Late Holocene Paleonvironmental Evolution of Two Coastal Lakes in Mediterranean Chile and Its Implications for Conservation Planning
Abstract
1. Introduction
2. Material and Methods
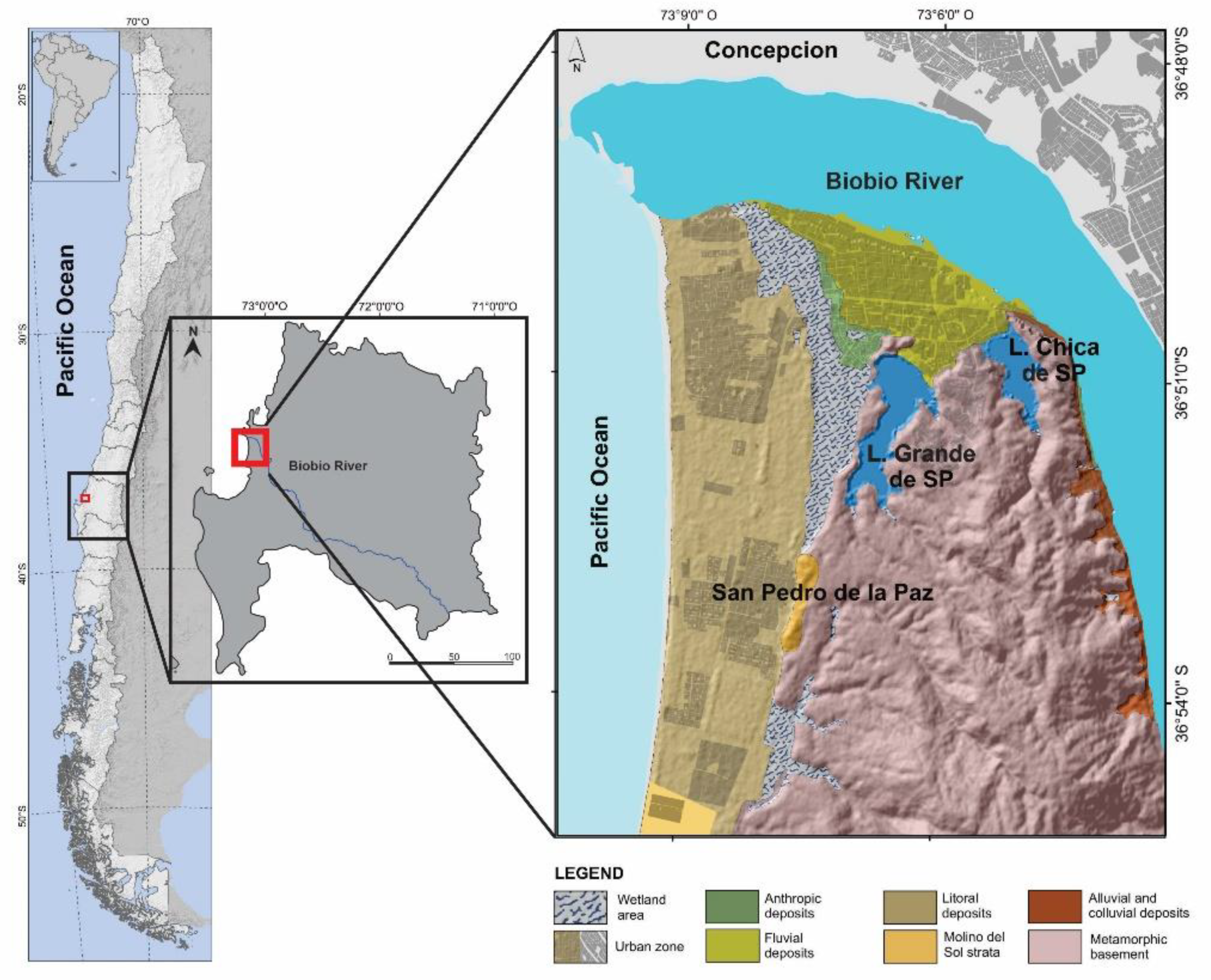
2.1. Study Area
2.2. Sampling and Sedimentological Analysis
2.3. Sediment Core Dating
2.4. Stable Isotopes
2.5. Pigment Analysis
3. Results
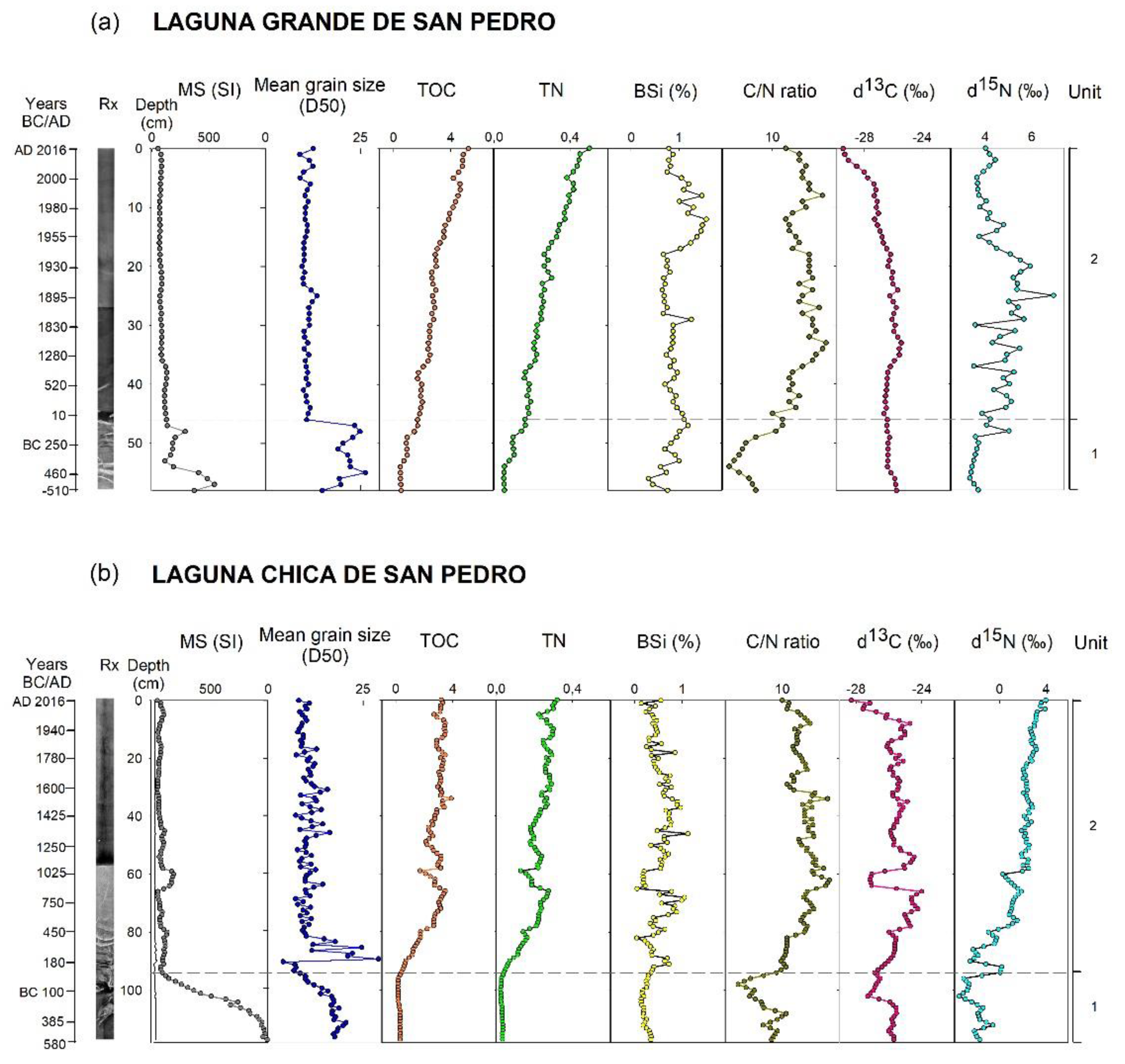
3.1. Age Model
3.2. Lithological Description
Laguna Chica de San Pedro (LCSP)
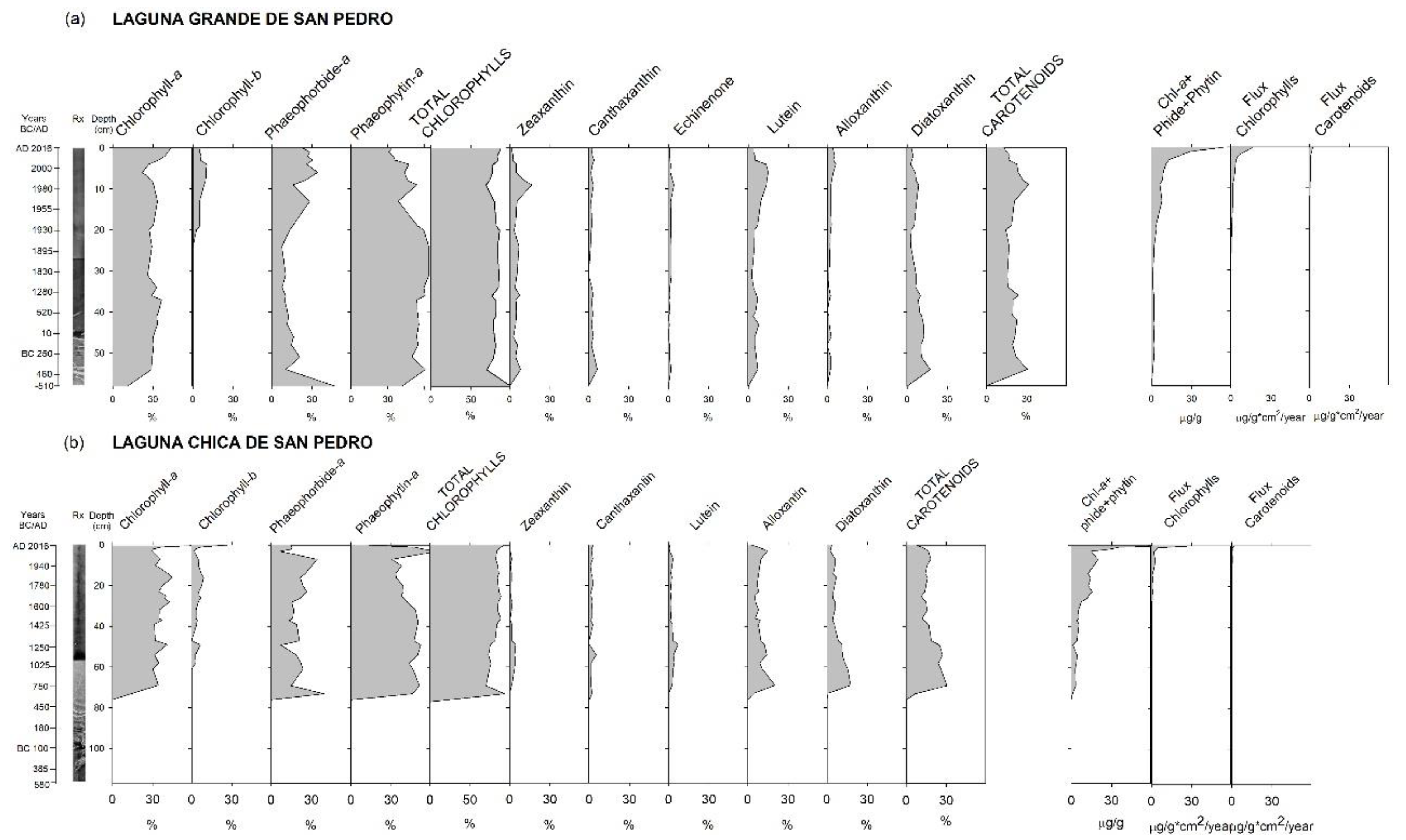
3.3. Fossil Pigments Analysis
4. Discussion
4.1. Changes in Sediment Deposition
4.2. Late Holocene Evolution of Lake Primary Production
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hakanson, L.; Jansson, M. Principles of Lake Sedimentology; Springer: Berlin/Heidelberg, Germany, 1983; Volume 330. [Google Scholar] [CrossRef]
- Bertrand, S.; Boës, X.; Castiaux, J.; Charlet, F.; Urrutia, R.; Espinoza, C.; Lepoint, G.; Charlier, B.; Fagel, N. Temporal evolution of sediment supply in Lago Puyehue (Southern Chile) during the last 600 yr and its climatic significance. Quat. Res. 2005, 64, 163–175. [Google Scholar] [CrossRef]
- Schindler, D.W. Lakes as sentinels and integrators for the effects of climate change on watersheds, airsheds, and landscapes. Limnol. Oceanogr. 2009, 54, 2349–2358. [Google Scholar] [CrossRef]
- Ahmad, K.; Davies, C. Stable isotope (δ13C and δ15N) based interpretation of organic matter source and paleoenvironmental conditions in Al-Azraq basin, Jordan. Appl. Geochem. 2017, 78, 49–60. [Google Scholar] [CrossRef]
- Maberly, S.C.; O’Donnell, R.A.; Woolway, R.I.; Cutler, M.E.J.; Gong, M.; Jones, I.D.; Merchant, C.J.; Miller, C.A.; Politi, E.; Scott, E.M.; et al. Global lake thermal regions shift under climate change. Nat. Commun. 2020, 11, 1232. [Google Scholar] [CrossRef] [PubMed]
- Birks, H.J.B. Multi-proxy studies in palaeolimnology. Veg. Hist. Archaeobot. 2006, 15, 235–251. [Google Scholar] [CrossRef]
- Leavitt, P.R.; Fritz, S.C.; Anderson, N.J.; Baker, P.A.; Blenckner, T.; Bunting, L.; Catalán, D.J.C.; Hobbs, W.O.; Jeppesen, E.; Korhola, A.; et al. Paleolimnological evidence of the effects on lakes of energy and mass transfer from climate and humans. Limnol. Oceanogr. 2009, 54, 2330–2348. [Google Scholar] [CrossRef]
- Villa-Martínez, R.; Villagrán, C. Historia de la vegetación de bosques pantanosos de la costa de Chile central durante el Holoceno medio y tardío. Rev. Chil. Hist. Nat. 1997, 70, 391–401. [Google Scholar]
- Jenny, B.; Valero-Garcés, B.L.; Villa-Martínez, R.; Urrutia, R.; Geyh, M.; Veit, H. Early to Mid-Holocene Aridity in Central Chile and the Southern Westerlies: The Laguna Aculeo Record (34°S). Quat. Res. 2002, 58, 160–170. [Google Scholar] [CrossRef]
- Maldonado, A.; Villagrán, C. Climate variability over the last 9900 cal yr BP from a swamp forest pollen record along the semiarid coast of Chile. Quat. Res. 2006, 66, 246–258. [Google Scholar] [CrossRef]
- Fletcher, M.-S.; Moreno, P.I. Vegetation, climate and fire regime changes in the Andean region of southern Chile (38°S) covaried with centennial-scale climate anomalies in the tropical Pacific over the last 1500 years. Quat. Sci. Rev. 2012, 46, 46–56. [Google Scholar] [CrossRef]
- Frugone-Álvarez, M.; Latorre, C.; Giralt, S.; Polanco-Martínez, J.; Bernárdez, P.; Oliva-Urcia, B.; Maldonado, A.; Carrevedo, M.L.; Moreno, A.; Huertas, A.D.; et al. A 7000-year high-resolution lake sediment record from coastal central Chile (Lago Vichuquén, 34°S): Implications for past sea level and environmental variability. J. Quat. Sci. 2017, 32, 830–844. [Google Scholar] [CrossRef]
- Cruces, F.; Urrutia, R.; Araneda, A.; Torres, L.; Cisternas, M.; Vyverman, W. Evolución trófica de Laguna Grande de San Pedro (VIII Región, Chile) durante el último siglo, mediante el análisis de registros sedimentarios. Rev. Chil. Hist. Nat. 2001, 74, 407–418. [Google Scholar] [CrossRef]
- Cisternas-Vega, M.; Torrejón-Godoy, F. Cambios de Uso del Suelo, Actividades Agropecuarias e Intervención Ambiental Temprana en una Localidad fronteriza de la Araucanía (S. XVI-XIX). Rev. Geogr. Norte Gd. 2002, 29, 83–94. [Google Scholar]
- Chirinos, L.; Rose, N.; Urrutia, R.; Muñoz, P.; Torrejón, F.; Torres, L.; Cruces, F.; Araneda, A.; Zaror, C. Environmental evidence of fossil fuel pollution in Laguna Chica de San Pedro lake sediments (Central Chile). Environ. Pollut. 2006, 141, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Fuentealba, C.; Henriquez, O. Rol de las poblaciones de Diplodon chilensis (Gray,1828) (Bivalvia: HYRIIDAE) en el estado trófico de la Laguna Chica de San Pedro (Chile). Commun. Soc. Malacol. Urug. 2009, 9, 195–200. Available online: https://www.redalyc.org/articulo.oa?id=52414008003 (accessed on 14 September 2019).
- Adán, L.; Rodrigo, M.; Navarro, X.; Campbell, R.; Quiroz, D.; Sánchez, M. Historia prehispánica de la región Centro-Sur de Chile: Cazadores-recolectores holocénicos y comunidades alfareras (ca. 10.000 años a.C. a 1.550 años d.C.). In Prehistoria en Chile: Desde sus Primeros Habitantes Hasta Los Incas; Falabella, F., Uribe, M., Sanhueza, L., Aldunate, C., Hidalgo, J., Eds.; Editorial Universitaria: Santiago, Chile, 2016; pp. 401–441. [Google Scholar]
- Torrejón, F.; Cisternas, M. Alteraciones del paisaje ecológico araucano por la asimilación mapuche de la agroganadería hispano-mediterránea (siglos XVI y XVII). Rev. Chil. Hist. Nat. 2002, 75, 729–736. [Google Scholar] [CrossRef]
- Torrejón, F.; Cisternas, M. Impacto ambiental temprano en la Araucanía deducido de crónicas españolas y estudios historiográficos. Bosque 2003, 24, 45–55. [Google Scholar] [CrossRef][Green Version]
- Parra, O. La eutroficación de la Laguna Grande de San Pedro, Concepción, Chile: Un caso de estudio. Ambient. Desarro. 1989, 1, 117–136. [Google Scholar]
- Cisternas, M.; Torres, L.; Urrutia, R.; Araneda, A.; Parra, O. Comparación ambiental, mediante registros sedimentarios, entre las condiciones prehispánicas y actuales de un sistema lacustre. Rev. Chil. Hist. Nat. 2000, 73, 151–162. [Google Scholar] [CrossRef]
- Urrutia, R.; Koen, S.; Cruces, F.; Pozo, K.; Becerra, J.; Araneda, A.; Vyverman, W.; Parra, O. Paleolimnological studies of Laguna Chica of San Pedro (VIII Region): Diatoms, hydrocarbons and fatty acid records. Rev. Chil. Hist. Nat. 2000, 73, 717–728. [Google Scholar] [CrossRef]
- Parra, O.; Valdovinos, C.; Urrutia, R.; Cisternas, M.; Habit, E.; Mardones, M. Caracterización y tendencias tróficas de cinco lagos costeros de Chile Central. Limnetica 2003, 22, 51–83. [Google Scholar]
- Almanza, V.; Parra, O.; Bicudo, C.E.D.M.; González, M.A.; Lopez, M.; Urrutia, R. Floraciones de fitoplancton y variación de la estructura comunitaria fitoplanctónica en tres lagos someros eutróficos de Chile Central. Gayana Bot. 2016, 73, 191–205. [Google Scholar] [CrossRef]
- Wanner, H.; Beer, J.; Bütikofer, J.; Crowley, T.J.; Cubasch, U.; Flückiger, J.; Goosse, H.; Grosjean, M.; Joos, F.; Kaplan, J.O.; et al. Mid- to Late Holocene climate change: An overview. Quat. Sci. Rev. 2008, 27, 1791–1828. [Google Scholar] [CrossRef]
- Paerl, H.W.; Paul, V.J. Climate change: Links to global expansion of harmful cyanobacteria. Water Res. 2012, 46, 1349–1363. [Google Scholar] [CrossRef] [PubMed]
- Woolway, R.I.; Kraemer, B.M.; Lenters, J.D.; Merchant, C.J.; O’Reilly, C.M.; Sharma, S. Global lake responses to climate change. Nat. Rev. Earth Environ. 2020, 1, 388–403. [Google Scholar] [CrossRef]
- Jenny, B.; Valero-Garcés, B.L.; Urrutia, R.; Kelts, K.; Veit, H.; Appleby, P.G.; Geyh, M. Moisture changes and fluctuations of the Westerlies in Mediterranean Central Chile during the last 2000 years: The Laguna Aculeo record (33°50′S). Quat. Int. 2002, 87, 3–18. [Google Scholar] [CrossRef]
- Leavitt, P.R.; Cumming, B.F.; Smol, J.P.; Reasoner, M.; Pienitz, R.; Hodgson, D.A. Climatic control of ultraviolet radiation effects on lakes. Limnol. Oceanogr. 2003, 48, 2062–2069. [Google Scholar] [CrossRef]
- Pal, S.; Gregory-Eaves, I.; Pick, F.R. Temporal trends in cyanobacteria revealed through DNA and pigment analyses of temperate lake sediment cores. J. Paleolimnol. 2015, 54, 87–101. [Google Scholar] [CrossRef]
- Züllig, H. Untersuchungen über die Stratigraphie von Carotinoiden im geschichteten Sediment von 10 Schweizer Seen zur Erkundung früherer Phytoplankton-Entfaltungen. Aquat. Sci. 1982, 44, 1–98. [Google Scholar] [CrossRef]
- Leavitt, P.R.; Hodgson, A. Sedimentary pigments. In Tracking Environmental Change Using Lake Sediments: Terrestrial, Algal and Siliceous Indicators; Smol, J.P., Birks, H.J.B., Last, W.M., Eds.; Kluwer Academic Publishers: New York, NY, USA, 2001; Volume 3, pp. 295–325. [Google Scholar] [CrossRef]
- Lami, A.; Musazzi, S.; Marchetto, A.; Buchaca, T.; Kernan, M.; Jeppesen, E.; Guilizzoni, P. Sedimentary pigments in 308 alpine lakes and their relation to environmental gradients. Adv. Limnol. 2009, 62, 247–268. [Google Scholar] [CrossRef]
- Bianchi, T.S.; Canuel, E.A. Chemical Biomarkers in Aquatic Ecosystems; Princeton University Press: Princeton, NJ, USA, 2011; Volume 392. [Google Scholar] [CrossRef]
- Gregory-Eaves, I.; Beisner, B.E. Palaeolimnological insights for biodiversity science: An emerging field. Freshw. Biol. 2011, 56, 2653–2661. [Google Scholar] [CrossRef]
- Schneider, T.; Rimer, D.; Butz, C.; Grosjean, M. A high-resolution pigment and productivity record from the varved Ponte Tresa basin (Lake Lugano, Switzerland) since 1919: Insight from an approach that combines hyperspectral imaging and high-performance liquid chromatography. J. Paleolimnol. 2018, 60, 381–398. [Google Scholar] [CrossRef]
- Sanchini, A.; Grosjean, M. Quantification of chlorophyll a, chlorophyll b and pheopigments a in lake sediments through deconvolution of bulk UV–VIS absorption spectra. J. Paleolimnol. 2020, 64, 243–256. [Google Scholar] [CrossRef]
- Sanchini, A.; Szidat, S.; Tylmann, W.; Vogel, H.; Wacnik, A.; Grosjean, M. A Holocene high-resolution record of aquatic productivity, seasonal anoxia and meromixis from varved sediments of Lake Łazduny, North-Eastern Poland: Insight from a novel multi-proxy approach. J. Quat. Sci. 2020, 35, 1070–1080. [Google Scholar] [CrossRef]
- Sanger, J.E. Fossil pigments in paleoecology and paleolimnology. Palaeogeogr. Palaeoclim. Palaeoecol. 1988, 62, 343–359. [Google Scholar] [CrossRef]
- Cuddington, K.; Leavitt, P.R. An individual-based model of pigment flux in lakes: Implications for organic biogeochemistry and paleoecology. Can. J. Fish. Aquat. Sci. 1999, 56, 1964–1977. [Google Scholar] [CrossRef]
- Kowalewska, G. Algal pigments in Baltic sediments as markers of ecosystem and climate changes. Clim. Res. 2001, 18, 89–96. [Google Scholar] [CrossRef]
- Reuss, N.; Leavitt, P.R.; Hall, R.I.; Bigler, C.; Hammarlund, D. Development and application of sedimentary pigments for assessing effects of climatic and environmental changes on subarctic lakes in northern Sweden. J. Paleolimnol. 2009, 43, 149–169. [Google Scholar] [CrossRef]
- Amann, B.; Lobsiger, S.; Fischer, D.; Tylmann, W.; Bonk, A.; Filipiak, J.; Grosjean, M. Spring temperature variability and eutrophication history inferred from sedimentary pigments in the varved sediments of Lake Żabińskie, north-eastern Poland, AD 1907–2008. Glob. Planet. Chang. 2014, 123, 86–96. [Google Scholar] [CrossRef]
- Tse, T.; Doig, L.; Leavitt, P.; Quiñones-Rivera, Z.; Codling, G.; Lucas, B.; Liber, K.; Giesy, J.; Wheater, H.; Jones, P. Long-term spatial trends in sedimentary algal pigments in a narrow river-valley reservoir, Lake Diefenbaker, Canada. J. Great Lakes Res. 2015, 41, 56–66. [Google Scholar] [CrossRef]
- Gu, B.; Schelske, C.L.; Brenner, M. Relationship between sediment and plankton isotope ratios (δ13C and δ15N) and primary productivity in Florida lakes. Can. J. Fish. Aquat. Sci. 1996, 53, 875–883. [Google Scholar] [CrossRef]
- Verleyen, E.; Hodgson, D.A.; Leavitt, P.R.; Sabbe, K.; Vyverman, W. Quantifying habitat-specific diatom production: A critical assessment using morphological and biogeochemical markers in Antarctic marine and lake sediments. Limnol. Oceanogr. 2004, 49, 1528–1539. [Google Scholar] [CrossRef]
- Deshpande, B.N.; Tremblay, R.; Pienitz, R.; Vincent, W.F. Sedimentary pigments as indicators of cyanobacterial dynamics in a hypereutrophic lake. J. Paleolimnol. 2014, 52, 171–184. [Google Scholar] [CrossRef]
- Talbot, M.R.; Johannessen, T. A high resolution palaeoclimatic record for the last 27,500 years in tropical West Africa from the carbon and nitrogen isotopic composition of lacustrine organic matter. Earth Planet. Sci. Lett. 1992, 110, 23–37. [Google Scholar] [CrossRef]
- Hassan, K.M.; Swinehart, J.B.; Spalding, R.F. Evidence for Holocene environmental change from C/N ratios, and δ13C and δ15N values in Swan Lake sediments, western Sand Hills, Nebraska. J. Paleolimnol. 1997, 18, 121–130. [Google Scholar] [CrossRef]
- Talbot, M.R.; Lærdal, T. The Late Pleistocene—Holocene palaeolimnology of Lake Victoria, East Africa, based upon elemental and isotopic analyses of sedimentary organic matter. J. Paleolimnol. 2000, 23, 141–164. [Google Scholar] [CrossRef]
- Routh, J.; Choudhary, P.; Meyers, P.A.; Kumar, B. A sediment record of recent nutrient loading and trophic state change in Lake Norrviken, Sweden. J. Paleolimnol. 2008, 42, 325–341. [Google Scholar] [CrossRef]
- Gell, P.; Bennion, H.; Battarbee, R. Paleolimnology and the restoration of aquatic systems. PAGES Newsl. 2009, 17, 119–120. [Google Scholar] [CrossRef][Green Version]
- Malone, T.C.; Newton, A. The Globalization of Cultural Eutrophication in the Coastal Ocean: Causes and Consequences. Front. Mar. Sci. 2020, 7, 670. [Google Scholar] [CrossRef]
- Cisternas, M.; Araneda, A.; Retamal, O.; Urrutia, R. Sedimentos como indicadores de eventos erosivos en una pequeña cuenca lacustre de Chile Central. Espac. Desarro. 1997, 9, 103–116. [Google Scholar]
- Fagel, N.; Bertrand, S.; Mattielli, N.; Gilson, D.; Chirinos, L.; Lepoint, G.; Urrutia, R. Geochemical evidence (C, N and Pb isotopes) of recent anthropogenic impact in south-central Chile from two environmentally distinct lake sediment records. J. Quat. Sci. 2010, 25, 1100–1112. [Google Scholar] [CrossRef]
- Martinez, C.; Rojas, C.; Rojas, O.; Quezada, J.; Lopez, P.; Ruiz, V. Crecimiento urbano sobre geoformas costeras de la llanura de San Pedro, área Metropolitana de Concepción. In En las Costas del Neoliberalismo: Naturaleza, Urbanización y Producción Inmobiliaria: Experiencias en Chile y Argentina; Hidalgo, R., Santana, D., Voltaire, A., Arenas, F., Salazar, A., Valdebenito, C., Alvarez, L., Eds.; No. 23; Geolibros: Santiago, Chile, 2016; pp. 287–312. [Google Scholar]
- Dobry, R.; Poblete, M. Densidades máximas y mínimas de las arenas Bio-Bio. Rev. IDIEM 1966, 5, 151–159. Available online: https://revistaidiem.uchile.cl/index.php/RIDIEM/article/view/38465 (accessed on 20 November 2020).
- Isla, F.I.; Flory, J.Q.; Martıinez, C.; Fernandez, A.; Jaque, E. The evolution of the Bío Bío delta and the coastal plains of the Arauco Gulf, Bío Bío Region: The Holocene sea-level curve of Chile. J. Coast. Res. 2012, 28, 102–111. [Google Scholar] [CrossRef]
- Mortlock, R.A.; Froelich, P.N. A simple method for the rapid determination of biogenic opal in pelagic marine sediments. Deep. Sea Res. Part A Oceanogr. Res. Pap. 1989, 36, 1415–1426. [Google Scholar] [CrossRef]
- Blott, S.J.; Pye, K. GRADISTAT: A grain size distribution and statistics package for the analysis of unconsolidated sediments. Earth Surf. Process. Landf. 2001, 26, 1237–1248. [Google Scholar] [CrossRef]
- Castro, J.M.T.; Vergara, C.; Alvarez, D.; Díaz, G.; Fierro, P.; Araneda, A.; Torrejón, F.; Rondanelli, M.; Fagel, N.; Urrutia, R. A new multi-proxy record of environmental change over the last 1000 years on Chiloé Island: Lake Pastahué, south-central Chile (42°S). Holocene 2018, 29, 421–431. [Google Scholar] [CrossRef]
- Ramsey, C.B. OxCal Program Version 3.10; Manual; Oxford Radiocarbon Accelerator Unit: Oxford, UK, 2005; Available online: https://c14.arch.ox.ac.uk/oxcal3/oxcal.htm (accessed on 10 October 2020).
- Hogg, A.G.; Hua, Q.; Blackwell, P.G.; Niu, M.; Buck, C.E.; Guilderson, T.P.; Heaton, T.J.; Palmer, J.G.; Reimer, P.J.; Reimer, R.W.; et al. SHCal13 Southern Hemisphere Calibration, 0–50,000 Years cal BP. Radiocarbon 2013, 55, 1889–1903. [Google Scholar] [CrossRef]
- Blaauw, M. Methods and code for ‘classical’ age-modelling of radiocarbon sequences. Quat. Geochronol. 2010, 5, 512–518. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; Version 2.6.2; R Foundation for Statistical Computing: Vienna, Austria, 2016; Available online: https://www.r-project.org/ (accessed on 14 September 2018).
- Freudenthal, T.; Wagner, T.; Wenzhöfer, F.; Zabel, M.; Wefer, G. Early diagenesis of organic matter from sediments of the eastern subtropical Atlantic: Evidence from stable nitrogen and carbon isotopes. Geochim. Cosmochim. Acta 2001, 65, 1795–1808. [Google Scholar] [CrossRef]
- Nyssen, F.; Brey, T.; Lepoint, G.; Bouquegneau, J.-M.; De Broyer, C.; Dauby, P. A stable isotope approach to the eastern Weddell Sea trophic web: Focus on benthic amphipods. Polar Biol. 2002, 25, 280–287. [Google Scholar] [CrossRef][Green Version]
- Vaslet, A.; Bouchon-Navaro, Y.; Harmelin-Vivien, M.; Lepoint, G.; Louis, M.; Bouchon, C. Foraging habits of reef fishes associated with mangroves and seagrass beds in a Caribbean lagoon: A stable isotope approach. Cienc. Mar. 2015, 41, 217–232. [Google Scholar] [CrossRef][Green Version]
- Van Heukelem, L.; Thomas, C.S. Computer-assisted high-performance liquid chromatography method development with applications to the isolation and analysis of phytoplankton pigments. J. Chromatogr. A 2001, 910, 31–49. [Google Scholar] [CrossRef]
- Meyer, I.; Van Daele, M.; Fiers, G.; Verleyen, E.; De Batist, M.; Verschuren, D. Sediment reflectance spectroscopy as a paleo-hydrological proxy in East Africa. Limnol. Oceanogr. Methods 2017, 16, 92–105. [Google Scholar] [CrossRef]
- Reuss, N. Sediment Pigments as Biomarkers of Environmental Change. Ph.D. Thesis, University of Copenhagen, København, Denmark, 2005. [Google Scholar]
- Verleyen, E.; Hodgson, D.A.; Sabbe, K.; Vyverman, W. Late Holocene changes in ultraviolet radiation penetration recorded in an East Antarctic lake. J. Paleolimnol. 2005, 34, 191–202. [Google Scholar] [CrossRef]
- Legendre, P.; Gallagher, E.D. Ecologically meaningful transformations for ordination of species data. Oecologia 2001, 129, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Stupar, Y.V.; Schäfer, J.; García, M.G.; Schmidt, S.; Piovano, E.; Blanc, G.; Huneau, F.; Le Coustumer, P. Historical mercury trends recorded in sediments from the Laguna del Plata, Córdoba, Argentina. Geochemistry 2014, 74, 353–363. [Google Scholar] [CrossRef]
- Veit, H. Southern Westerlies during the Holocene deduced from geomorphological and pedological studies in the Norte Chico, Northern Chile (27–33°S). Palaeogeogr. Palaeoclim. Palaeoecol. 1996, 123, 107–119. [Google Scholar] [CrossRef]
- Link, O.; Brox-Escudero, L.M.; González, J.; Aguayo, M.; Torrejón, F.; Montalva, G.; Eguibar-Galán, M.Á. A paleo-hydro-geomorphological perspective on urban flood risk assessment. Hydrol. Process. 2019, 33, 3169–3183. [Google Scholar] [CrossRef]
- Aronson, J.; Del Pozo, A.; Ovalle, C.; Avendaño, J.; Lavin, A.; Etienne, M. Land Use Changes and Conflicts in Central Chile. In Landscape Disturbance and Biodiversity in Mediterranean-Type Ecosystems; Rundel, P.W., Montenegro, R.G., Jaksic, F.M., Eds.; Ecological Studies: Berlin, Germany, 1998; pp. 155–168. [Google Scholar] [CrossRef]
- Koch, A.; Brierley, C.; Maslin, M.M.; Lewis, S.L. Earth system impacts of the European arrival and Great Dying in the Americas after 1492. Quat. Sci. Rev. 2019, 207, 13–36. [Google Scholar] [CrossRef]
- Cisternas, M.; Araneda, A.; Martínez, P.; Pérez, S. Effects of historical land use on sediment yield from a lacustrine wa-tershed in central Chile. Earth Surf. Process. Landf. 2001, 26, 63–76. [Google Scholar] [CrossRef]
- Basualto, S.; del Valle, J.; Gil, M.V.; Figueroa, R.; Parra, O.; Gonzalez, A.; Stehr, A. Modelos de gestión, conflictos y mediación en cuencas hidrográficas: Los casos de España y Brasil y su aplicabilidad a Chile. Aqua-LAC 2019, 11, 66–76. [Google Scholar] [CrossRef]


| Laguna Chica | Laguna Grande | |
|---|---|---|
| Latitude | 36°51′ | 36°51′ |
| Longitude | 73°05′ | 73°06′ |
| Altitude (m.a.s.l.) | 5 | 4 |
| Lake area (km2) | 0.82 | 1.55 |
| Watershed area (km2) | 4.5 | 12.7 |
| Maximum depth (m) | 18 | 13.5 |
| Volume (m3 × 106) | 8.64 | 12.9 |
| Native forest and shreds | 27.1% | 5.7% |
| Forest plantations | 48.9% | 52.4% |
| Agriculture | 3.4% | 1.0% |
| Urban area | 4.9% | 4.1% |
| Water transparency (m) | 5.2 (3.8–6.8) | 3.7 (3.0–6.1) |
| Water temperature °C (min-max) | 17.4 (12.5–24.0) | 18.1 (12.2–24.0) |
| Annual mean precipitation (mm) | 1.235 mm | 1.235 mm |
| pH | 7.0 (6.5–7.73) | 7.0 (6.5-7.6) |
| Conductivity (µS/cm) | 71 (50–90) | 84 (35–107) |
| Dissolved oxygen (mg/L) | 9.2 (5.9–10.9) | 8.8 (4.9–10.7) |
| Alkalinity (meq/L CaCO3) | 0.4 (0.4–0.5) | 0.5 (0.5–0.6) |
| TDS (mg/L) | 42.3 | 52.6 |
| Total phosphorus TP (µg/L) | 20 | 50 |
| Total nitrogen TN (µg/L) | 170 (60–320) | 230 (80–340) |
| Chlorophyll a (µg/L) | 1.20 | 6.42 |
| Primary productivity (mgC/m3/hr) | 2.44 | 22.68 |
| Trophic state | Mesotrophic-Eutrophic | Eutrophic-Hypereutrophic |
| Macrophytes | Abundant | Major abundance |
| Summer stratification | Yes | No |
| Phytoplankton richness | 39 | 57 |
| Main phytoplankton groups | Bacillariophyceae Chlorophyceae Desmideaceae Cyanobacteria | Chlorophyceae Bacillariophyceae Desmideaceae Cyanobacteria |
| Reports of Cyanobacteria | Microcystis sp. (blooms) Dolichospermum sp. Merismopedia sp. Oscillatoria sp. Aphanocapsa sp. | Microcystis sp. (blooms) Dolichospermum sp. Pseudoanabaena sp. Gomphospheria sp. Chroococcus sp. Snowella sp. |
| Lab Code | Lake | Core Depth (cm) | 14C yr BP ±1σ | Calibrate Age BC/AD | ||
|---|---|---|---|---|---|---|
| Min | Mean | Max | ||||
| ETH–70456 | Laguna grande | 41 | 1707 ± 23 | 339 | 385 | 431 |
| ETH–70457 | Laguna grande | 51 | 2276 ± 24 | −325 | −267 | −209 |
| ETH–70458 | Laguna grande | 71 | 3467 ± 25 | −1780 | −1707 | −1635 |
| D-AMS 025916 | Laguna Chica | 54.5 | 936 ± 26 | 1129 | 1172 | 1216 |
| D-AMS 025914 | Laguna Chica | 116.5 | 2482 ± 43 | −599 | −503 | −407 |
| Laguna Grande de San Pedro | |||
| Clay (%) | Silt (%) | Sand (%) | |
| UNIT 2 (46–0 cm) | 7.12 ± 0.83 | 90.75 ± 1.95 | 2.03 ± 2.38 |
| UNIT 1 (60–47 cm) | 4.64 ± 0.91 | 80.88 ± 4.13 | 14.48 ± 3.76 |
| Laguna Chica de San Pedro | |||
| Clay (%) | Silt (%) | Sand (%) | |
| UNIT 2 (93–0 cm) | 7.05 ± 2.45 | 90.12 ± 4.09 | 2.83 ± 2.72 |
| UNIT 1 (120–94cm) | 4.68 ± 1.62 | 87.82 ± 2.86 | 7.50 ± 2.65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montes, I.-Y.; Banegas-Medina, A.; Fagel, N.; El Ouahabi, M.; Verleyen, E.; Alvarez, D.; Torrejón, F.; Schmidt, S.; Lepoint, G.; Diaz, G.; et al. Late Holocene Paleonvironmental Evolution of Two Coastal Lakes in Mediterranean Chile and Its Implications for Conservation Planning. Appl. Sci. 2021, 11, 3478. https://doi.org/10.3390/app11083478
Montes I-Y, Banegas-Medina A, Fagel N, El Ouahabi M, Verleyen E, Alvarez D, Torrejón F, Schmidt S, Lepoint G, Diaz G, et al. Late Holocene Paleonvironmental Evolution of Two Coastal Lakes in Mediterranean Chile and Its Implications for Conservation Planning. Applied Sciences. 2021; 11(8):3478. https://doi.org/10.3390/app11083478
Chicago/Turabian StyleMontes, Isis-Yelena, Andy Banegas-Medina, Nathalie Fagel, Meriam El Ouahabi, Elie Verleyen, Denisse Alvarez, Fernando Torrejón, Sabine Schmidt, Gilles Lepoint, Gustavo Diaz, and et al. 2021. "Late Holocene Paleonvironmental Evolution of Two Coastal Lakes in Mediterranean Chile and Its Implications for Conservation Planning" Applied Sciences 11, no. 8: 3478. https://doi.org/10.3390/app11083478
APA StyleMontes, I.-Y., Banegas-Medina, A., Fagel, N., El Ouahabi, M., Verleyen, E., Alvarez, D., Torrejón, F., Schmidt, S., Lepoint, G., Diaz, G., Pedreros, P., & Urrutia, R. (2021). Late Holocene Paleonvironmental Evolution of Two Coastal Lakes in Mediterranean Chile and Its Implications for Conservation Planning. Applied Sciences, 11(8), 3478. https://doi.org/10.3390/app11083478








