Abstract
Background: This study investigated selenium nanoparticles’ protective effects (SE-NPs) against carbon tetrachloride (CCl4)-induced hepatic injury in rats. Methods: Rats were divided into four groups (n = 8). Group 1 rats received the vehicle solution only. Group 2 received a single intraperitoneal injection of 1 mL/kg CCl4 in liquid paraffin (1:1 v/v). Group 3 was treated with SE-NPs (2.5 mg/kg) twice a week for three weeks before receiving CCl4 challenge. Oxidative stress, liver function, liver histopathology and serum lipid levels were evaluated. Results: Plasma concentrations of aspartate transaminase (AST), gamma-glutamyl transferase (GGT), alkaline phosphatase (ALP), urea, creatinine, malondialdehyde (MDA) and the toxicity marker, lactate dehydrogenase (LDH), were significantly elevated in rats treated with CCl4 compared to the controls. CCl4 also caused a significant decline in liver glutathione (GSH) concentration. SE-NP pretreatment significantly improved the level of AST, urea, creatinine, MDA, LDH, and GSH in the CCl4-injected rats towards the control levels. Conclusions: SE-NPs restored both liver function and hepatic structure in CCl4 treated rats. SE-NPs exhibit an ability to counter markers of liver injury induced by CCl4 and restore oxidative stability to lipid profiles and liver structure and function.
1. Introduction
Liver damage or hepatotoxicity is one of the most common side effects of any chemical drug or medicine-based treatment. It can be caused by herbal medicines [1], chemicals, metabolic intermediates and viruses. Despite a large body of research, hepatotoxicity still a significant clinical challenge. Chemical-induced liver toxicity is attributed to the classical mechanism, including apoptosis along with an upsurge in cytokine concentration and oxidative stress [2]. Moreover, ROS (Reactive oxygen species)-mediated lipid peroxidation also plays a part in various liver and kidney pathological conditions and injuries; it has caused subsequent liver fibrogenesis in many in vivo studies [3]. Carbon tetrachloride (CCl4) is a well-known hepatotoxicant [4]. This chemical is used in animal models to mimic pathophysiological lesions observed in humans. CCl4 toxicity is mediated by metabolites that react with antioxidant enzymes [5]. It also raises the extent of inflammatory cytokines, including tumor necrosis factor-alpha (TNF-α), interleukin-1 β (IL-1β), nuclear factor (NF-κB). It is well established that CCl4 elicits free radicals and causes macrophages’ activation, leading to the production of inflammatory and profibrogenic mediators [6]. Free radical production is the primary step in the sequence of events leading to membrane lipid peroxidation, apoptosis, and necrosis [7]. Antioxidants provide substantial protection against CCl4-induced hepatic toxicity [8]. Previous studies show that folic acid and melatonin are potent antioxidants in dealing with oxidative stress in vivo [9,10].
Selenium is an essential element for living organisms. It affects the activity of glutathione peroxidase and helps protect cells and tissues from oxidative damage. It is used as a dietary supplement [11] and supports immune responses to prevent various diseases [12,13]. Cancer and immune dysfunction are associated with modest selenium deficiency and altered expression and single nucleotide polymorphisms of some selenoproteins [11,14]. The impact of selenium on health and disease, dietary selenium requirements for support of cognitive and immune functions, and cancer prevention at the molecular level is hot in contemporary research [15]. Cells of the immune system, especially T lymphocytes and macrophages, express most of the 25 genes encoding human selenoproteins, with GPx isoenzymes, GPx1 and GPx4, exhibiting the highest expression levels in both cell types [16]. Selenium derivatives are detoxifying agents toward mercury, cadmium, lead, and many other elements [12]. The study on selenium interacting with other toxic elements in vivo is a hot-spot of contemporary research [17]. The suitability of selenium nanoparticles (SE-NPs) in such a study is attributed to their chemical stability, biocompatibility, and low toxicity [18]. Thus, selenium may be useful against hepatotoxicity as demonstrated in this study.
2. Materials and Methods
2.1. Fabrication and Characterization of Stabilized Nano-Selenium
Analytical grade chemicals (Sigma Aldrich, St. Louis, MO, USA), including citric acid, ascorbic acid, and selenium dioxide, were used to prepare the nano-selenium. The selenium dioxide was used as a selenium precursor. In contrast, citric acid and ascorbic acid were employed as stabilizing and reducing agents, respectively, during the nucleation and formation of stabilized nano-selenium particles following the previously described method [19,20]. For this, the selenium oxide and citric acid were mixed in an aqueous medium to form a 200-mL homogenous solution under stirring with a magnetic stirrer at room temperature for two hours. Then 25 mL of ascorbic acid (0.1M) was added to the mixture dropwise in 2 h under vigorous magnetic stirring. The stirring was continued for an additional 2 h at room temperature. In the final stage, the temperature was increased to 80 °C for 15 h. Finally, the mixture was centrifuged, and nano-selenium was collected after drying at 80 °C. The surface morphology of the prepared nano-selenium was examined by SEM, and EDS characterization was applied to identify the surface structural elements. In addition, the size of the nano-selenium particles and the image of the whole formed nano-selenium embedded citric acid were observed by TEM.
2.2. Animals and Experimental Design
Twenty-four male rats (Rattus norvegicus) weighing 150–170 g (20 ± 1 weeks) were obtained from the College of Sciences, King Saud University. The rearing and caring of the rats were conducted as per our previously published work [5]. The Animal Ethics Committee approved the study protocol in the Zoology Department in the College of Science at King Saud University (KSU-SE-20-38). Rats were divided into three groups (n = 8). Rats from group 1 served as the control and only received the vehicle solution. Group 2 received a single intraperitoneal (IP) injection of 1 mL/kg CCl4 in liquid paraffin (1:1 v/v) [21]. Group 3 was treated with 2.5 mg/kg selenium nanoparticles (SE-NPs) twice a week for three weeks [22] before receiving the CCl4 challenge.
2.2.1. Blood and Liver Samples
Two days after treatment, animals from all groups were necropsied under light anesthesia. Blood was drawn by puncturing the retro-orbital veins in capillary tubes (heparin-coated) until the animals’ death. Plasma was separated after centrifugation at 200× g for 10 min in Eppendorf tubes and stored at −30 °C. The sample was used to determine levels of liver enzymes and lipid profiling.
The liver was removed, washed with saline, and cut into two parts; one part was used for the histological study and the other for assessing lipid peroxidation (malondialdehyde (MDA)) and glutathione (GSH). The samples were processed and prepared as homogenates as done earlier [5].
2.2.2. Estimation of Lipid Peroxidation and an Assay of Reduced Glutathione
Lipid peroxidation was evaluated via measurements of the malondialdehyde (MDA) [23]. The homogenized tissue was read at an absorbance of 532 nm. The standard, 1,1,3,3- tetra methoxy propane was applied to determine the concentration of TBARS (Thiobarbituric acid reactive substances) (MDA per mg protein). An amount of 0.5 mL supernatant with 0.5 mL TCA (Trichloroacetic acid) and 0.5 mL TBA were mixed. Samples were incubated for 30 min in boiling water and then immediately cooled. Samples were centrifuged at 4000 rpm for 10 min at 4 °C. Absorbance was measured at the wavelength of 530 nm in 1 mL of the supernatant. According to the manufacturer, the protein concentration was measured at 500 nm in tissue supernatant (Quimica, Clinica Aplicada S.A., Amposta, Spain).
The reduced glutathione was estimated as described previously [24]. A mixture of 1 mL of tissue supernatant and 1 mL of sulfosalicylic acid was incubated for one hour. Then, it was centrifuged for two min at 1200 rpm. The supernatant was diluted with 1.1 mL of PBS and 200 µL of 5,5-dithiobis-2-nitrobenzoic acid (DTNB). The protein concentration was measured in 500 µL of the supernatant for each sample and at a wavelength of 412 nm, according to the reagent manufacturer (Quimica, Clinica Aplicada S.A., Amposta, Spain).
2.2.3. Liver Function Tests and Lipid Profile
Levels of aspartate transaminase (AST), lactate dehydrogenase (LDH), and protein were measured in plasma samples. The measurements were performed with Bio Merieux kits, France, following the manufacturer protocols. Their levels were measured kinetically by the colorimetric method (UV/Visible-Model-80-2106-00 spectrophotometer, Pharmacia Biotech, Cambridge, England).
Lipid profiling, including cholesterol, triglycerides, HDL (High density lipoproteins), and LDL (Low density lipoproteins), were estimated in plasma samples by commercial kits (Salucea Company, Etten-Leur, The Netherlands).
2.2.4. Histological Study
Tissues were fixed firstly in 10% neutral buffered formalin and then embedded in paraffin. Their sections were prepared and stained with hematoxylin–eosin (H&E). Moreover, Mallory trichrome was used for staining collagen deposits in separate slides. The sections were analyzed blind using a Leica DMRB/E light microscope (Heerbrugg, Switzerland).
2.3. Statistical Analysis
MINITAB software (MINITAB, State College, PA, USA, Version 13.1, 2002) was employed for statistical analysis. Data were treated with Anderson–Darling tests and for variance homogeneity. Furthermore, one-way ANOVA and Tukey’s method for pairwise comparisons were implemented to determine the overall effects of each treatment. Results were expressed as arithmetic mean (M) ± standard deviation (SD). Only statistically significant differences, with p < 0.05, found between a treatment group and the control or between a treatment group and the CCl4 group were considered.
3. Results
3.1. Characteristics of Fabricated Nano-Selenium
The SEM noticed the morphology and shapes of the prepared nano-selenium particles with a magnification of 6000 (Figure 1A) and 10,000 (Figure 1B), which indicate the formation of the nano-selenium with uniform particles with edges and that the forms are not entirely spherical forms. The EDS analysis (Figure 2 upper) showed the elemental nano-selenium surface composition formed from approximately 64.79% selenium and 35.21% carbon, which is indication of formation of stabilized nano-selenium combination with an organic matrix. The nano-selenium particles were formed with sizes of about 100–150 nm and embedded in the organic matrix to create structures of sizes between 200 and 300 nm with different shapes, as shown in Figure 2 lower.
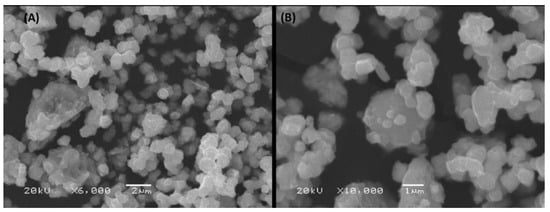
Figure 1.
SEM of stabilized nano-selenium with magnifications of 6000 (A) and 10,000 (B).
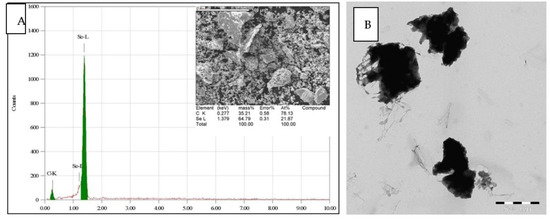
Figure 2.
(A) EDS elemental analysis of stabilized nano-selenium. (B) The TEM image of nano-selenium.
3.2. Bioactivity of SE-NPs
3.2.1. Stress Markers
- (a)
- MDA
Free radical production is the primary step in the sequence of events leading to membrane lipid peroxidation, apoptosis as well as necrosis. MDA is an indicative parameter of the lipid peroxidation. Here, the MDA was dramatically increased in the CCl4 group rats in comparison to the control GSHs. CCl4 group rats exhibited a 176.92% increase in MDA levels over controls, and CCl4 + SE-NP group animals showed MDA levels 63.71% lower than CCl4 group rats (Figure 3). Thus, SE-NPs were found to restore the MDA concentration CCl4 group in comparison to the controls.
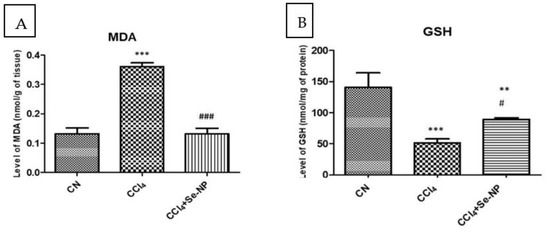
Figure 3.
Hepatic oxidative stress parameters (malondialdehyde (A) and reduced glutathione (B)) in rat liver in different treatment groups. * (p-value < 0.05) indicates significant differences in other rat groups in comparison to control (CN) group animals. # (p-value < 0.05) indicates significant differences in different rat groups compared to the CCl4 group animals. Also ** and ## indicate p ≤ 0.005 while *** and ### indicate p ≤ 0.001.
- (b)
- GSHs
Antioxidants provide substantial protection against CCl4-induced hepatic toxicity. The GSHs declined in the CCl4 group rats in comparison to the control GSHs. CCl4 rats showed a 63.55% reduction in GSHs compared to controls. SE-NPs was found to significantly restore the GSHs in CCl4 animals in comparison to the control GSHs. The GSHs showed a 73.84% increase in CCl4-SE-NPs over levels in the CCl4 group (Figure 3).
3.2.2. Liver Function Tests
After evaluating oxidative stress, the hepatic function parameters (plasma aspartate aminotransferase and lactate dehydrogenase, gamma-glutamyltransferase and alkaline phosphatase) in different rat groups were estimated.
- (a)
- AST
The AST is one of the strong biomarkers determining the liver function and structure. Here, we found a remarkably very significant (p value < 0.01) increase in the AST concentration of CCl4 rats in comparison to control rats. Plasma levels of AST in CCl4 treated rats were 804.40% higher than controls, and Se-NP treatment reduced this increase to 421.43% as compared to controls. Pre-administration of Se-NP treatment before CCl4 challenge decreased AST activity by 27.29% compared to CCl4 treatment alone (Figure 4).
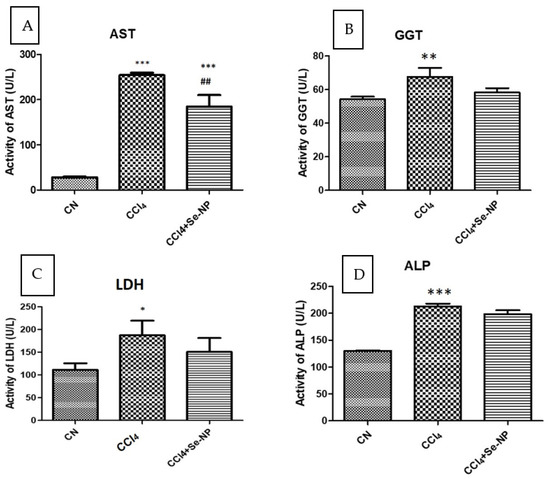
Figure 4.
Hepatic function parameters ((plasma aspartate aminotransferase (A) and lactate dehydrogenase (B), gamma-glutamyltransferase (C) and alkaline phosphatase (D)) in different rat groups. * (p-value < 0.05) indicates significant differences in rat groups in comparison to control (CN) group animals. # (p-value < 0.05) reveals significant differences in different rat groups compared to CCl4 group animals. Also ** and ## indicate p ≤ 0.005 while *** and ### indicate p ≤ 0.001.
- (b)
- Lactate DH
Lactate dehydrogenase (LDH) is expressed extensively in body tissues, in particular liver cells. It is released during hepatic damage, making it a marker of common tissue injuries and diseases involving hepatic toxicity. LDH is a bioindicator for necrotic progression in the living tissue. As expected, LDH was dramatically increased in the CCl4 rats due to the toxicity compared to the control rats. LDH activity was enhanced by 68.16% in CCl4 group animals compared to controls, and CCl4 + Se-NP treated reduced this increase by 19.52% compared to CCl4 group animals (Figure 4).
- (c)
- Gamma-glutamyltransferase
In the cell membranes of many tissues, gamma-glutamyl transferase (GGT) transfers amino acids and the glutamyl moiety of the glutathione across the cell membrane to various acceptor molecules including water, and peptides, leaving the cysteine product to preserve oxidative stability inside the cell. The concentration of GGT was shown to be significantly (p-value < 0.05) increased due to CCl4 toxicity in comparison to the control rats. On the contrary, the GGT level was restored approximately to normal levels in the CCl4 rats treated with Se-NPs in comparison to the control (Figure 4).
- (d)
- Alkaline phosphatase
Alkaline phosphatase (ALP) is of the critical indicators on liver health. Its concentration was found to behave like that of the GGT level in the CCl4 group. Because of the CCl4 toxicity, ALP level was significantly (p-value < 0.05) elevated in the CCl4 rats compared to the control level. Se-NPs were found to partially but not significantly restore the ALP level in CCl4 rats (Figure 4).
3.2.3. Lipid Profiling and Glucose Concentration
- (a)
- Cholesterol
CCl4 group showed a 17.53% increase in cholesterol levels, and CCl4 + Se-NP treated rats exhibited a decrease of 7.18% (Figure 5).
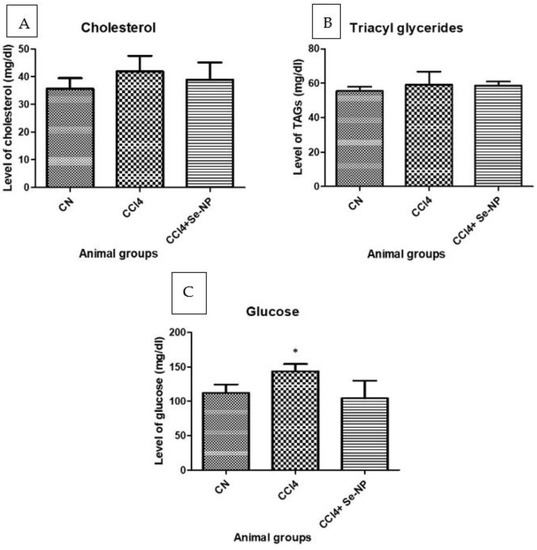
Figure 5.
Showing level of total cholesterol (A), triglycerides (B), and glucose concentrations (C) in the plasma from different rat groups. * (p-value < 0.05) indicates significant differences in different rat groups in comparison to control (CN) group animals.
- (b)
- TAGs
CCl4 displayed a 6.69% increase in TAG (Triglycerides) level, and CCl4 + Se-NP treatment produced a decrease of 0.96% compared to the CCl4 group (Figure 5).
- (c)
- Glucose
CCl4 treated rats showed a 28.21% increase in glucose levels, and Se-NP treatment reduced this increase by 27.36% compared to controls (Figure 5).
3.3. Liver Histology
Examination of histopathological sections revealed that CCl4 challenge caused severe hepatic damage. Injuries included disturbed hepatic structure, narrow hepatic sinusoids, infiltration with inflammatory cells, and eosinophilic hepatocytes. Unlike renal tissues, selenium NPs did not produce any apparent improvement in histopathology of hepatic tissues in CCl4 treated rats (Figure 6).
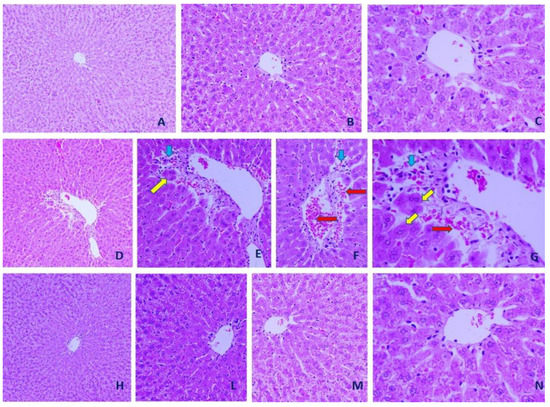
Figure 6.
Representative microscopic images from control, CCl4 and CCl4 + selenium nanoparticles (SE-NPs) hepatic tissues. (A–C): Control hepatic tissues (×200, ×400, and ×1000, respectively). (D–G) CCl4 hepatic tissues (×200, ×400, ×400, and ×1000, respectively). (H–N) CCl4 + SE-NPs hepatic tissues (×200, ×400, ×400, and ×1000, respectively). Hepatocytes (yellow arrows), inflammatory cells (blue arrows) and hemorrhage (red arrow) are shown (hematoxylin–eosin (H&E)).
3.4. Effect of Se-NP on CCl4-Induced Damage to Nuclear DNA
Comet assay is a reliable method for measuring damage to nuclear DNA in the targeted tissues. DNA in liver tissue in CCl4 treated rats showed a 133.33% increase in tail-length of nuclear DNA as compared with controls. CCl4 + Se-NP reduced this increase by 38.29% compared to CCl4 (Figure 7).
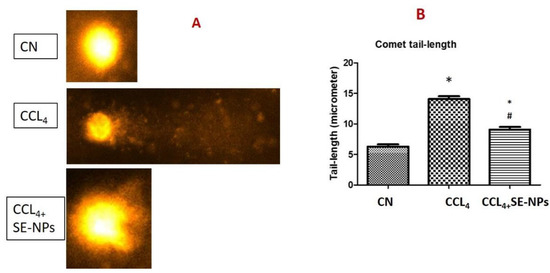
Figure 7.
Representative photos (A) and data analysis (B) of the Comet tail length from different rat groups. * (p-value < 0.05) indicates significant differences in different rat groups in comparison to control (CN) group animals. # (p-value < 0.05) indicates significant differences in different rat groups in comparison to the CCl4 group animals.
4. Discussion
This study is to investigate the effectiveness of SE-NPs in protection against CCl4-induced hepatotoxicity. CCl4, an established hepatotoxicant, is converted to various metabolites, including radicals like CCl3. They are involved in liver disease, cirrhosis, and hepatic carcinoma [25]. Its exposure to rats causes hepatic injuries due to the generated free radicals. It also significantly enhances the release of ALT (alanine transaminase) and AST (aspartate transaminase) to plasma and prominent alteration in lipid profiles [26]. Selenium, as an antioxidant, is a necessary trace element in humans and is suggested as a dietary supplement for its health benefits [27]. Nanotechnology is developed as an amalgamation of chemistry, engineering, biology, and medicine. Nanoparticles have proven useful for the early detection of tumors and the development of novel treatments [28]. We have previously found that Se-NPs have strong anti-diabetic and anti-oxidant properties in gestational diabetic mothers in rat models [29]. The Se-NPs regulated inflammation-mediated complications in diabetic mothers and pups. Ag-NPs showed a potential role in preventing the deleterious effects on DNA in nephrotoxicity [30].
Our findings show that administration of SE-NPs significantly counters hepatotoxicity caused by CCl4, which might be due to antioxidant activity. SE-NPs might scavenge free radicals as reported in other investigations, which in turn lowers serum cholesterol, triglycerides and lipid peroxide [31]. The CCl4 rats exhibited a profound elevation in total cholesterol and triglycerides levels. Hyperlipidemia was detected due to the hepatotoxic effects of CCl4. Hyperlipidemia is a common feature in patients with liver disease. Thus, the cardiovascular risk indices may also be increased. It has been observed that hypercholesterolemia is associated with disturbed angiogenesis, which delayed the tissue healing process [32]. SE-NPs treatment of CCL4 rats produced marked amelioration of the tested lipids. Thus, it can be suggested that the alleviation of lipids may be involved, at least in part, in the enhanced tissue healing observed in CCL4 rats treated with SE-NPs.
Hyperlipidemia interferes with liver functions. Oxidative stress damages the stability and integrity of biological membranes and increases permeability, resulting in the outflow of cytoplasm enzymes such as ALT, ALP, and AST into the blood [33]. Thus, the ALT, ALP and AST activities in the serum are essential indices for evaluating liver injury. In the current experiment, serum ALT activity, ALP, and AST in the CCL4 group were significantly higher than that in the normal rats, suggesting that CCL4 exerted a toxic effect on CCl4-injected rats. Recent results indicated that oxidative stress and lipid peroxidation elevated inflammation and enzyme and GGT activity [34].
On the other hand, breaking down extracellular glutathione and making GGT component amino acids available to the cells has an essential physiological role in counteracting oxidative stress [35]. Indeed, conditions that increase serum GGT lead to increased free radical production and the threat of glutathione depletion [35]. It is more likely that CCL4 increased GGT in a similar mechanism in the CCl4-injected rats. The antioxidant treatment may inhibit disease progression. Therefore, treatment of CCl4-injected rats with SE-NPs restored the liver function-enzyme concentration with the GGT level.
The hepatotoxicity of CCl4 is attributed to the generation of free radicals. They activate the Kupffer cells and macrophages that further elicit inflammatory mediators causing fibrosis. The heightened release of free radicals is the first step in a chain of events leading to lipid peroxidation of membraned cell organelles, resulting in apoptosis and necrosis [6]. The elevation in cytokine concentrations and oxidative stress might be the possible chemically-induced liver apoptosis mechanisms [7]. In addition, ROS-mediated lipid peroxidation is the main reason behind the pathogenesis of various liver toxicity and liver fibrosis followed by histological distortion and DNA damage in the target tissues in the experimental animals [8].
CCl4 causes ROS-mediated biotransformation leading to moderate-to-severe toxicity that disturbs cellular redox balance and compromises structural settings in the target tissues [36]. ROS increases lipid peroxidation (MDA levels) and compromises levels of GSH [9] in the target tissues. The histological evaluation of kidney sections further agrees with these conclusions. Results are consistent with the nephrotoxic effect of potassium bromate [37]. Furthermore, CCl4 is converted to highly reactive metabolites that can affect the cellular components (proteins, enzymes, lipids, and nucleic acids) and cellular structures. Moreover, free radicals generated during metabolism and biotransformation of CCl4 cause chromosomal aberrations, DNA-base transversions, DNA adduct formation, and 8-OH dG. Administration of CCl4 caused genotoxicity and DNA fragmentation in hepatic tissues in the male Sprague–Dawley rats [38]. This activity can explain the present study results, in which comet assays show a noticeable increase in DNA tail length in CCl4-treated rats. Hence, CCl4 damages the biological functions of critical biomolecules that disrupt cellular structure and function [39]. The elevated concentration of MDA is a strong index of oxidative stress in CCl4-challenged rats. However, its decreased concentration was observed in the CCl4 administered rats treated with SE-NPs. Thus, the SE-NPs can effectively protect against lipid peroxidation.
SE-NPs have shown antioxidant protection in vitro and in vivo studies [29,31]. All the organisms have effective defense mechanisms that prevent and neutralize damage incurred by free radicals. The NPs restore the activity of GSH and catalase significantly. GSH acts as a non-enzymatic antioxidant that reduces H2O2, hydroperoxides, and xenobiotic toxicity [40]. In contrast, a significant decrease in MDA in hepatic tissues is confirmed after pretreatment with SE-NPs and hence protects against hepatic lipid peroxidation incurred by CCl4. Antioxidant activity and antilipidemic effects of SE-NPs have been reported to modulate blood pressure and alleviate the damage to target organs [41]. GSH is an essential determinant for cell survival or death during disturbed oxidative stress [42].
Inflammatory macrophages and dendritic cells are a physiological defense mechanism for injury and toxicity that are still inadequately addressed by the immune system [43]. Infiltration of inflammatory cells, if prolonged, can exacerbate fibrosis [44]. We previously found that carcinogens trigger all critical pro-inflammatory cytokines (IL-1, 2, 6, and 7) as well as transcription factors (NF-kB and TNF-α) in target organs [45]. Immune cells then become aggressive, causing extensive inflammation and fibrosis in the liver, as evidenced in histopathological findings. SE-NPs were found to remarkably ameliorate inflammatory infiltration with the immune cells in the hepatic tissues (Figure 6).
NF-kB might be downregulated by SE-NPs that induce oxidative stability, although this activity was not addressed in the present study. This amelioration of hepatic seems to be mediated by the ceasing of oxidative stress and suppression of NF-κB, the critical regulator of inflammatory response. Downregulation of NF-κB would exert diminishing production of pro-inflammatory cytokines.
Hepatic injury markers are significantly low in the animals treated with any antioxidant treatment. SE-NPs also noticeably attenuated the plasma liver enzymes induced by CCl4, which led to the restoration of the key enzymes’ activities to normal activity. The effect of SE-NPs was further confirmed through histopathological examinations. Hence, pretreatment with SE-NPs has broad anti-inflammatory effects that can attenuate the allergies and inflammation in CCl4-challenged rats.
This study, hence, confirms the hypolipidemic effect of SE-NPs in CCl4-treated rats. It is evident from the low concentrations of cholesterol and triglycerides after the NPs treatment. Similar findings have been reported in many animal model studies [5]. This effect of NPs may be attributed to the enhanced catabolism of cholesterol to bile acids [46] and suppressing cholesterol synthesis and LDL receptor activity.
5. Conclusions
Use of nanotechnology may allow the development of novel therapeutic strategies. Our findings provide evidence of possible antioxidant and anti-inflammatory effects of SE-NPs. This combination restored normal oxidative stress balance, perhaps by inducing oxidative stability and reducing tissue damage. SE-NPs treatment produced significant improvement in liver function and histological architecture after CCl4 challenge. The size of NPs, only several hundred nanometers, may allow close and frequent interaction with biomolecules present both on cell surfaces and inside cells. Currently, no data exist to determine if SE-NPs’ activity toward CCl4-induced hepatic injury might be clinically useful. The present study establishes a possible use of SE-NPs to treat CCl4-induced hepatic toxicity in vivo. However, further research is warranted to elucidate the in-depth mechanisms.
Author Contributions
H.E. designed this study, analyzed the data, prepared the figures, and prepared and finalized the manuscript. M.A.H. Prepared and characterized the nanoparticles. J.A.-T. and I.H. performed the animal handling, treatment, sample preparation and conducted all biochemical studies. A.M.R. and I.M.A. revised the manuscript. Writing–review and editing, A.M.A. All authors have read and agreed to the published version of the manuscript.
Funding
Researchers Supporting Project number (RSP-2020/225), King Saud University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
All procedures were conducted in accordance with guidelines for the care and use of experimental animals by the Committee for the Purpose of Control and Supervision of Experiments on Animals and the National Institutes of Health. The present work was approved by the KSU ethical committee under approval number KSU-SE-20-38.
Data Availability Statement
The data used to support the findings of this study are included in the article.
Acknowledgments
This work was supported by Researchers Supporting Project number (RSP-2020/225), King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Teschke, R.; Frenzel, C.; Glass, X.; Schulze, J.; Eickhoff, A. Herbal hepatotoxicity: A critical review. Br. J. Clin. Pharmacol. 2013, 75, 630–636. [Google Scholar] [CrossRef]
- Brattin, W.J.; Glende, E.A., Jr.; Recknagel, R.O. Pathological mechanisms in carbon tetrachloride hepatotoxicity. J. Free Radic. Biol. Med. 1985, 1, 27–38. [Google Scholar] [CrossRef]
- Hassan, I.; Ebaid, H.; Alhazza, M.I.; Al-Tamimi, J.; Aman, S.; Abdel-Mageed, A.M. Copper mediates anti-inflammatory and antifibrotic activity of Gleevec in hepatocellular carcinoma-induced male rats. Canad. J. Gastroenterol. Hepatol. 2019, 2019, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ramaiah, S.K.; Rittling, S. Role of osteopontin in regulating hepatic inflammatory responses and toxic liver injury. Expert Opin. Drug Metab. Toxicol. 2007, 3, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Rikans, L.E.; Hornbrook, K.R.; Cai, Y. Carbon tetrachloride hepatotoxicity as a function of age in female Fischer 344 rats. Mech. Ageing Dev. 1994, 76, 89–99. [Google Scholar] [CrossRef]
- Ebaid, H.; Bashandy, S.A.; Alhazza, I.M.; Rady, A.; El-Shehry, S. Folic acid and melatonin ameliorate carbon tetrachloride-induced hepatic injury, oxidative stress and inflammation in rats. Nutr. Metab. 2013, 10, 20. [Google Scholar] [CrossRef]
- Basu, S. Carbon tetrachloride-induced lipid peroxidation: Eicosanoid formation and their regulation by antioxidant nutrients. Toxico 2003, 189, 113–127. [Google Scholar] [CrossRef]
- Sheweita, S.; Abd El-Gabar, M.; Bastawy, M. Carbon tetrachloride changes the activity of cytochrome P450 system in the liver of male rats: Role of antioxidants. Toxicology 2001, 169, 83–92. [Google Scholar] [CrossRef]
- Ebaid, H.; Bashandy, S.A.; Abdel-Mageed, A.M.; Al-Tamimi, J.; Hassan, I.; Alhazza, I.M. Folic acid and melatonin mitigate diabetic nephropathy in rats via inhibition of oxidative stress. Nutr. Metab. 2020, 17, 6. [Google Scholar] [CrossRef]
- Letelier, M.E.; Jara-Sandoval, J.; Molina-Berrios, A.; Faundez, M.; Aracena-Parks, P.; Aguilera, F. Melatonin protects the cytochrome P450 system through a novel antioxidant mechanism. Chem. Biol. Interact. 2010, 185, 208–214. [Google Scholar] [CrossRef]
- Fairweather-Tait, S.J.; Bao, Y.; Broadley, M.R.; Collings, R.; Ford, D.; Hesketh, J.E.; Hurst, R. Selenium in human health and disease. Antioxid. Redox Signal. 2011, 14, 1337–1383. [Google Scholar] [CrossRef] [PubMed]
- Masukawa, T. Pharmacological and toxicological aspects of inorganic and organic selenium compounds. Org. Selenium Tellurium Compd. 1987, 2, 377–392. [Google Scholar]
- Weekley, C.M.; Harris, H.H. Which form is that? The importance of selenium speciation and metabolism in the prevention and treatment of disease. Chem. Soc. Rev. 2013, 42, 8870–8894. [Google Scholar] [CrossRef] [PubMed]
- Steinbrenner, H.; Sies, H. Selenium homeostasis and antioxidant selenoproteins in brain: Implications for disorders in the central nervous system. Arch. Biochem. Biophys. 2013, 536, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Steinbrenner, H.; Speckmann, B.; Sies, H. Toward understanding success and failures in the use of selenium for cancer prevention. Antioxid. Redox Sign. 2013, 19, 181–191. [Google Scholar] [CrossRef]
- Huang, Z.; Rose, A.H.; Hoffmann, P.R. The role of selenium in inflammation and immunity: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Sign. 2012, 16, 705–743. [Google Scholar] [CrossRef]
- Kędziorski, A.; Nakonieczny, M.; Swierczek, E.; Szulinska, E. Cadmium-selenium antagonism and detoxifying enzymes in insects. Fresenius J. Anal. Chem. 1996, 354, 571–575. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, J.; Yu, H. Elemental selenium at nano size possesses lower toxicity without compromising the fundamental effect on selenoenzymes: Comparison with selenomethionine in mice. Free Radic. Biol. Med. 2007, 42, 1524–1533. [Google Scholar] [CrossRef]
- Jia, X.; Liu, Q.; Zou, S.; Xu, X.; Zhang, L. Construction of selenium nanoparticles/β-glucan composites for enhancement of the antitumor activity. Carbohydr. Polym. 2015, 117, 434–442. [Google Scholar] [CrossRef]
- Yan, J.K.; Qiu, W.Y.; Wang, Y.Y.; Wang, W.H.; Yang, Y.; Zhang, H.N. Fabrication and stabilization of biocompatible selenium nanoparticles by carboxylic curdlans with various molecular properties. Carbohydr. Polym. 2018, 179, 19–27. [Google Scholar] [CrossRef]
- Makni, M.; Chtourou, Y.; Fetoui, H.; Garoui, E.M.; Boudawara, T.; Zeghal, N. Evaluation of the antioxidant, anti-inflammatory and hepatoprotective properties of vanillin in carbon tetrachloride-treated rats. Eur. J. Pharmacol. 2011, 668, 133–139. [Google Scholar] [CrossRef]
- Malek, F.A.; Moritz, K.-U.; Fanghanel, J.; Bienengraber, V. Reduction of procarbazine-induced cleft palates by prenatal folic acid supplementation in rats. Pathol. Res. Pract. 2004, 200, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar]
- Jollow, D.J.; Mitchell, J.R.; Zampaglione, N.; Gillette, J.R. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 1974, 11, 151–169. [Google Scholar] [CrossRef]
- Khan, R.A.; Khan, M.R.; Sahreen, S. CCl4-induced hepatotoxicity: Protective effect of rutin on p53, CYP2E1 and the antioxidative status in rat. BMC Complement. Altern. Med. 2012, 12, 178. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.Y.; Chen, Y.L.; Yang, S.C.; Huang, G.C.; Tsi, D.; Huang, C.C.; Chen, J.R.; Li, J.S. Effect of schisandrin B and sesamin mixture on CCl4-induced hepatic oxidative stress in rats. Phytother. Res. 2009, 23, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Tan, H.Y.; Li, S.; Xu, Y.; Guo, W.; Feng, Y. Supplementation of micronutrient selenium in metabolic diseases: Its role as an antioxidant. Oxidative Med. Cell. Longev. 2017. [Google Scholar] [CrossRef]
- Cai, W.; Gao, T.; Hong, H.; Sun, J. Applications of gold nanoparticles in cancer nanotechnology. Nanotechnol. Sci. Appl. 2008, 1, 17. [Google Scholar] [CrossRef]
- Hassan, I.; Ebaid, H.; Al-Tamimi, J.; Habila, M.A.; Alhazza, I.M.; Rady, A.M. Selenium nanoparticles mitigate diabetic nephropathy and pancreatopathy in rat offspring via inhibition of oxidative stress. J. King Saud Univ. Sci. 2021, 33, 101265. [Google Scholar] [CrossRef]
- Ebaid Al-Tamimi, J.; Habila, M.A.; Hassan, I.; Rady, A.M.; Alhazza, I.M. Potential therapeutic effect of synthesized AgNP using curcumin extract on CCl4-induced nephrotoxicity in male mice. J. King Saud Univ. Scien. 2021, 101356. [Google Scholar] [CrossRef]
- Steinbrenner, H.; Al-Quraishy, S.; Dkhil, M.A.; Wunderlich, F.; Sies, H. Dietary selenium in adjuvant therapy of viral and bacterial infections. Adv. Nutr. 2015, 6, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.J.; Ho, H.V.; Kwan, H.H.; Fajardo, L.F.; Cooke, J.P. Angiogenesis is impaired by hypercholesterolemia role of asymmetric dimethylarginine. Circulation 2000, 102, 1414–1419. [Google Scholar] [CrossRef] [PubMed]
- Ebaid, H.; Habila, M.; Hassan, I.; Al-Tamimi, J.; Omar, M.S.; Rady, A.; Alhazza, I.M. Comb Chem High 2020. Curcumin-containing Silver Nanoparticles prevent carbon tetrachlorideinduced hepatotoxicity in mice. Throughput Screen. 2020, 23, 10. [Google Scholar] [CrossRef]
- Cheraghi, M.; Ahmadvand, H.; Maleki, A.; Babaeenezhad, E.; Shakiba, S.; Hassanzadeh, F. Oxidative Stress Status and Liver Markers in Coronary Heart Disease. Rep. Biochem. Mol. Biol. 2019, 8, 49–55. [Google Scholar]
- Whitfield, J.B. Gamma glutamyl transferase. Crit. Rev. Clin. Lab. Sci. 2001, 38, 263–355. [Google Scholar] [CrossRef]
- Shanmugavel, V.; Santhi, K.K.; Kurup, A.H.; Kalakandan, S.; Anandharaj, A.; Rawson, A. Potassium bromate: Effects on bread components, health, environment and method of analysis: A review. Food Chem. 2020, 311, 125964. [Google Scholar] [CrossRef]
- Alhazza, I.M.; Hassan, I.; Ebaid, H.; Al-Tamimi, J.; Alwasel, S.H. Chemopreventive effect of riboflavin on the potassium bromate–induced renal toxicity in vivo. Naunyn Schmiedebergs Arch. Pharmacol. 2020, 1, 10. [Google Scholar] [CrossRef]
- Alkreathy, H.M.; Khan, R.A.; Khan, M.R.; Sahreen, S. CCl4 induced genotoxicity and DNA oxidative damages in rats: Hepatoprotective effect of Sonchus arvensis. BMC Complement. Altern. Med. 2014, 14, 452. [Google Scholar] [CrossRef]
- Hassan, I.; Husain, F.M.; Khan, R.A.; Ebaid, H.; Al-Tamimi, J.; Alhazza, I.M.; Aman, S.; Ibrahim, K.E. Ameliorative effect of zinc oxide nanoparticles against potassium bromate-mediated toxicity in Swiss albino rats. Environ. Sci. Pollut. Res. 2019, 26, 9966–9980. [Google Scholar] [CrossRef]
- Ebaid, H.; Salem, A.; Sayed, A.; Metwalli, A. Whey protein enhances normal inflammatory responses during cutaneous wound healing in diabetic rats. Lipids Health Dis. 2011, 10, 1–10. [Google Scholar] [CrossRef]
- Paulis, L.; Simko, F. Blood pressure modulation and cardiovascular protection by melatonin: Potential mechanisms behind. Physiol. Res. 2007, 56, 6. [Google Scholar]
- Ahmed, R.R.; Mahmoud, A.; Ahmed, O.M.; Metwalli, A.; Ebaid, H. Up-regulation of Hsp72 and keratin16 mediates wound healing in streptozotocin diabetic rats. Biol. Res. 2015, 48, 54. [Google Scholar] [CrossRef]
- Lee, S.B.; Kalluri, R. Mechanistic connection between inflammation and fibrosis. Kidney Int. 2010, 78, S22–S26. [Google Scholar] [CrossRef] [PubMed]
- Wallace, E.; Gewin, L. Imatinib: Novel treatment of immune-mediated kidney injury. J. Am. Soc. Nephrol. 2013, 24, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.; Tang, P. Effect of melatonin on the maintenance of cholesterol homeostasis in the rat. Endocr. Res. 1995, 21, 681–696. [Google Scholar] [CrossRef] [PubMed]
- Mullerwieland, D.; Behnke, B.; Koopmann, K.; Krone, W. Melatonin inhibits LDL receptor activity and cholesterol-synthesis in freshly isolated human mononuclear leukocytes. Biochem. Biophys. Res. Commun. 1994, 203, 416–421. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).