Comparison of Four Dental Pulp-Capping Agents by Cone-Beam Computed Tomography and Histological Techniques—A Split-Mouth Design Ex Vivo Study
Abstract
1. Introduction
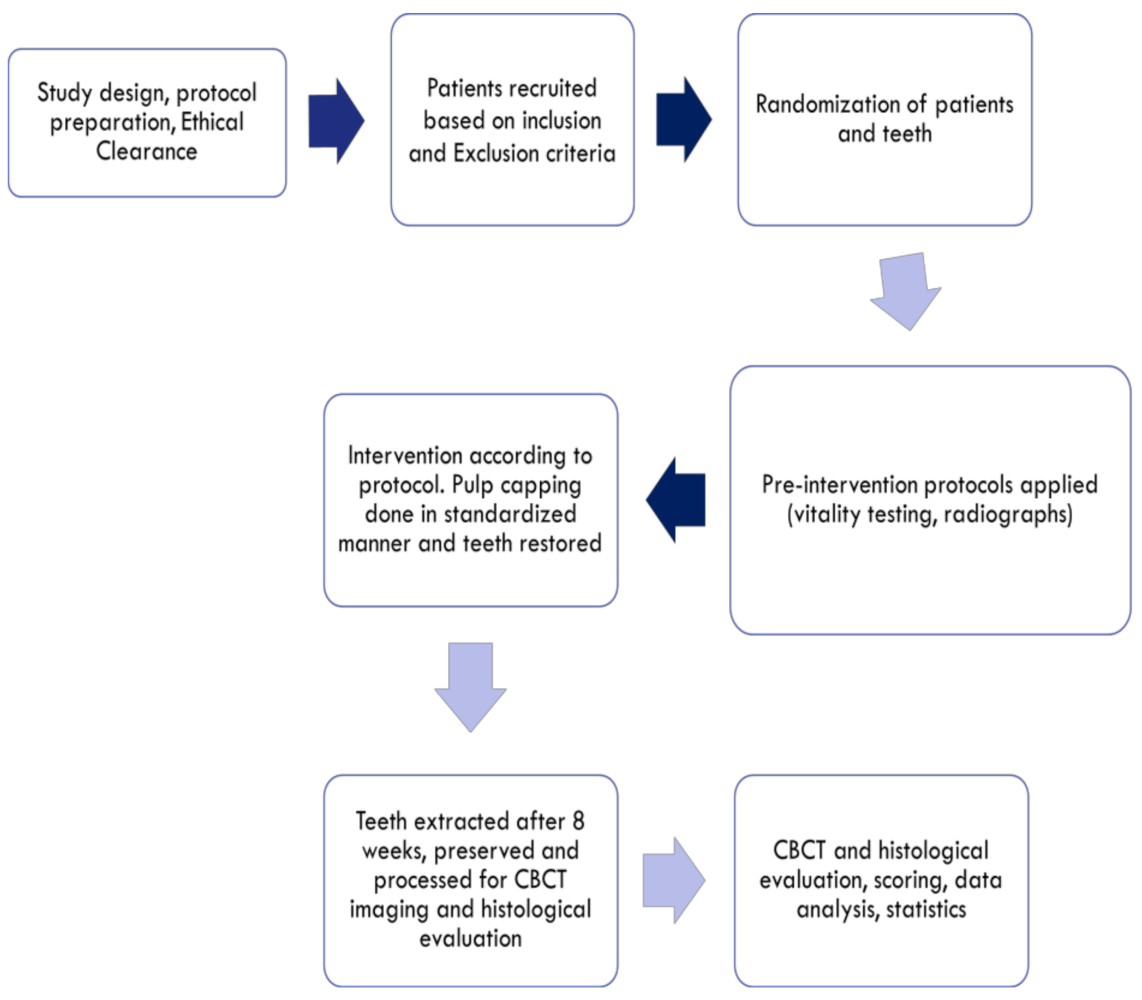
2. Materials and Methods
2.1. Study Design
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Interventions
2.5. Preparation of Teeth for Analysis
2.6. CBCT and Histological Scoring
2.7. Statistical Analysis and Report
3. Results
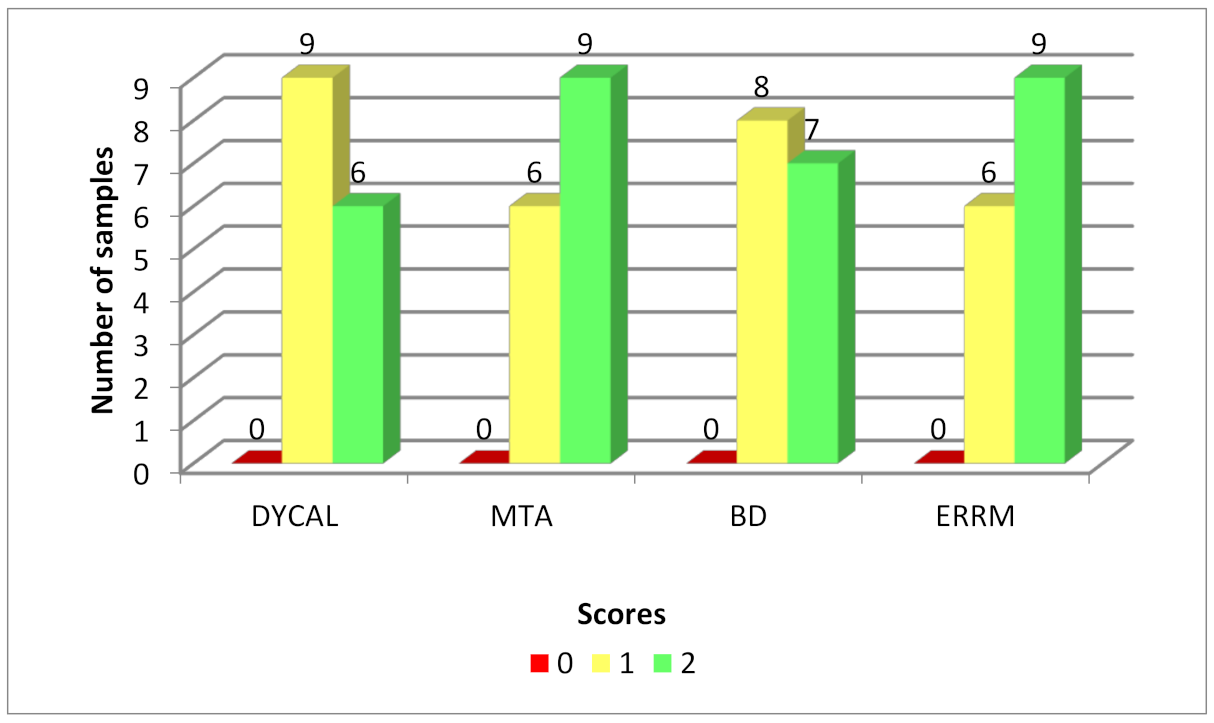
3.1. CBCT
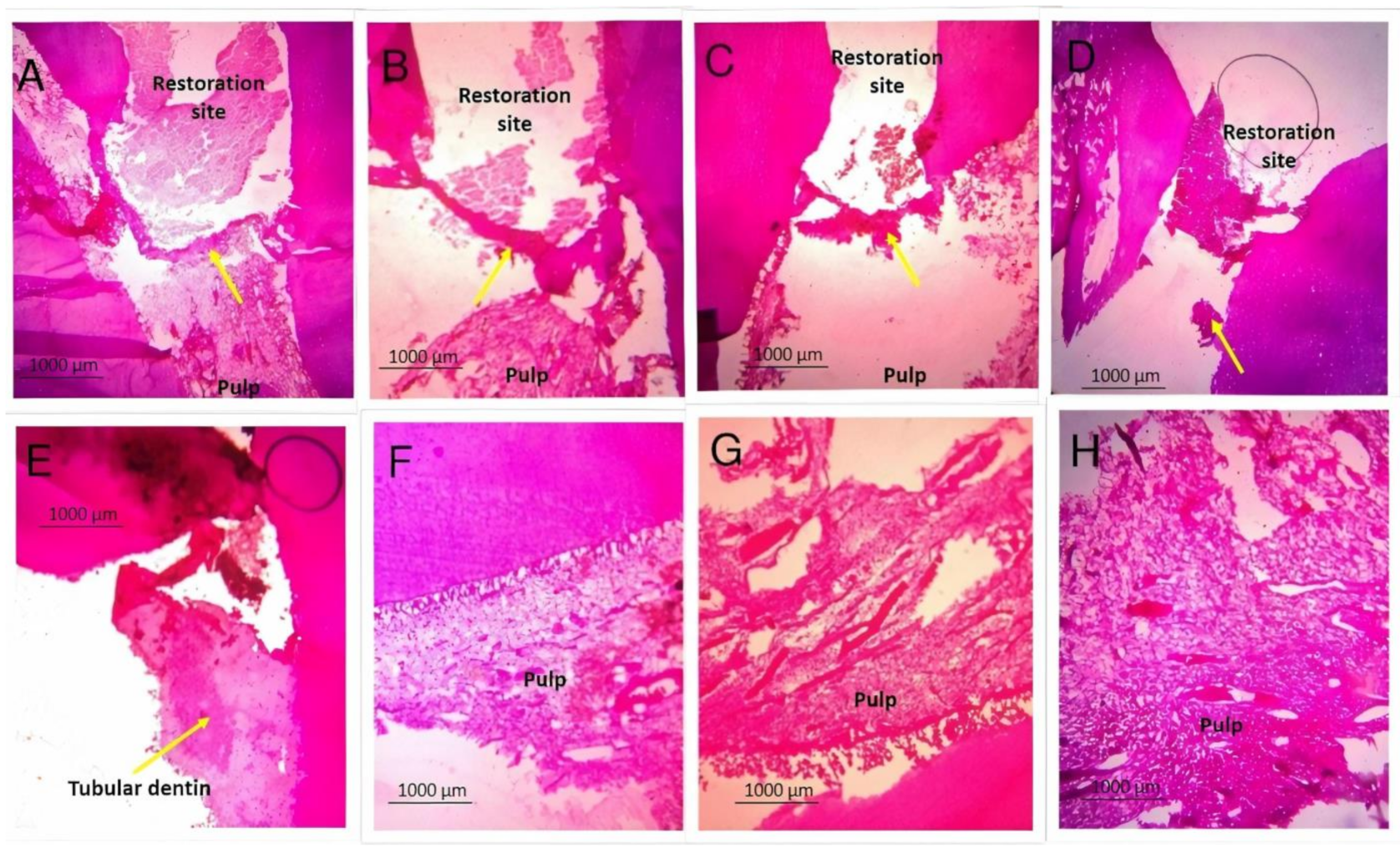
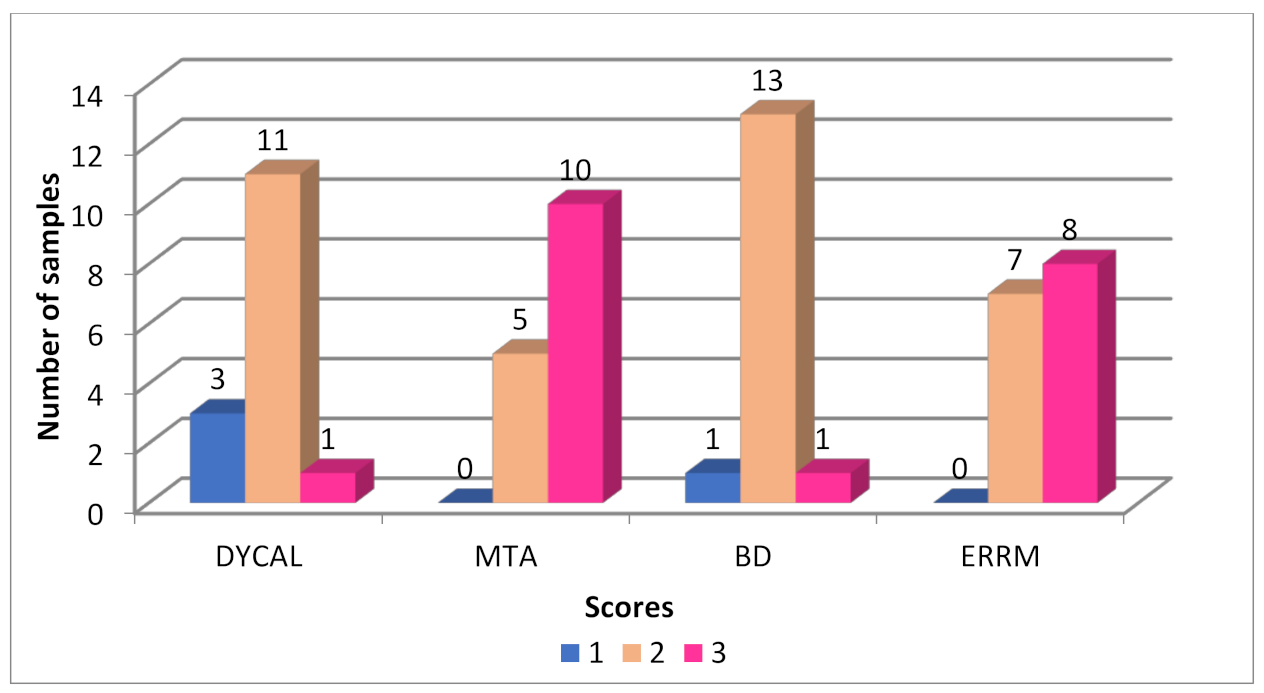
3.2. Histopathology of the Dentinal Bridge
3.3. Histopathology of the Pulpal Response
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hilton, T.J. Keys to Clinical Success with Pulp Capping: A Review of the Literature. Oper. Dent. 2009, 34, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Monea, A.; Stoica, A. Histological Evaluation of Indirect Pulp Capping Procedures With Calcium Hydroxide and Mineral Trioxide Aggregate. Eur. Sci. J. 2014, 10, 1–10. [Google Scholar]
- Maria De Lourdes, R.A.; Holland, R.; Reis, A.; Bortoluzzi, M.C.; Murata, S.S.; Dezan, E.; Souza, V.; Alessandro, L.D. Evaluation of Mineral Trioxide Aggregate and Calcium Hydroxide Cement as Pulp-Capping Agents in Human Teeth. J. Endod. 2008, 34, 1–6. [Google Scholar]
- Grawish, M.E.; Mahmoud, S.H.; El-Negoly, S.A.; El-Din, A.M.Z.; El-Zekrid, M.H.; Grawish, L.M.; Grawish, H.M. Biodentine versus mineral trioxide aggregate as a direct pulp capping material for human mature permanent teeth—A systematic review. J. Conserv. Dent. 2018, 21, 466–473. [Google Scholar] [CrossRef]
- Giacaman, R.A.; Muñoz-Sandoval, C.; Neuhaus, K.W.; Fontana, M.; Chałas, R. Evidence-based strategies for the minimally invasive treatment of carious lesions: Review of the literature. Adv. Clin. Exp. Med. 2018, 27, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- De Queiroz, A.M.; Assed, S.; Leonardo, M.R.; Nelson-Filho, P.; Da Silva, L.A.B. MTA and calcium hydroxide for pulp capping. J. Appl. Oral Sci. 2005, 13, 126–130. [Google Scholar] [CrossRef][Green Version]
- Mente, J.; Geletneky, B.; Ohle, M.; Koch, M.J.; Ding, P.G.F.; Wolff, D.; Dreyhaupt, J.; Martin, N.; Staehle, H.J.; Pfefferle, T. Mineral Trioxide Aggregate or Calcium Hydroxide Direct Pulp Capping: An Analysis of the Clinical Treatment Outcome. J. Endod. 2010, 36, 806–813. [Google Scholar] [CrossRef]
- Accorinte, M.L.R.; Loguercio, A.D.; Reis, A.; Carneiro, E.; Grande, R.H.M.; Murata, S.S.; Holland, R. Response of Human Dental Pulp Capped with MTA and Calcium Hydroxide Powder. Oper. Dent. 2008, 33, 488–495. [Google Scholar] [CrossRef]
- Cox, C.F.; Sübay, R.K.; Ostro, E.; Suzuki, S.; Suzuki, S.H. Tunnel Defects in Dentin Bridges: Their Formation Following Direct Pulp Capping. Oper. Dent. 1996, 21, 4–11. [Google Scholar]
- Koh, E.T.; McDonald, F.; Ford, T.R.P.; Torabinejad, M. Cellular response to mineral trioxide aggregate. J. Endod. 1998, 24, 543–547. [Google Scholar] [CrossRef]
- Parirokh, M.; Torabinejad, M. Mineral Trioxide Aggregate: A Comprehensive Literature Review—Part I: Chemical, Physical, and Antibacterial Properties. J. Endod. 2010, 36, 16–27. [Google Scholar] [CrossRef]
- Min, K.-S.; Kim, H.-I.; Park, H.-J.; Pi, S.-H.; Hong, C.-U.; Kim, E.-C. Human Pulp Cells Response to Portland Cement In Vitro. J. Endod. 2007, 33, 163–166. [Google Scholar] [CrossRef] [PubMed]
- E Iwamoto, C.; Adachi, E.; Pameijer, C.H.; Barnes, D.; E Romberg, E.; Jefferies, S. Clinical and histological evaluation of white ProRoot MTA in direct pulp capping. Am. J. Dent. 2006, 19, 85–90. [Google Scholar]
- Shayegan, A.; Petein, M.; Vanden Abbeele, A. The Use of Beta-Tricalcium Phosphate, White MTA, White Portland Cement and Calcium Hydroxide For Direct Pulp Capping of Primary Pig Teeth. Dent. Traumatol. 2009, 25, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Palma, P.J.; Marques, J.A.; Santos, J.; Falacho, R.I.; Sequeira, D.; Diogo, P.; Caramelo, F.; Ramos, J.C.; Santos, J.M. Tooth Discoloration after Regenerative Endodontic Procedures with Calcium Silicate-Based Cements—An Ex Vivo Study. Appl. Sci. 2020, 10, 5793. [Google Scholar] [CrossRef]
- Camilleri, J.; Borg, J.; Damidot, D.; Salvadori, E.; Pilecki, P.; Zaslansky, P.; Darvell, B.W. Colour and chemical stability of bismuth oxide in dental materials with solutions used in routine clinical practice. PLoS ONE 2020, 15, e0240634. [Google Scholar] [CrossRef]
- Brave, D.; Ali Nasseh, A.; Koch, K. A Review of Bioceramic Technology in Endodontics. Roots 2012, 4, 6–12. [Google Scholar]
- Abusrewil, S.M.; McLean, W.; Scott, J.A. The use of Bioceramics as root-end filling materials in periradicular surgery: A literature review. Saudi Dent. J. 2018, 30, 273–282. [Google Scholar] [CrossRef]
- Emara, R.; Elhennawy, K.; Schwendicke, F. Effects of calcium silicate cements on dental pulp cells: A systematic review. J. Dent. 2018, 77, 18–36. [Google Scholar] [CrossRef] [PubMed]
- Didilescu, A.C.; Cristache, C.M.; Andrei, M.; Voicu, G.; Perlea, P. The effect of dental pulp-capping materials on hard-tissue barrier formation. J. Am. Dent. Assoc. 2018, 149, 903–917. [Google Scholar] [CrossRef]
- Tran, X.; Gorin, C.; Willig, C.; Baroukh, B.; Pellat, B.; Decup, F.; Vital, S.O.; Chaussain, C.; Boukpessi, T. Effect of a Calcium-silicate-based Restorative Cement on Pulp Repair. J. Dent. Res. 2012, 91, 1166–1171. [Google Scholar] [CrossRef]
- Palma, P.J.; Marques, J.A.; Antunes, M.; Falacho, R.I.; Sequeira, D.; Roseiro, L.; Santos, J.M.; Ramos, J.C. Effect of restorative timing on shear bond strength of composite resin/calcium silicate–based cements adhesive interfaces. Clin. Oral Investig. 2020, 1–9. [Google Scholar] [CrossRef]
- Hashem, D.; Mannocci, F.; Patel, S.; Manoharan, A.; Brown, J.E.; Watson, T.F.; Banerjee, A. Clinical and Radiographic Assessment of The Efficacy of Calcium Silicate Indirect Pulp Capping. A Randomized Controlled Clinical Trial. J. Dent. Res. 2015, 94, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Biodentine Active Biosilicate Technology Scientific File, Septodont, Paris, France. Available online: Www.Oraverse.Com/Bio/Img/Biodentine™-Scientificfile.Pdf (accessed on 12 March 2018).
- Malkondu, Ö.; Kazandağ, M.K.; Kazazoğlu, E. A Review on Biodentine, A Contemporary Dentine Replacement and Repair Material. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Rajasekharan, S.; Martens, L.C.; Cauwels, R.G.; Verbeeck, R.M. Biodentine Material Characteristics and Clinical Applications: A Review of the Literature. Eur. Arch. Paediatr. Dent. 2014, 15, 147–158. [Google Scholar] [CrossRef]
- Solanki, N.P.; Venkappa, K.K.; Shah, N.C. Biocompatibility and Sealing Ability of Mineral Trioxide Aggregate and Biodentine™ As Root-End Filling Material: A Systematic Review. J. Conserv. Dent. 2018, 21, 10–15. [Google Scholar]
- Brizuela, C.; Ormeño, A.; Cabrera, C.; Cabezas, R.; Silva, C.I.; Ramírez, V.; Mercade, M. Direct Pulp Capping With Calcium Hydroxide, Mineral Trioxide Aggregate, and Biodentine in Permanent Young Teeth with Caries: A Randomized Clinical Trial. J. Endod. 2017, 43, 1776–1780. [Google Scholar] [CrossRef] [PubMed]
- Laurent, P.; Camps, J.; About, I. BiodentineTM induces TGF-β1 release from human pulp cells and early dental pulp mineralization. Int. Endod. J. 2011, 45, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Corral Nuñez, C.M.; Bosomworth, H.J.; Field, C.; Whitworth, J.M.; Valentine, R.A. Biodentine and Mineral Trioxide Aggregate Induce Similar Cellular Responses in A Fibroblast Cell Line. J. Endod. 2014, 40, 406–411. [Google Scholar] [CrossRef]
- Tomás-Catalá, C.J.; Collado-González, M.; García-Bernal, D.; Oñate-Sánchez, R.E.; Forner, L.; Llena, C.; Lozano, A.; Moraleda, J.M.; Rodríguez-Lozano, F.J. Biocompatibility of New Pulp-capping Materials NeoMTA Plus, MTA Repair HP, and Biodentine on Human Dental Pulp Stem Cells. J. Endod. 2018, 44, 126–132. [Google Scholar] [CrossRef]
- Nowicka, A.; Wilk, G.; Lipski, M.; Kołecki, J.; Buczkowska-Radlińska, J. Tomographic Evaluation of Reparative Dentin Formation after Direct Pulp Capping with Ca[OH]2, MTA, Biodentine, and Dentin Bonding System In Human Teeth. J. Endod. 2015, 41, 1234–1240. [Google Scholar] [CrossRef] [PubMed]
- Nowicka, A.; Lipski, M.; Parafiniuk, M.; Sporniak-Tutak, K.; Lichota, D.; Kosierkiewicz, A.; Kaczmarek, W.; Buczkowska-Radlińska, J. Response of Human Dental Pulp Capped with Biodentine and Mineral Trioxide Aggregate. J. Endod. 2013, 39, 743–747. [Google Scholar] [CrossRef]
- Kim, J.; Song, Y.-S.; Min, K.-S.; Kim, S.-H.; Koh, J.-T.; Lee, B.-N.; Chang, H.-S.; Hwang, I.-N.; Oh, W.-M.; Hwang, Y.-C. Evaluation of reparative dentin formation of ProRoot MTA, Biodentine and BioAggregate using micro-CT and immunohistochemistry. Restor. Dent. Endod. 2016, 41, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Endosequence Root Repair Material Product Brochure. Available online: Http://Brasselerusadental.Com/Wp-Content/Uploads/Sites/9/2015/03/B_3238A_RRM-DFU.Pdf (accessed on 1 April 2019).
- Duarte, M.A.; Marciano, M.A.; Vivan, R.R.; Tanomaru Filho, M.; Tanomaru, J.M.; Camilleri, J. Tricalcium Silicate-Based Cements: Properties and Modifications. Brazil Oral Res. 2018, 32 (Suppl. 1). [Google Scholar] [CrossRef] [PubMed]
- Deepthi, V.; Mallikarjun, E.; Nagesh, B.; Mandava, P. Effect of acidic pH on microhardness and microstructure of theraCal LC, endosequence, mineral trioxide aggregate, and biodentine when used as root repair material. J. Conserv. Dent. 2018, 21, 408–412. [Google Scholar] [CrossRef]
- Khar, A.; Gite, R.; Chandak, M.; Sawant, S.; Dass, A. Comparative Evaluation of Response of Human Dental Pulp On Direct Pulp Capping With MTA, ERRM [Endosequence Root Repair Putty Material]. IOSR J. oDent. Med Sci. 2016, 15, 52–57. [Google Scholar]
- Parirokh, M.; Torabinejad, M.; Dummer, P.M.H. Mineral trioxide aggregate and other bioactive endodontic cements: An updated overview—Part I: Vital pulp therapy. Int. Endod. J. 2017, 51, 177–205. [Google Scholar] [CrossRef]
- Moinzadeh, A.T.; Portoles, C.A.; Wismayer, P.S.; Camilleri, J. Bioactivity Potential of EndoSequence BC RRM Putty. J. Endod. 2016, 42, 615–621. [Google Scholar] [CrossRef] [PubMed]


| Biocompatibility | The agent should not be injurious to tissues or elicit deleterious inflammatory processes. |
| Workability | The material must be easily prepared and applied to the exposed pulp tissue. It must have some adhesive capability to bind to the dentin and the restoration. |
| Insolubility | The material as a whole must be stable and insoluble in tissue fluids. |
| Radiopacity | The material must contain a radiopaque component for radiographic assessment. |
| Bioactivity | The material must be able to stimulate reparative dentin formation, i.e., a dentinal bridge, thereby sealing the pulp. Preferably tubular dentin. |
| Anti-caries | The material may also release fluoride and prevent further caries. |
| Antimicrobial | The material must prevent microbial activity. |
| Cone-Beam CT | Scores | p-Value | Post Hoc Test | ||||||
| 0 | 1 | 2 | |||||||
| CH | 0 | 9 | 6 | 0.62 | Not applicable | ||||
| MTA | 0 | 6 | 9 | ||||||
| BD | 0 | 8 | 7 | ||||||
| ERRM | 0 | 6 | 9 | ||||||
| Histopathology | Scores | p-Value | Conover p-Values, Further Adjusted by the Benjamini–Hochberg FDR Method | ||||||
| 1 | 2 | 3 | |||||||
| CH | 3 | 11 | 1 | 0.001 | BD | CH | ERRM | ||
| MTA | 0 | 5 | 10 | CH | 0.56 | ||||
| BD | 1 | 13 | 1 | ERRM | 0.004 | 0.001 | |||
| ERRM | 0 | 7 | 8 | MTA | 0.0006 | 0.0002 | 0.49 | ||
| Pulpal Response | Scores | p-Value | |||||||
| 0 | 1 | 2 | 3 | ||||||
| CH | 3 | 11 | 1 | 0 | 0.00005 | BD | CH | ERRM | |
| MTA | 10 | 5 | 0 | 0 | CH | 0.028 | |||
| BD | 1 | 7 | 7 | 0 | ERRM | 0.00004 | 0.024 | ||
| ERRM | 9 | 6 | 0 | 0 | MTA | 0.00002 | 0.012 | 0.71 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muruganandhan, J.; Sujatha, G.; Poorni, S.; Srinivasan, M.R.; Boreak, N.; Al-Kahtani, A.; Mashyakhy, M.; Chohan, H.; Bhandi, S.; Raj, A.T.; et al. Comparison of Four Dental Pulp-Capping Agents by Cone-Beam Computed Tomography and Histological Techniques—A Split-Mouth Design Ex Vivo Study. Appl. Sci. 2021, 11, 3045. https://doi.org/10.3390/app11073045
Muruganandhan J, Sujatha G, Poorni S, Srinivasan MR, Boreak N, Al-Kahtani A, Mashyakhy M, Chohan H, Bhandi S, Raj AT, et al. Comparison of Four Dental Pulp-Capping Agents by Cone-Beam Computed Tomography and Histological Techniques—A Split-Mouth Design Ex Vivo Study. Applied Sciences. 2021; 11(7):3045. https://doi.org/10.3390/app11073045
Chicago/Turabian StyleMuruganandhan, Jayanandan, Govindarajan Sujatha, Saravanan Poorni, Manali Ramakrishnan Srinivasan, Nezar Boreak, Ahmed Al-Kahtani, Mohammed Mashyakhy, Hitesh Chohan, Shilpa Bhandi, A. Thirumal Raj, and et al. 2021. "Comparison of Four Dental Pulp-Capping Agents by Cone-Beam Computed Tomography and Histological Techniques—A Split-Mouth Design Ex Vivo Study" Applied Sciences 11, no. 7: 3045. https://doi.org/10.3390/app11073045
APA StyleMuruganandhan, J., Sujatha, G., Poorni, S., Srinivasan, M. R., Boreak, N., Al-Kahtani, A., Mashyakhy, M., Chohan, H., Bhandi, S., Raj, A. T., Zanza, A., Testarelli, L., & Patil, S. (2021). Comparison of Four Dental Pulp-Capping Agents by Cone-Beam Computed Tomography and Histological Techniques—A Split-Mouth Design Ex Vivo Study. Applied Sciences, 11(7), 3045. https://doi.org/10.3390/app11073045









