Multiple Brain Abscesses of Odontogenic Origin. May Oral Microbiota Affect Their Development? A Review of the Current Literature
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Data Collection
2.3. Outcomes
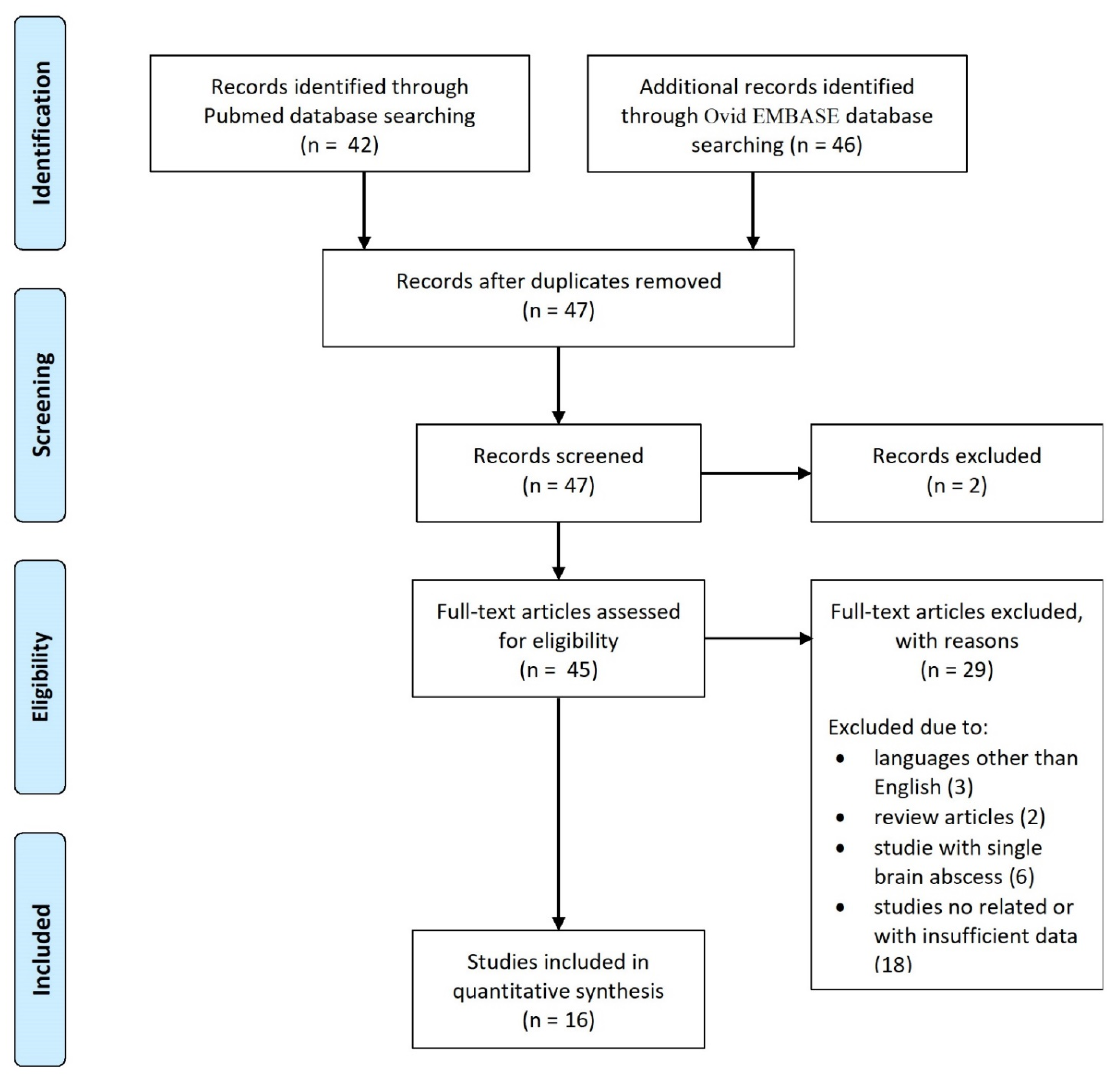
3. Results
3.1. Literature Review
3.2. Demographic, Clinical and Radiological Characteristics
3.3. Pathogens, Treatment and Clinical Outcome
4. Discussion
4.1. History and Epidemiology
4.2. Pathogen Features and Clinical Presentation
4.3. Differential Diagnosis and Outcome
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kichenbrand, C.; Marchal, A.; Mouraret, A.; Hasnaoui, N.; Guillet, J.; Rech, F.; Phulpin, B. Brain abscesses and intracranial empyema due to dental pathogens: Case series. Int. J. Surg. Case Rep. 2020, 69, 35–38. [Google Scholar] [CrossRef]
- Alvis Miranda, H.; Castellar-Leones, S.M.; Elzain, M.A.; Moscote-Salazar, L.R. Brain abscess: Current management. J. Neurosci. Rural Pract. 2013, 4 (Suppl. 1), S67–S81. [Google Scholar]
- Mathisen, G.E.; Johnson, J.P. Brain abscess. Clin. Infect. Dis. 1997, 25, 763–779. [Google Scholar] [CrossRef]
- Menon, S.; Bharadwaj, R.; Chowdhary, A.; Kaundinya, D.V.; Palande, D.A. Current epidemiology of intracranial abscesses: A prospective 5 year study. J. Med. Microbiol. 2008, 57, 1259–1268. [Google Scholar] [CrossRef]
- Olsen, I. Update on bacteraemia related to dental procedures. Transfus. Apher. Sci. 2008, 39, 173–178. [Google Scholar] [CrossRef] [PubMed]
- AlHarmi, R.A.; Henari, D.F.; Jadah, R.H.S.; AlKhayyat, H.M. A brain populated with space-occupying lesions: Identifying the culprit. BMJ Case Rep. 2018, 24. [Google Scholar] [CrossRef] [PubMed]
- Hollin, S.A.; Hayashi, H.; Gross, S.W. Intracranial abscesses of odontogenic origin. Oral Surg. Oral Med. Oral Pathol. 1967, 23, 277–293. [Google Scholar] [CrossRef]
- MacEwan, W. Pyogenic Infective Diseases of the Brain and Spinal Cord; Maclehose Press: Glasgow, UK, 1893. [Google Scholar]
- Sulyanto, R.M.; Thompson, Z.A.; Beall, C.J.; Leys, E.J.; Griffen, A.L. The Predominant Oral Microbiota Is Acquired Early in an Organized Pattern. Sci. Rep. 2019, 22, 10550. [Google Scholar] [CrossRef] [PubMed]
- Liébana-Ureña, J.; González, M.P.; Liébana, M.J.; Parra, L. Composición y ecología de la microbiota oral. In Microbiología Oral; Liébana-Ureña, J., Ed.; McGraw-Hill: Madrid, Spain, 2002; pp. 514–525. [Google Scholar]
- Dewhirst, F.E. The oral microbiome: Critical for understanding oral health and disease. J. Calif. Dent. Assoc. 2016, 44, 409–410. [Google Scholar] [PubMed]
- Costalonga, M.; Herzberg, M.C. The oral microbiome and the immunobiologyof periodontal disease and caries. Immunol. Lett. 2014, 162, 22–38. [Google Scholar] [CrossRef]
- Kumar, P.S. From focal sepsis to periodontal medicine: A century of exploringthe role of the oral microbiome in systemic disease. J. Physiol. 2017, 595, 465–476. [Google Scholar] [CrossRef]
- Sami, A.; Elimairi, I.; Stanton, C.; Ross, R.P.; Ryan, C.A. The Role of the Microbiome in Oral Squamous Cell Carcinoma with Insight into the Microbiome-Treatment Axis. Int. J. Mol. Sci. 2020, 21, 8061. [Google Scholar]
- Montemurro, N.; Perrini, P.; Rapone, B. Clinical Risk and Overall Survival in Patients with Diabetes Mellitus, Hyperglycemia and Glioblastoma Multiforme. A Review of the Current Literature. Int. J. Environ. Res. Public Health 2020, 17, 8501. [Google Scholar] [CrossRef]
- Wang, L.; Ganly, I. The oral microbiome and oral cancer. Clin. Lab. Med. 2014, 34, 711–719. [Google Scholar] [CrossRef]
- Graves, D.T.; Correa, J.D.; Silva, T.A. The oral microbiota is modified by systemic diseases. J. Dent. Res. 2019, 98, 148–156. [Google Scholar] [CrossRef]
- Chen, B.; Zhao, Y.; Li, S.; Yang, L.; Wang, H.; Wang, T.; Bin, S.; Gai, Z.; Heng, X.; Zhang, C.; et al. Variations in oral microbiome profiles in rheumatoid arthritis and osteoarthritis with potential biomarkers for arthritis screening. Sci. Rep. 2018, 20, 17126. [Google Scholar] [CrossRef]
- Cobb, C.M.; Kelly, P.J.; Williams, K.B.; Babbar, S.; Angolkar, M.; Derman, R.J. The oral microbiome and adverse pregnancy outcomes. Int. J. Women Health 2017, 9, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Montemurro, N. Glioblastoma Multiforme and Genetic Mutations: The Issue Is Not Over Yet. An Overview of the Current Literature. J. Neurol. Surg. A Cent. Eur. Neurosurg. 2020, 81, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Isola, G.; Polizzi, A.; Alibrandi, A.; Williams, R.C.; Lo Giudice, A. Analysis of galectin-3 levels as a source of coronary heart disease risk during periodontitis. J. Periodontal Res. 2021, 28–32. [Google Scholar] [CrossRef]
- Isola, G.; Polizzi, A.; Patini, R.; Ferlito, S.; Alibrandi, A.; Palazzo, G. Association among serum and salivary A. actinomycetemcomitans specific immunoglobulin antibodies and periodontitis. BMC Oral Health 2020, 20, 283. [Google Scholar] [CrossRef] [PubMed]
- Eickholz, P.; Schröder, M.; Asendorf, A.; Schacher, B.; Oremek, G.M.; Kaiser, F.; Wohlfeil, M.; Nibali, L. Effect of nonsurgical periodontal therapy on haematological parameters in grades B and C periodontitis: An exploratory analysis. Clin. Oral Investig. 2020, 24, 4291–4299. [Google Scholar] [CrossRef]
- Kebschull, M.; Papapanou, P.N. Periodontal microbial complexes associated with specific cell and tissue responses. J. Clin. Periodontol. 2011, 11, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Azenha, M.R.; Homsi, G.; Garcia, I.R., Jr. Multiple brain abscess from dental origin: Case report and literature review. Oral Maxillofac. Surg. 2012, 16, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Chacko, A.G.; Chandy, M.J. Diagnostic and staged stereotactic aspiration of multiple bihemispheric pyogenic brain abscesses. Surg. Neurol. 1997, 48, 278–282. [Google Scholar] [CrossRef]
- Clifton, T.C.; Kalamchi, S. A case of odontogenic brain abscess arising from covert dental sepsis. Ann. R. Coll. Surg. Engl. 2012, 94, e41–e43. [Google Scholar] [CrossRef]
- Ewald, C.; Kuhn, S.; Kalff, R. Pyogenic infections of the central nervous system secondary to dental affections—A report of six cases. Neurosurg. Rev. 2006, 29, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Gemelli, N.A.; Boccalatte, L.A.; Ciarrocchi, N.M. Multiple Brain Abscesses Due to Odontogenic Infection. Neurocrit. Care. 2020, 33, 604–606. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.H.; Ro, S.S.; Lee, S.W.; Jeon, J.Y.; Park, C.J.; Hwang, K.G. Multiple brain abscesses treated by extraction of the maxillary molars with chronic apical lesion to remove the source of infection. Maxillofac. Plast. Reconstr. Surg. 2019, 41, 25. [Google Scholar] [CrossRef] [PubMed]
- Kuijper, E.J.; Wiggerts, H.O.; Jonker, G.J.; Schaal, K.P.; de Gans, J. Disseminated actinomycosis due to Actinomyces meyeri and Actinobacillus actinomycetemcomitans. Scand. J. Infect. Dis. 1992, 24, 667–672. [Google Scholar] [CrossRef]
- Marks, P.V.; Patel, K.S.; Mee, E.W. Multiple brain abscesses secondary to dental caries and severe periodontal disease. Br. J. Oral Maxillofac. Surg. 1988, 26, 244–247. [Google Scholar] [CrossRef]
- Martiny, D.; Dauby, N.; Konopnicki, D.; Kampouridis, S.; Jissendi Tchofo, P.; Horoi, M.; Vlaes, L.; Retore, P.; Hallin, M.; Vandenberg, O. MALDI-TOF MS contribution to the diagnosis of Campylobacter rectus multiple skull base and brain abscesses. New Microbes New Infect. 2017, 19, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Pallesen, L.P.; Schaefer, J.; Reuner, U.; Leonhardt, H.; Engellandt, K.; Schneider, H.; Reichmann, H.; Puetz, V. Multiple brain abscesses in an immunocompetent patient after undergoing professional tooth cleaning. J. Am. Dent. Assoc. 2014, 145, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Rahamat-Langendoen, J.C.; van Vonderen, M.G.; Engström, L.J.; Manson, W.L.; van Winkelhoff, A.J.; Mooi-Kokenberg, E.A. Brain abscess associated with Aggregatibacter actinomycetemcomitans: Case report and review of literature. J. Clin. Periodontol. 2011, 38, 702–706. [Google Scholar] [CrossRef] [PubMed]
- Ryan, D.; Lippert, W.C.; Fenwick, A.J.; Bhatt, M.; Jones, C.R. Central Nervous System Blastomycosis With Multiple Brain Abscesses Presenting as Right Upper Extremity Weakness. Neurohospitalist 2019, 9, 230–234. [Google Scholar] [CrossRef]
- Stepanović, S.; Tosić, T.; Savić, B.; Jovanović, M.; K’ouas, G.; Carlier, J.P. Brain abscess due to Actinobacillus actinomycetemcomitans. APMIS 2005, 113, 225–228. [Google Scholar] [CrossRef]
- Viviano, M.; Cocca, S. Multiple brain abscesses after professional tooth cleaning: Case report and literature review. J. Stomatol. Oral Maxillofac. Surg. 2018, 119, 432–435. [Google Scholar] [CrossRef]
- Wu, P.C.; Tu, M.S.; Lin, P.H.; Chen, Y.S.; Tsai, H.C. Prevotella brain abscesses and stroke following dental extraction in a young patient: A case report and review of the literature. Intern. Med. 2014, 53, 1881–1887. [Google Scholar] [CrossRef] [PubMed]
- Nathoo, N.; Nadvi, S.S.; Narotam, P.K.; van Dellen, J.R. Brain abscess: Management and outcome analysis of a computed tomography era experience with 973 patients. World Neurosurg. 2011, 75, 716–726. [Google Scholar] [CrossRef] [PubMed]
- Muzumdar, D.; Jhawar, S.; Goel, A. Brain abscess: An overview. Int. J. Surg. 2011, 9, 136–144. [Google Scholar] [CrossRef]
- Nathoo, N.; Narotam, P.K.; Nadvi, S.; van Dellen, J.R. Taming an old enemy: A profile of intracranial suppuration. World Neurosurg. 2012, 77, 484–490. [Google Scholar] [CrossRef]
- Perrini, P.; Montemurro, N.; Iannelli, A. The contribution of Carlo Giacomini (1840–1898): The limbus Giacomini and beyond. Neurosurgery 2013, 72, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Mut, M.; Dinç, G.; Naderi, S. On the report of the first successful surgical treatment of brain abscess in the Ottoman Empire by Dr. Cemil Topuzlu in 1891. Neurosurgery 2007, 61, 869–872. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tronstad, L.; Olsen, I. Brain abscesses caused by oral infection. Endod. Dent. Traumatol. 1999, 15, 95–101. [Google Scholar] [CrossRef]
- Montemurro, N.; Anania, Y.; Cagnazzo, F.; Perrini, P. Survival outcomes in patients with recurrent glioblastoma treated with Laser Interstitial Thermal Therapy (LITT): A systematic review. Clin. Neurol. Neurosurg. 2020, 195, 105942. [Google Scholar] [CrossRef]
- Laulajainen-Hongisto, A.; Lempinen, L.; Färkkilä, E.; Saat, R.; Markkola, A.; Leskinen, K.; Blomstedt, G.; Aarnisalo, A.A.; Jero, J. Intracranial abscesses over the last four decades; changes in aetiology, diagnostics, treatment and outcome. Infect. Dis. 2016, 48, 310–316. [Google Scholar] [CrossRef]
- Erdogan, E.; Cansever, T. Pyogenic brain abscess. Neurosurg. Focus 2008, 24, E2. [Google Scholar] [CrossRef]
- Perrini, P.; Montemurro, N.; Caniglia, M.; Lazzarotti, G.; Benedetto, N. Wrapping of intracranial aneurysms: Single-center series and systematic review of the literature. Br. J. Neurosurg. 2015, 29, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Montemurro, N.; Perrini, P. Will COVID-19 change neurosurgical clinical practice? Br. J. Neurosurg. 2020, 1, 1–2. [Google Scholar] [CrossRef]
- Montemurro, N. Intracranial hemorrhage and COVID-19, but please do not forget “old diseases” and elective surgery. Brain Behav. Immun. 2021, 92, 207–208. [Google Scholar] [CrossRef] [PubMed]
- Montemurro, N.; Perrini, P.; Mangini, V.; Galli, M.; Papini, A. The Y-shaped trabecular bone structure in the odontoid process of the axis: A CT scan study in 54 healthy subjects and biomechanical considerations. J. Neurosurg. Spine. 2019, 1, 1–8. [Google Scholar] [CrossRef]
- Jevon, P.; Abdelrahman, A.; Pigadas, N. Management of odontogenic infections and sepsis: An update. Br. Dent. J. 2020, 229, 363–370. [Google Scholar] [CrossRef]
- Prasad, K.N.; Mishra, A.M.; Gupta, D.; Husain, N.; Husain, M.; Gupta, R.K. Analysis of microbial etiology and mortality in patients with brain abscess. J. Infect. 2006, 53, 221–227. [Google Scholar] [CrossRef]
- Matthews, D. Impact of everyday oral activities on the risk of bacteraemia is unclear. Evid. Based Dent. 2012, 13, 80. [Google Scholar] [CrossRef] [PubMed]
- Mark, W.J.; Rossetti, B.J.; Rieken, C.W.; Dewhirst, F.E.; Borisy, G.G. Biogeographyof a human oral microbiome at the micron scale. Proc. Natl. Acad. Sci. USA 2016, 113, E791–E800. [Google Scholar] [CrossRef]
- Steinberg, J.P.; Burd, E.M. Other gramnegative and gram-variable bacilli. In Principles and Practice of Infectious Diseases; Mandell, G.L., Bennet, J.E., Dolin, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 3015–3017. [Google Scholar]
- Akashi, M.; Tanaka, K.; Kusumoto, J.; Furudoi, S.; Hosoda, K.; Komori, T. Brain Abscess Potentially Resulting from Odontogenic Focus: Report of Three Cases and a Literature Review. J. Maxillofac. Oral Surg. 2017, 16, 58–64. [Google Scholar] [CrossRef]
- Pokrowiecki, R.; Mielczarek, A.; Zaręba, T.; Tyski, S. Oral microbiome and peri-implant diseases: Where are we now? Ther. Clin. Risk Manag. 2017, 13, 1529–1542. [Google Scholar] [CrossRef] [PubMed]
- Dhir, S. Biofilm and dental implant: The microbial link. J. Indian Soc. Periodontol. 2013, 17, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Rapone, B.; Corsalini, M.; Converti, I.; Loverro, M.T.; Gnoni, A.; Trerotoli, P.; Ferrara, E. Does Periodontal Inflammation Affect Type 1 Diabetes in Childhood and Adolescence? A Meta-Analysis. Front. Endocrinol. 2020, 11, 278. [Google Scholar] [CrossRef]
- Bahl, R.; Sandhu, S.; Singh, K.; Sahai, N.; Gupta, M. Odontogenic infections: Microbiology and management. Contemp. Clin. Dent. 2014, 5, 307–311. [Google Scholar] [CrossRef]
- Sheehan, J.P.; Jane, J.A.; Ray, D.K.; Goodkin, H.P. Brain abscess in children. Neurosurg. Focus. 2008, 24, E6. [Google Scholar] [CrossRef]
- Schroeder, B.O.; Bäckhed, F. Signals from the gut microbiota to distant organs in physiology and disease. Nat. Med. 2016, 22, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Guarro, J.; Kantarcioglu, A.S.; Horré, R.; Rodriguez-Tudela, J.L.; Cuenca Estrella, M.; Berenguer, J.; de Hoog, G.S. Scedosporium apiospermum: Changing clinical spectrum of a therapy-refractory opportunist. Med. Mycol. 2006, 44, 295–327. [Google Scholar] [CrossRef]
- Oberoi, S.S.; Dhingra, C.; Sharma, G.; Sardana, D. Antibiotics in dental practice: How justified are we. Int. Dent. J. 2015, 65, 4–10. [Google Scholar] [CrossRef]
- Quaglia, E.; Moscufo, L.; Corsalini, M.; Coscia, D.; Sportelli, P.; Cantatore, F.; De Rinaldis, C.; Rapone, B.; Carossa, M.; Carossa, S. Polyamide vs. silk sutures in the healing of postextraction sockets: A split mouth study. Oral Implantol. 2018, 11, 115–120. [Google Scholar]
- Montemurro, N.; Santoro, G.; Marani, W.; Petrella, G. Posttraumatic synchronous double acute epidural hematomas: Two craniotomies, single skin incision. Surg. Neurol. Int. 2020, 11, 435. [Google Scholar] [CrossRef] [PubMed]
- Von Graevenitz, A.; Zbinden, R. Actinobacillus, Capnocytophaga, Eikenella, Kingella, Pasteurella, and other fastidious or rarely encountered gram-negative rods. In Manual of Clinical Microbiology; Murray, P.R., Baron, E.J., Eds.; Wiley Online Library: Hoboken, NJ, USA, 2007; pp. 621–636. [Google Scholar]
- Rapone, B.; Ferrara, E.; Converti, I.; Loverro, M.; Loverro, M.T.; Gnoni, A.; Petruzzi, M.; Corsalini, M.; Scacco, S.; Di Naro, E. Exploring the role of Fusobacterium nucleatum in preterm birth: A narrative review. Open Access Maced. J. Med. Sci. 2020, 8, 1–7. [Google Scholar] [CrossRef]
- Rapone, B.; Ferrara, E.; Montemurro, N.; Converti, I.; Loverro, M.; Loverro, M.T.; Gnoni, A.; Scacco, S.; Siculella, L.; Corsalini, M.; et al. Oral Microbiome and Preterm Birth: Correlation or Coincidence? A Narrative Review. Open Access Maced. J. Med. Sci. 2020, 8, 123–132. [Google Scholar] [CrossRef]
- Rapone, B.; Ferrara, E.; Corsalini, M.; Converti, I.; Grassi, F.R.; Santacroce, L.; Topi, S.; Gnoni, A.; Scacco, S.; Scarano, A.; et al. The Effect of Gaseous Ozone Therapy in Conjunction with Periodontal Treatment on Glycated Hemoglobin Level in Subjects with Type 2 Diabetes Mellitus: An Unmasked Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2020, 17, 5467. [Google Scholar] [CrossRef]
- Bryan, N.S.; Tribble, G.; Angelov, N. Oral microbiome and nitric oxide: The missing link in the management of blood pressure. Curr. Hypertens. Rep. 2017, 19, 33. [Google Scholar] [CrossRef] [PubMed]
- Fanelli, G.N.; Grassini, D.; Ortenzi, V.; Pasqualetti, F.; Montemurro, N.; Perrini, P.; Naccarato, A.G.; Scatena, C. Decipher the Glioblastoma Microenvironment: The First Milestone for New Groundbreaking Therapeutic Strategies. Genes 2021, 12, 445. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Haake, S.K.; Mannon, P.; Lemon, K.P.; Waldron, L.; Gevers, D.; Huttenhower, C.; Izard, J. Composition of the adult digestive tract bacterial microbiome based on seven mouth surfaces, tonsils, throat and stool samples. Genome Biol. 2012, 13, R42. [Google Scholar] [CrossRef]
- Montemurro, N.; Herbet, G.; Duffau, H. Right Cortical and Axonal Structures Eliciting Ocular Deviation During Electrical Stimulation Mapping in Awake Patients. Brain Topogr. 2016, 29, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Allan, R. Tunkel, in Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 8th ed.; Science Direct: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Perrini, P.; Pieri, F.; Montemurro, N.; Tiezzi, G.; Parenti, G.F. Thoracic extradural haematoma after epidural anaesthesia. Neurol. Sci. 2010, 31, 87–88. [Google Scholar] [CrossRef]
- Corsalini, M.; Di Venere, D.; Sportelli, P.; Magazzino, D.; Ripa, M.; Cantatore, F.; Cagnetta, C.; De Rinaldis, C.; Montemurro, N.; De Giacomo, A.; et al. Evaluation of prosthetic quality and masticatory efficiency in patients with total removable prosthesis: Study of 12 cases. Oral Implantol. 2018, 11, 230–240. [Google Scholar]
- Segura-Egea, J.J.; Gould, K.; Şen, B.H.; Jonasson, P.; Cotti, E.; Mazzoni, A.; Sunay, H.; Tjäderhane, L.; Dummer, P.M.H. Antibiotics in Endodontics: A review. Int. Endod. J. 2017, 50, 1169–1184. [Google Scholar] [CrossRef]
- Oguntebi, B.; Slee, A.M.; Tanzer, J.M.; Langeland, K. Predominant microflora associated with human dental periapical abscesses. J. Clin. Microbiol. 1982, 15, 964–966. [Google Scholar] [CrossRef] [PubMed]
| PubMed Search Accessed on 5 January 2021 (42 Articles) | Embase Search Accessed on 5 January 2021 (46 Articles) |
|---|---|
| (multiple) AND (brain abscess OR brain abscesses OR cerebral abscess OR cerebral abscesses) AND (odontogenic OR dental OR dental origin) | (‘multiple’) AND (‘brain abscess’ OR ‘brain abscesses’ OR ‘cerebral abscess’ OR ‘cerebral abscesses’) AND (‘odontogenic’ OR ‘dental’ OR ‘dental origin’) |
| Authors | Year | Age/Sex | Neurological Clinical Presentation | N° of BAs | BAs Location | Pathogens | Surgical Treatment | Medical Treatment | Follow-Up (Months) | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Marks et al. [33] | 1988 | 26/M | confusion and reduced consciousness | 5 | Right frontal (3), right parietal (2) | Streptococcus viridans | - | - | - | Death |
| Kuijper et al. [32] | 1992 | 44/M | right arm paresis | 2 | Left frontoparietal region, right occipital | Actinomyces meyeri, Aggregatibacter actinomycetemcomitans | Drainage of the lesions | Amoxicillin | 12 | Good |
| Chacko & Chandy [27] | 1997 | 60/M | drowsy, confusion | 6 | Right frontal (1), left and right parietal lobes (5) | - | Drainage of the lesions | Penicillin, chloramphenicol, metronidazole, ampicillin, trimethoprim-sulfamethoxazole | 4 | Good |
| Stepanović et al. [38] | 2005 | 47/M | headache, nausea, vomiting, progressive left hemiparesis | 3 | Right occipital (3) | Aggregatibacter actinomycetemcomitans | Drainage of the lesions | Ceftriaxone, amikacin, metronidazole | 1 | Good |
| Ewald et al. [29] | 2006 | 49/M | right arm paresis, left leg paresis, seizures | more than 2 | - | Fusobacterium nucleatum | Drainage of the lesions | Clindamycin, metronidazole, cefuroxim | 12 | Good |
| Rahamat-Langendoen et al. [36] | 2011 | 42/M | confusion and reduced consciousness | 3 | Right parietal (2), left frontal (1) | Aggregatibacter actinomycetemcomitans | Drainage of the lesions | Antibiotic treatment | 12 | Good |
| Azenha et al. [26] | 2012 | 70/M | headache, dizziness, nausea, mild fever, left hemiparesis | 3 | Left frontal, right occipital, left thalamic | Streptococcus viridians, Bacteroides | Drainage of the lesions | Ceftriaxone, amoxicillin, metronidazole | 12 | Good |
| Clifton et al. [28] | 2012 | 56/M | nausea, vomiting | 2 | Right frontal, left frontal | - | Drainage of the lesions and dental extraction | Vancomycin, aciclovir, ceftriaxone | 3 | Good |
| Wu et al. [40] | 2014 | 32/M | left arm paresis, left facial palsy | 3 | Right frontal, right temporal, right basal ganglia | Prevotella denticola | Drainage of the lesions | Cefepime, penicillin, metronidazole | 6 | Good |
| Pallesen et al. [35] | 2014 | 55/M | left leg paresis | 3 | Right frontal, right occipital, left occipital | Streptococcus intermedius, Staphylococcus warneri | Drainage of the lesions | Ceftriaxone, vancomycin | 6 | Left leg paresis |
| Martiny et al. [34] | 2017 | 66/M | dysarthria, diplopia, nystagmus, right peripheral facial palsy, right deafness | 3 | Right cerebellopontine angle, cerebellum | Campylobacter rectus | Drainage of the lesions and dental extraction | Meropenem, doxycycline | 10 | Good |
| AlHarmi et al. [6] | 2018 | 8/F | fever, left hemiparesis, hyporeflexia | 5 | Right frontal (3), left parietal (2) | - | Drainage of the lesions | Meropenem, vancomycin | 1 | Left hemiparesis |
| Viviano & Cocca [39] | 2018 | 28/M | headache, nuchal pain, vomiting, confusion | 2 | Left parietal, left occipital | Streptococcus intermedius, Actinomyces | Drainage of the lesions | Vancomycin, ceftriaxone, metronidazole, clindamycin, ampicillin | 6 | Good |
| Ryan et al. [37] | 2019 | 79/M | progressive right arm paresis | 2 | Left parietal, right frontal | Blastomyces dermatitidis | Drainage of the lesions and dental extraction | Vancomycin, ceftriaxone, metronidazole | 6 | Good |
| Jung et al. [31] | 2019 | 45/M | right facial spasms, tingling of the right arm, paresthesia, dysarthria | 3 | Right frontal (2), left frontal (1) | Streptococcus anginosus | Drainage of the lesions and dental extraction | Metronidazole, cefotaxime, ceftriaxone, Vancomycin, Augmentin | 3 | Good |
| Gemelli et al. [30] | 2020 | 71/F | confusion, disorientation in time and space with inattention | more than 10 | Frontal and parietal lobes | Aggregatibacter aphrophilus | Dental extractions | Vancomycin, piperacillin/tazobactam, ceftriaxone | 2 | Good |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montemurro, N.; Perrini, P.; Marani, W.; Chaurasia, B.; Corsalini, M.; Scarano, A.; Rapone, B. Multiple Brain Abscesses of Odontogenic Origin. May Oral Microbiota Affect Their Development? A Review of the Current Literature. Appl. Sci. 2021, 11, 3316. https://doi.org/10.3390/app11083316
Montemurro N, Perrini P, Marani W, Chaurasia B, Corsalini M, Scarano A, Rapone B. Multiple Brain Abscesses of Odontogenic Origin. May Oral Microbiota Affect Their Development? A Review of the Current Literature. Applied Sciences. 2021; 11(8):3316. https://doi.org/10.3390/app11083316
Chicago/Turabian StyleMontemurro, Nicola, Paolo Perrini, Walter Marani, Bipin Chaurasia, Massimo Corsalini, Antonio Scarano, and Biagio Rapone. 2021. "Multiple Brain Abscesses of Odontogenic Origin. May Oral Microbiota Affect Their Development? A Review of the Current Literature" Applied Sciences 11, no. 8: 3316. https://doi.org/10.3390/app11083316
APA StyleMontemurro, N., Perrini, P., Marani, W., Chaurasia, B., Corsalini, M., Scarano, A., & Rapone, B. (2021). Multiple Brain Abscesses of Odontogenic Origin. May Oral Microbiota Affect Their Development? A Review of the Current Literature. Applied Sciences, 11(8), 3316. https://doi.org/10.3390/app11083316











