Effects of Custom-Made Insole Materials on Frictional Stress and Contact Pressure in Diabetic Foot with Neuropathy: Results from a Finite Element Analysis
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
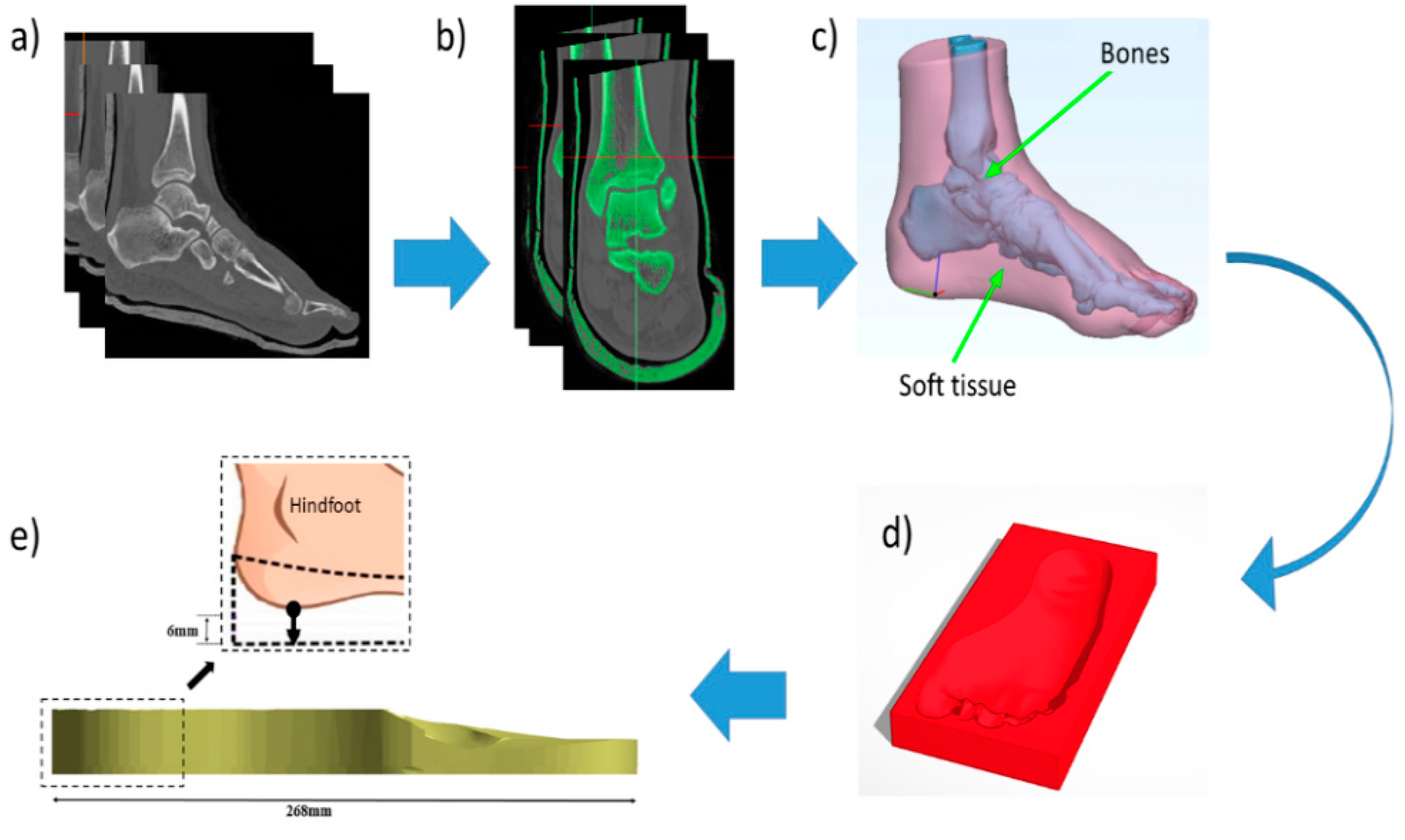
2.1. Finite Element Model Generation
2.2. Material Properties
2.3. Meshing
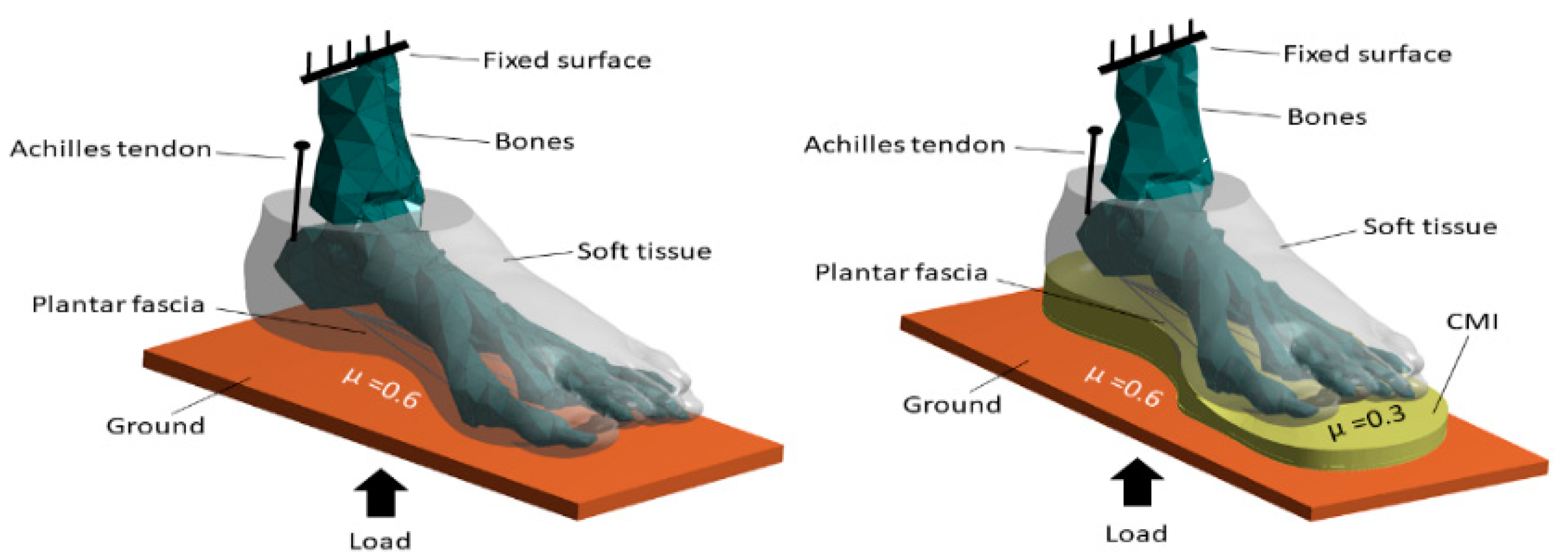
2.4. Loading and Boundary Conditions
2.5. Validation
3. Results
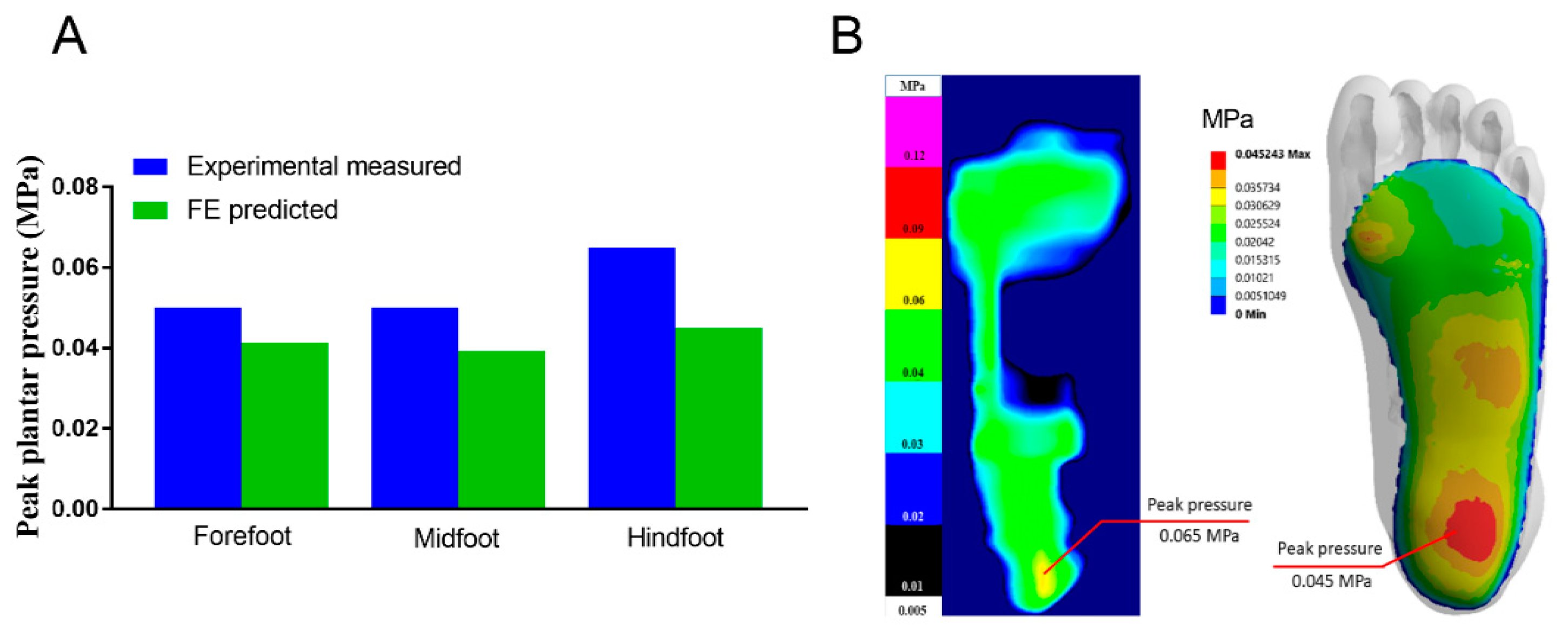
3.1. Mesh Convergence and Model Validation
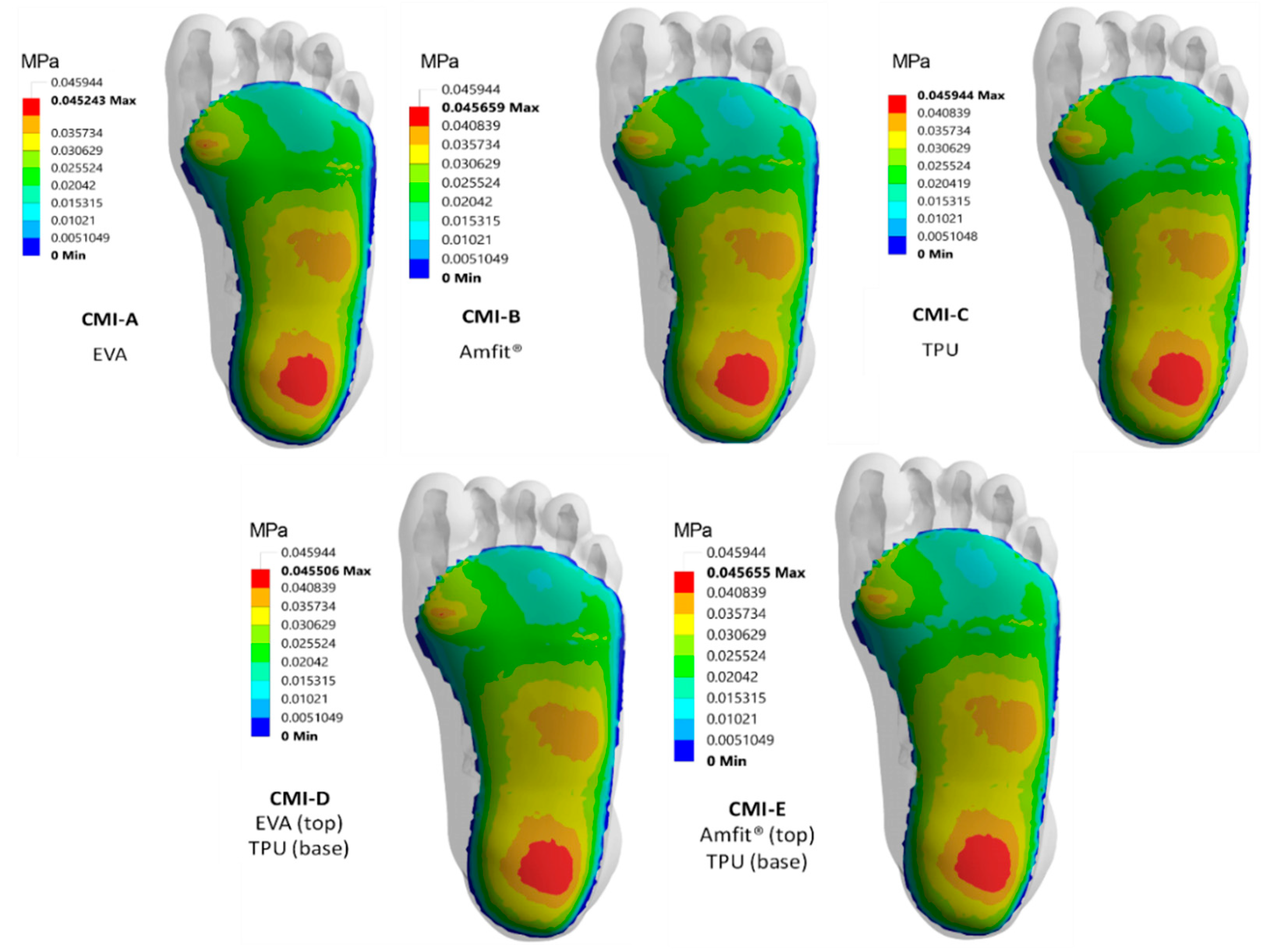
3.2. Contact Pressure Distribution
3.3. Regional Peak Contact Pressure
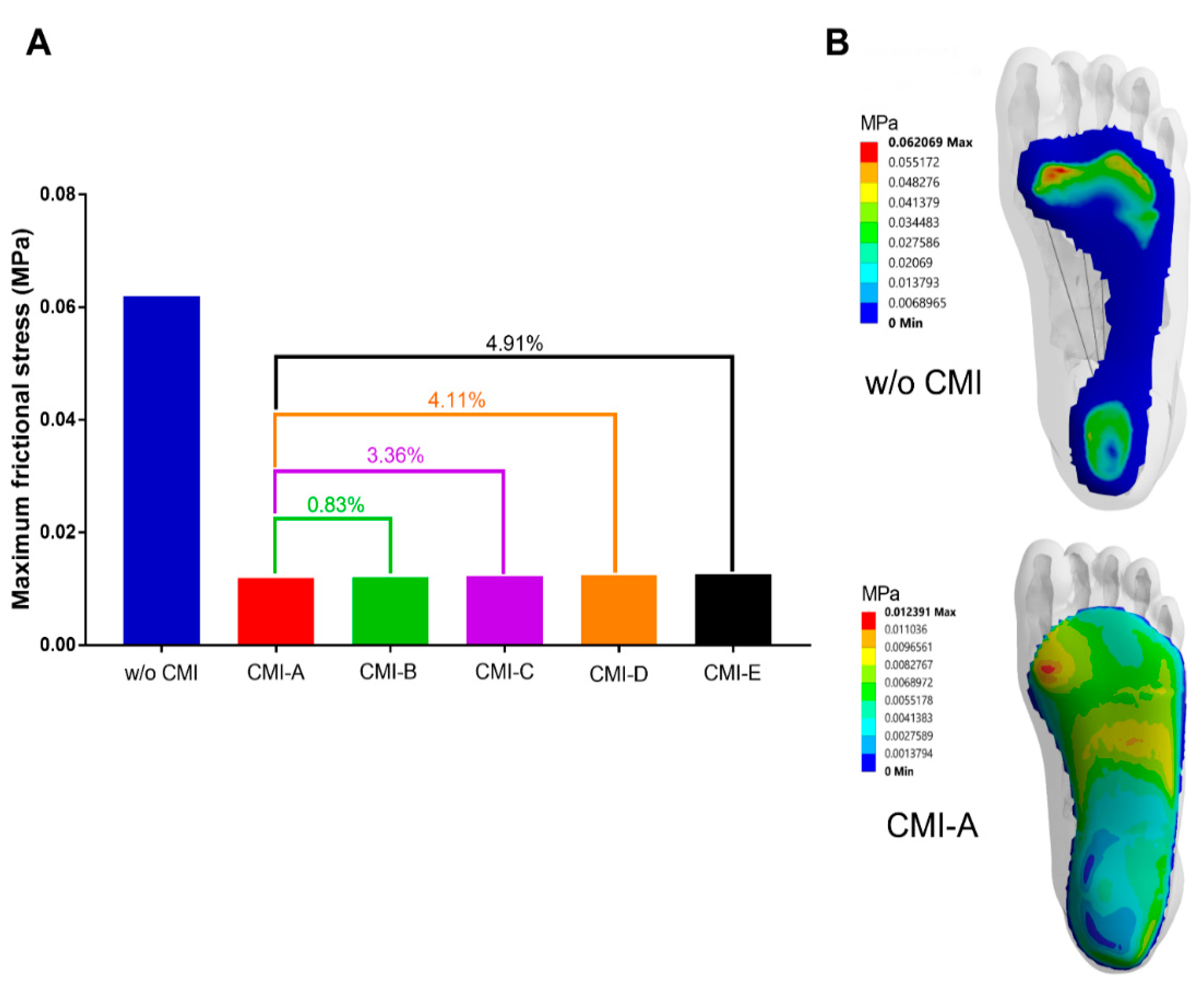
3.4. Frictional Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Yazdanpanah, L.; Shahbazian, H.; Nazari, I.; Arti, H.R.; Ahmadi, F.; Mohammadianinejad, S.E.; Cheraghian, B.; Hesam, S. Incidence and Risk Factors of Diabetic Foot Ulcer: A Population-Based Diabetic Foot Cohort (ADFC Study)-Two-Year Follow-Up Study. Int. J. Endocrinol. 2018, 2018, 7631659. [Google Scholar] [CrossRef]
- Yazdanpanah, L.; Nasiri, M.; Adarvishi, S. Literature review on the management of diabetic foot ulcer. World J. Diabetes 2015, 6, 37–53. [Google Scholar] [CrossRef]
- Armstrong, D.G.; Boulton, A.J.M.; Bus, S.A. Diabetic Foot Ulcers and Their Recurrence. N. Engl. J. Med. 2017, 376, 2367–2375. [Google Scholar] [CrossRef] [PubMed]
- Pemayun, T.G.D.; Naibaho, R.M. Clinical profile and outcome of diabetic foot ulcer, a view from tertiary care hospital in Semarang, Indonesia. Diabet Foot Ankle 2017, 8, 1312974. [Google Scholar] [CrossRef]
- Markakis, K.; Bowling, F.L.; Boulton, A.J. The diabetic foot in 2015: An overview. Diabetes Metab. Res. Rev. 2016, 32 (Suppl. 1), 169–178. [Google Scholar] [CrossRef]
- Schaper, N.C.; Van Netten, J.J.; Apelqvist, J.; Lipsky, B.A.; Bakker, K. International Working Group on the Diabetic Foot. Prevention and management of foot problems in diabetes: A Summary Guidance for Daily Practice 2015, based on the IWGDF Guidance Documents. Diabetes Metab. Res. Rev. 2016, 32 (Suppl. 1), 7–15. [Google Scholar] [CrossRef] [PubMed]
- Boulton, A.J.; Kirsner, R.S.; Vileikyte, L. Clinical practice. Neuropathic diabetic foot ulcers. N. Engl. J. Med. 2004, 351, 48–55. [Google Scholar] [CrossRef]
- Frykberg, R.G. Diabetic foot ulcers: Pathogenesis and management. Am. Fam. Phys. 2002, 66, 1655–1662. [Google Scholar]
- Grimm, A.; Kastenbauer, T.; Sauseng, S.; Sokol, G.; Irsigler, K. Progression and distribution of plantar pressure in Type 2 diabetic patients. Diabetes Nutr. Metab. 2004, 17, 108–113. [Google Scholar] [PubMed]
- Cheng, Q.; Lazzarini, P.A.; Gibb, M.; Derhy, P.H.; Kinnear, E.M.; Burn, E.; Graves, N.; Norman, R.E. A cost-effectiveness analysis of optimal care for diabetic foot ulcers in Australia. Int. Wound J. 2017, 14, 616–628. [Google Scholar] [CrossRef] [PubMed]
- Norman, G.; Westby, M.J.; Vedhara, K.; Game, F.; Cullum, N.A. Effectiveness of psychosocial interventions for the prevention and treatment of foot ulcers in people with diabetes: A systematic review. Diabet Med. 2020, 37, 1256–1265. [Google Scholar] [CrossRef] [PubMed]
- Zimny, S.; Schatz, H.; Pfohl, M. Determinants and estimation of healing times in diabetic foot ulcers. J. Diabetes Complicat. 2002, 16, 327–332. [Google Scholar] [CrossRef]
- Martin, I.d.S.; Beraldo, A.A.; Passeri, S.M.; Freitas, M.C.F.; Pace, A.E. Causas referidas para o desenvolvimento de úlceras em pés de pessoas com diabetes mellitus. Acta Paul. Enferm. 2012, 25, 218–224. [Google Scholar] [CrossRef]
- Vella, L.; Gatt, A.; Formosa, C. Does Baseline Hemoglobin A1c Level Predict Diabetic Foot Ulcer Outcome or Wound Healing Time? J. Am. Podiatr. Med. Assoc. 2017, 107, 272–279. [Google Scholar] [CrossRef]
- Hanson, D.; Langemo, D.K.; Anderson, J.; Thompson, P.; Hunter, S. Friction and shear considerations in pressure ulcer development. Adv. Ski. Wound Care 2010, 23, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Nouman, M.; Dissaneewate, T.; Leelasamran, W.; Chatpun, S. The insole materials influence the plantar pressure distributions in diabetic foot with neuropathy during different walking activities. Gait Posture 2019, 74, 154–161. [Google Scholar] [CrossRef]
- Nouman, M.; Leelasamran, W.; Chatpun, S. Effectiveness of Total Contact Orthosis for Plantar Pressure Redistribution in Neuropathic Diabetic Patients During Different Walking Activities. Foot Ankle Int. 2017, 38, 901–908. [Google Scholar] [CrossRef]
- Cavanagh, P.R. Therapeutic footwear for people with diabetes. Diabetes Metab. Res. Rev. 2004, 20 (Suppl. 1), S51–S55. [Google Scholar] [CrossRef] [PubMed]
- Bus, S.A.; Ulbrecht, J.S.; Cavanagh, P.R. Pressure relief and load redistribution by custom-made insoles in diabetic patients with neuropathy and foot deformity. Clin. Biomech. (BristolAvon) 2004, 19, 629–638. [Google Scholar] [CrossRef]
- Praet, S.F.; Louwerens, J.W. The influence of shoe design on plantar pressures in neuropathic feet. Diabetes Care 2003, 26, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Cheung, J.T.; Zhang, M. A 3-dimensional finite element model of the human foot and ankle for insole design. Arch. Phys. Med. Rehabil. 2005, 86, 353–358. [Google Scholar] [CrossRef]
- Fontanella, C.G.; Carniel, E.L.; Forestiero, A.; Natali, A.N. Investigation of the mechanical behaviour of the foot skin. Ski. Res. Technol. 2014, 20, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Dian, W.; Ping, C. Finite Element Analysis of the Expression of Plantar Pressure Distribution in the Injury of the Lateral Ligament of the Ankle. Nano Biomed. Eng. 2019, 11, 290–296. [Google Scholar] [CrossRef]
- Ghassemi, A.; Mossayebi, A.R.; Jamshidi, N.; Naemi, R.; Karimi, M.T. Manufacturing and finite element assessment of a novel pressure reducing insole for Diabetic Neuropathic patients. Australas Phys. Eng. Sci. Med. 2015, 38, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Gerrard, J.M.; Bonanno, D.R.; Whittaker, G.A.; Landorf, K.B. Effect of different orthotic materials on plantar pressures: A systematic review. J. Foot Ankle Res. 2020, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Barwick, A.; Butterworth, P.; Nancarrow, S. Footwear and insole design features that reduce neuropathic plantar forefoot ulcer risk in people with diabetes: A systematic literature review. J. Foot Ankle Res. 2020, 13, 30. [Google Scholar] [CrossRef]
- Chatzistergos, P.; Gatt, A.; Formosa, C.; Farrugia, K.; Chockalingam, N. Optimised cushioning in diabetic footwear can significantly enhance their capacity to reduce plantar pressure. Gait Posture 2020, 79. [Google Scholar] [CrossRef]
- Sun, X.; Lam, W.K.; Zhang, X.; Wang, J.; Fu, W. Systematic Review of the Role of Footwear Constructions in Running Biomechanics: Implications for Running-Related Injury and Performance. J. Sports Sci. Med. 2020, 19, 20–37. [Google Scholar]
- Flores, D.V.; Mejia Gomez, C.; Fernandez Hernando, M.; Davis, M.A.; Pathria, M.N. Adult Acquired Flatfoot Deformity: Anatomy, Biomechanics, Staging, and Imaging Findings. Radiographics 2019, 39, 1437–1460. [Google Scholar] [CrossRef]
- Natali, A.N.; Forestiero, A.; Carniel, E.L.; Pavan, P.G.; Dal Zovo, C. Investigation of foot plantar pressure: Experimental and numerical analysis. Med. Biol. Eng. Comput. 2010, 48, 1167–1174. [Google Scholar] [CrossRef]
- Brilakis, E.; Kaselouris, E.; Xypnitos, F.; Provatidis, C.G.; Efstathopoulos, N. Effects of Foot Posture on Fifth Metatarsal Fracture Healing: A Finite Element Study. J. Foot Ankle Surg. 2012, 51, 720–728. [Google Scholar] [CrossRef]
- Chen, W.P.; Ju, C.W.; Tang, F.T. Effects of total contact insoles on the plantar stress redistribution: A finite element analysis. Clin. Biomech. (BristolAvon) 2003, 18, S17–S24. [Google Scholar] [CrossRef]
- Chen, Y.N.; Chang, C.W.; Li, C.T.; Chang, C.H.; Lin, C.F. Finite element analysis of plantar fascia during walking: A quasi-static simulation. Foot Ankle Int. 2015, 36, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G. Finite element analysis of a model of a therapeutic shoe: Effect of material selection for the outsole. Bio-Med. Mater. Eng. 2003, 13, 75–81. [Google Scholar]
- Lo, W.T.; Yick, K.; Ng, Z.; Yip, J. Numerical simulation of orthotic insole deformation for diabetic foot. J. Fiber Bioeng. Inform. 2015, 8, 401–411. [Google Scholar] [CrossRef]
- Frick, A.; Rochman, A. Characterization of TPU-elastomers by thermal analysis (DSC). Polym. Test. 2004, 23, 413–417. [Google Scholar] [CrossRef]
- Su, S.; Mo, Z.; Guo, J.; Fan, Y. The Effect of Arch Height and Material Hardness of Personalized Insole on Correction and Tissues of Flatfoot. J. Healthc. Eng. 2017, 2017, 8614341. [Google Scholar] [CrossRef]
- Qian, Z.; Ren, L.; Ding, Y.; Hutchinson, J.R.; Ren, L. A dynamic finite element analysis of human foot complex in the sagittal plane during level walking. PLoS ONE 2013, 8, e79424. [Google Scholar] [CrossRef]
- Zhang, M.; Mak, A.F. In vivo friction properties of human skin. Prosthet. Orthot. Int. 1999, 23, 135–141. [Google Scholar] [CrossRef]
- Tang, L.; Wang, L.; Bao, W.N.; Zhu, S.Y.; Li, D.C.; Zhao, N.X.; Liu, C.Z. Functional gradient structural design of customized diabetic insoles. J. Mech. Behav. Biomed. 2019, 94, 279–287. [Google Scholar] [CrossRef]
- Mates, M. Atlas of anatomy: General anatomy and musculoskeletal system. Occup. Ther. Health Care 2008, 22, 76–77. [Google Scholar] [CrossRef]
- Goske, S.; Erdemir, A.; Petre, M.; Budhabhatti, S.; Cavanagh, P.R. Reduction of plantar heel pressures: Insole design using finite element analysis. J. Biomech. 2006, 39, 2363–2370. [Google Scholar] [CrossRef]
- Albert, S.; Rinoie, C. Effect of custom orthotics on plantar pressure distribution in the pronated diabetic foot. J. Foot Ankle Surg. 1994, 33, 598–604. [Google Scholar]
- Kitaoka, H.B.; Luo, Z.P.; Kura, H.; An, K.N. Effect of foot orthoses on 3-dimensional kinematics of flatfoot: A cadaveric study. Arch. Phys. Med. Rehabil. 2002, 83, 876–879. [Google Scholar] [CrossRef]
- Caselli, A.; Pham, H.; Giurini, J.; Armstrong, D.; Veves, A. The Forefoot-to-Rearfoot Plantar Pressure Ratio Is Increased in Severe Diabetic Neuropathy and Can Predict Foot Ulceration. Diabetes Care 2002, 25, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Cheung, J.T.; Zhang, M. Parametric design of pressure-relieving foot orthosis using statistics-based finite element method. Med. Eng. Phys. 2008, 30, 269–277. [Google Scholar] [CrossRef]
- Nicolopoulos, C.S.; Black, J.; Anderson, E.G. Foot orthoses materials. Foot 2000, 10, 1–3. [Google Scholar] [CrossRef]
- Hsu, Y.C.; Gung, Y.W.; Shih, S.L.; Feng, C.K.; Wei, S.H.; Yu, C.H.; Chen, C.S. Using an optimization approach to design an insole for lowering plantar fascia stress--a finite element study. Ann. Biomed. Eng. 2008, 36, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Davia-Aracil, M.; Hinojo-Pérez, J.J.; Jimeno-Morenilla, A.; Mora-Mora, H. 3D printing of functional anatomical insoles. Comput. Ind. 2018, 95, 38–53. [Google Scholar] [CrossRef]
- Leber, C.; Evanski, P.M. A comparison of shoe insole materials in plantar pressure relief. Prosthet. Orthot. Int. 1986, 10, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Fauli, A.C.; Andres, C.L.; Rosas, N.P.; Fernandez, M.J.; Parreno, E.M.; Barcelo, C.O. Physical evaluation of insole materials used to treat the diabetic foot. J. Am. Podiatr. Med. Assoc. 2008, 98, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Foto, J.G.; Birke, J.A. Evaluation of Multidensity Orthotic Materials Used in Footwear for Patients with Diabetes. Foot Ankle Int. 1998, 19, 836–841. [Google Scholar] [CrossRef]
- Amemiya, A.; Noguchi, H.; Oe, M.; Takehara, K.; Ohashi, Y.; Suzuki, R.; Yamauchi, T.; Kadowaki, T.; Sanada, H.; Mori, T. Shear Stress-Normal Stress (Pressure) Ratio Decides Forming Callus in Patients with Diabetic Neuropathy. J. Diabetes Res. 2016, 2016, 3157123. [Google Scholar] [CrossRef] [PubMed]
- Cong, Y.; Cheung, J.T.; Leung, A.K.; Zhang, M. Effect of heel height on in-shoe localized triaxial stresses. J. Biomech. 2011, 44, 2267–2272. [Google Scholar] [CrossRef] [PubMed]
- Doupis, J.; Gaber Amin, N. Diabetic foot disease: From the evaluation of the "foot at risk" to the novel diabetic ulcer treatment modalities. World J. Diabetes 2016, 7. [Google Scholar] [CrossRef]
| Components | Young’s Modulus (MPa) | Poisson’s Ratio | References |
|---|---|---|---|
| Bone | 7300 | 0.3 | Brilakis et al. [31] |
| Soft tissue | 0.19 | 0.49 | Chen et al. [32] |
| Plantar fascia | 350 | 0.35 | Chen et al. [33] |
| EVA (CMI-A 6 mm) | 5 | 0.40 | Lewis et al. [34] |
| Amfit® (CMI-B 6 mm) | 8.97 | 0.39 | Lo et al. [35] |
| TPU (CMI-C 6 mm) | 11 | 0.45 | Frick et al. [36] |
| Ground | 2,100,000 | 0.3 | Su et al. [37] |
| Type | Top Layer | Base Layer |
|---|---|---|
| CMI-D | EVA (3 mm) | TPU (3 mm) |
| CMI-E | Amfit® (3 mm) | TPU (3 mm) |
| CMIs | Peak Contact Pressure (kPa) | Ratio | ||||
|---|---|---|---|---|---|---|
| FF | MF | HF | MF/FF | HF/FF | MF/HF | |
| w/o | 275.0 | - | 279.0 | - | 0.98 | - |
| CMI-A | 41.3 | 39.3 | 45.2 | 0.95 | 0.91 | 0.87 |
| CMI-B | 40.2 | 39.5 | 45.6 | 0.98 | 0.88 | 0.87 |
| CMI-C | 39.7 | 39.4 | 45.9 | 0.99 | 0.86 | 0.86 |
| CMI-D | 41.3 | 39.2 | 45.5 | 0.95 | 0.90 | 0.86 |
| CMI-E | 40.1 | 39.4 | 45.6 | 0.98 | 0.87 | 0.86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nouman, M.; Dissaneewate, T.; Chong, D.Y.R.; Chatpun, S. Effects of Custom-Made Insole Materials on Frictional Stress and Contact Pressure in Diabetic Foot with Neuropathy: Results from a Finite Element Analysis. Appl. Sci. 2021, 11, 3412. https://doi.org/10.3390/app11083412
Nouman M, Dissaneewate T, Chong DYR, Chatpun S. Effects of Custom-Made Insole Materials on Frictional Stress and Contact Pressure in Diabetic Foot with Neuropathy: Results from a Finite Element Analysis. Applied Sciences. 2021; 11(8):3412. https://doi.org/10.3390/app11083412
Chicago/Turabian StyleNouman, Muhammad, Tulaya Dissaneewate, Desmond Y. R. Chong, and Surapong Chatpun. 2021. "Effects of Custom-Made Insole Materials on Frictional Stress and Contact Pressure in Diabetic Foot with Neuropathy: Results from a Finite Element Analysis" Applied Sciences 11, no. 8: 3412. https://doi.org/10.3390/app11083412
APA StyleNouman, M., Dissaneewate, T., Chong, D. Y. R., & Chatpun, S. (2021). Effects of Custom-Made Insole Materials on Frictional Stress and Contact Pressure in Diabetic Foot with Neuropathy: Results from a Finite Element Analysis. Applied Sciences, 11(8), 3412. https://doi.org/10.3390/app11083412









