioactive compounds to be used as nutraceuticals and food ingredients: An overview: Production of nutraceutical compounds: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 5–23. [Google Scholar] [CrossRef]
- Godefroid, S.; Van De Vyver, A.; Stoffelen, P.; Vanderborght, T. Effectiveness of dry heat as a seed sterilisation technique: Implications for ex situ conservation. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2017, 151, 1054–1061. [Google Scholar] [CrossRef]
- Fayose, F.; Huan, Z. Heat pump drying of fruits and vegetables: Principles and potentials for Sub-Saharan Africa. Int. J. Food Sci. 2016, 2016, 1–8. [Google Scholar] [CrossRef]
- Gottlieb, A.A.; Maziarz, G.A.; Tamaki, N.; Sutcliffe, S.B. The Effects of dialyzable products from human leukocyte extracts on cutaneous delayed-hypersensitivity response. J. Immunol. 1980, 124, 885–892. [Google Scholar]
- Krishnaveni, M. A Review on transfer factor an immune modulator. Drug Invent. Today 2013, 5, 153–156. [Google Scholar] [CrossRef]
- Liu, Y.; Nair, M.G. An efficient and economical MTT assay for determining the antioxidant activity of plant natural product extracts and pure compounds. J. Nat. Prod. 2010, 73, 1193–1195. [Google Scholar] [CrossRef]
- Benchabane, O.; Hazzit, M.; Mouhouche, F.; Baaliouamer, A. Influence of extraction duration on the chemical composition and biological activities of essential oil of Thymus pallescens de Noé. Arab. J. Sci. Eng. 2015, 40, 1855–1865. [Google Scholar] [CrossRef]
- Franco-Molina, M.A.; Mendoza-Gamboa, E.; Zapata-Benavides, P.; Vera-García, M.E.; Castillo-Tello, P.; de la Fuente, A.G.; Mendoza, R.D.; Garza, R.G.; Támez-Guerra, R.S.; Rodríguez-Padilla, C. IMMUNEPOTENT CRP (bovine dialyzable leukocyte extract) adjuvant immunotherapy: A phase I study in non-small cell lung cancer patients. Cytotherapy 2008, 10, 490–496. [Google Scholar] [CrossRef]
- Lara, H.; Turrent, L.I.; Garza-Treviño, E.N.; Tamez-Guerra, R.; Rodriguez-Padilla, C. Clinical and immunological assessment in breast cancer patients receiving anticancer therapy and bovine dialyzable leukocyte extract as an adjuvant. Exp. Med. 2010, 1, 425–431. [Google Scholar] [CrossRef]
- Franco-Molina, M.A.; Mendoza-Gamboa, E.; Coronado-Cerda, E.E.; Zarate-Triviño, D.; Arizpe-Coronado, J.E.; Zapata-Benavides, P.; Ramos Zayas, Y.; Tamez-Guerra, R.; Rodríguez-Padilla, C. Clinical trial evaluating the effectiveness of biocompound IMMUNEPOTENT CRP in the third-molar extraction. Biotechnol. Biotechnol. Equip. 2017, 31, 182–186. [Google Scholar] [CrossRef][Green Version]
- Franco-Molina, M.A.; Mendoza-Gamboa, E.; Zapata-Benavides, P.; Castillo-Tello, P.; Isaza-Brando, C.E.; Zamora-Avila, D.; Rivera-Morales, L.G.; Miranda-Hernández, D.F.; Sierra-Rivera, C.A.; Vera-García, M.E.; et al. Antiangiogenic and antitumor effects of IMMUNEPOTENT CRP in murine melanoma. Immunopharmacol. Immunotoxicol. 2010, 32, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Stuart, A.V.; Sutherland, G.B.B.M. Effect of hydrogen bonding on the deformation frequencies of the hydroxyl group in alcohols. J. Chem. Phys. 1956, 24, 559–570. [Google Scholar] [CrossRef]
- Steiner, T. C–H···O hydrogen bonding in crystals. Crystallogr. Rev. 2003, 9, 177–228. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and Zeta Potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Franco-Molina, M.A.; Mendoza-Gamboa, E.; Castillo-León, L.; Tamez-Guerra, R.S.; Rodríguez-Padilla, C. Bovine dialyzable leukocyte extract modulates the nitric oxide and pro-inflammatory cytokine production in lipopolysaccharide-stimulated murine peritoneal macrophages in vitro. J. Med. Food 2005, 8, 20–26. [Google Scholar] [CrossRef]
- Moisés, A. Franco-Molina Anti-Inflammatory and antioxidant effects of IMMUNEPOTENT CRP in lipopolysaccharide (LPS)-stimulated human macrophages. Afr. J. Microbiol. Res. 2011, 5. [Google Scholar] [CrossRef]
- Tadesse, S.A.; Emire, S.A. Production and processing of antioxidant bioactive peptides: A driving force for the functional food market. Heliyon 2020, 6, e04765. [Google Scholar] [CrossRef] [PubMed]
- Daliri, E.; Oh, D.; Lee, B. Bioactive peptides. Foods 2017, 6, 32. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).