Rising Temperature Is a More Important Driver Than Increasing Carbon Dioxide Concentrations in the Trait Responses of Enhalus acoroides Seedlings
Abstract
1. Introduction
2. Material and Methods
2.1. Collection and Maintenance of Seagrass Seedlings
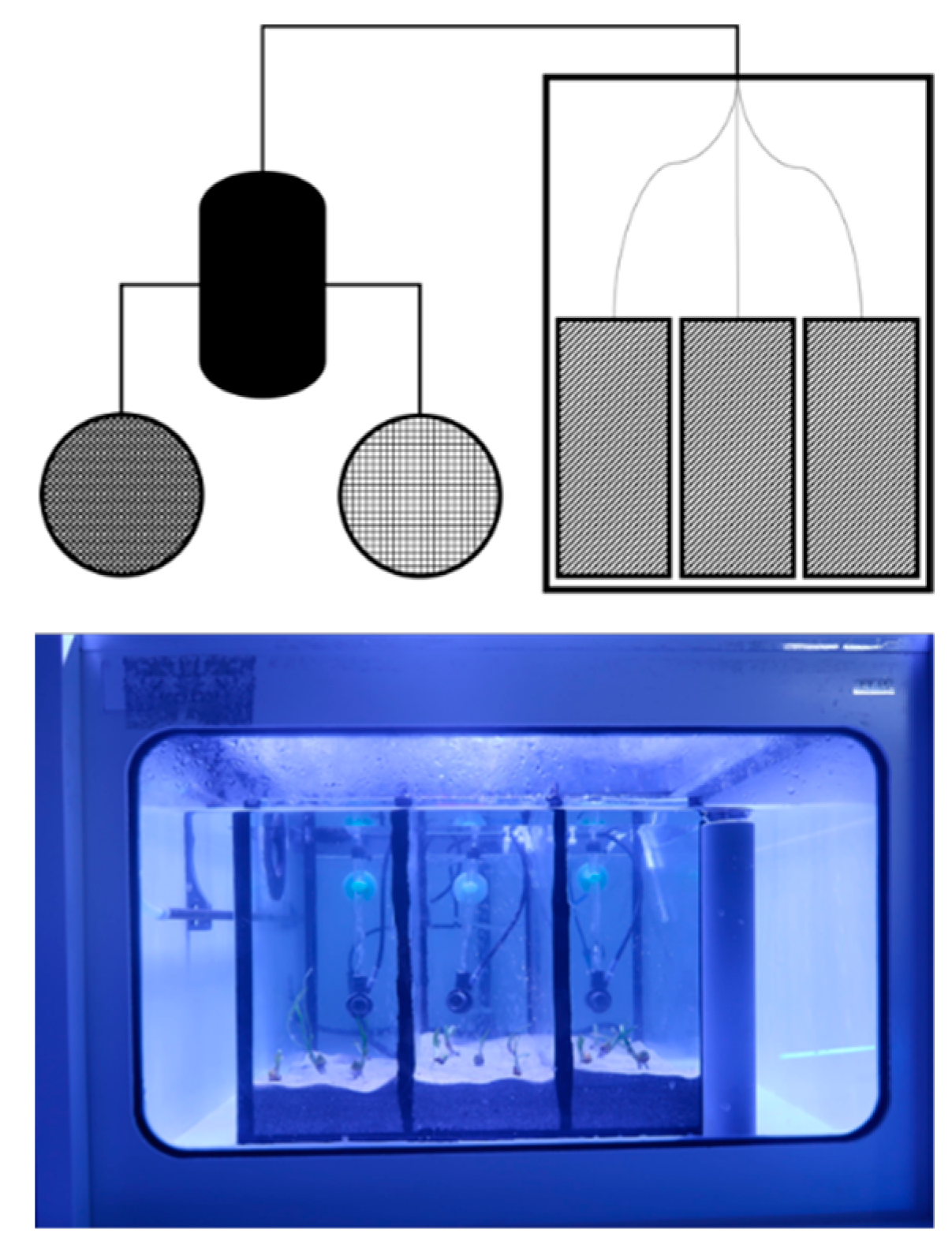
2.2. Experimental Design and Setup
2.3. Water Sampling
2.4. Seedling Trait Responses to Treatments
2.4.1. Morphological Traits
2.4.2. Biochemical Traits
2.4.3. Physiological Traits
2.5. Statistical Analysis
3. Results
3.1. Water Parameters
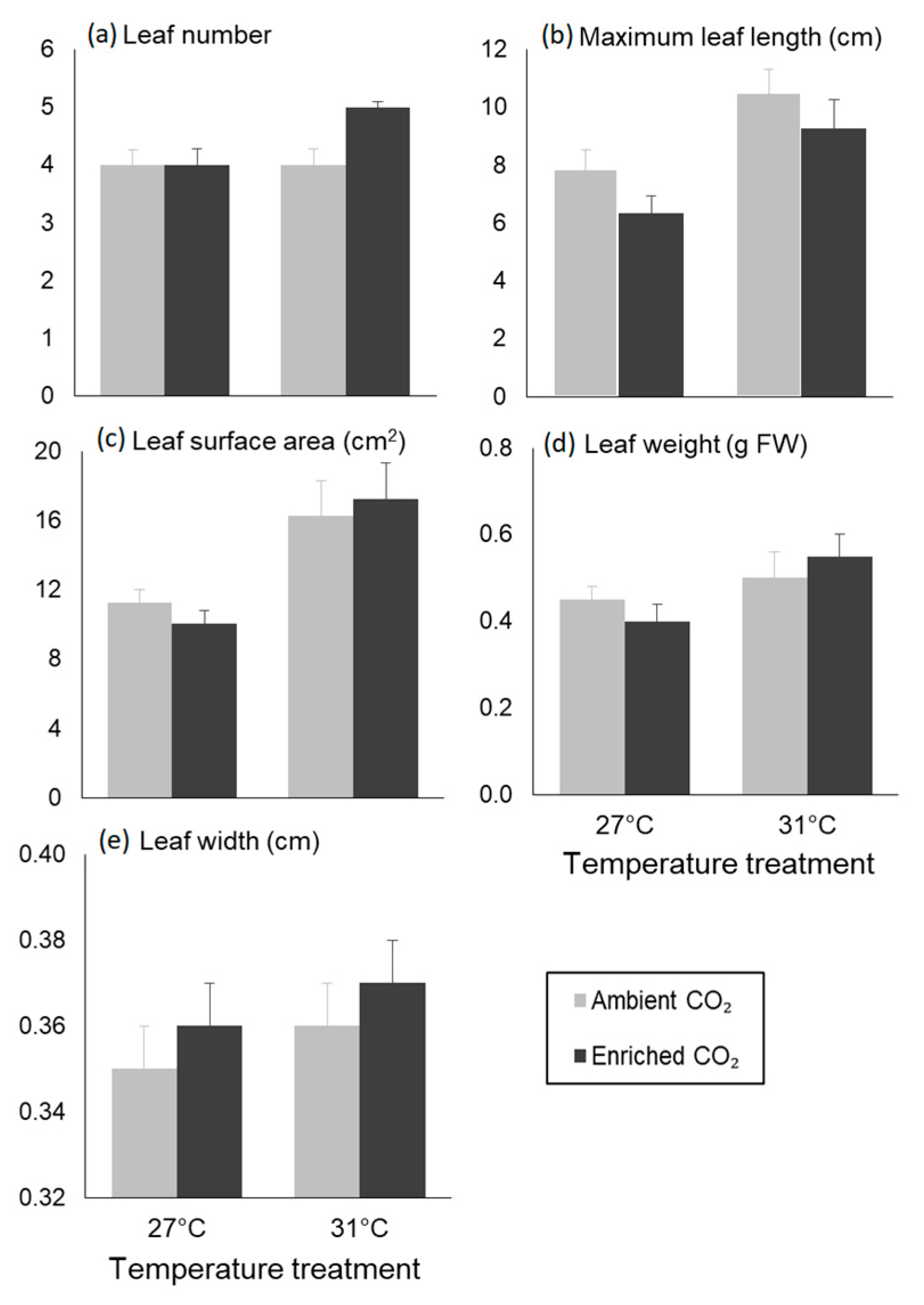
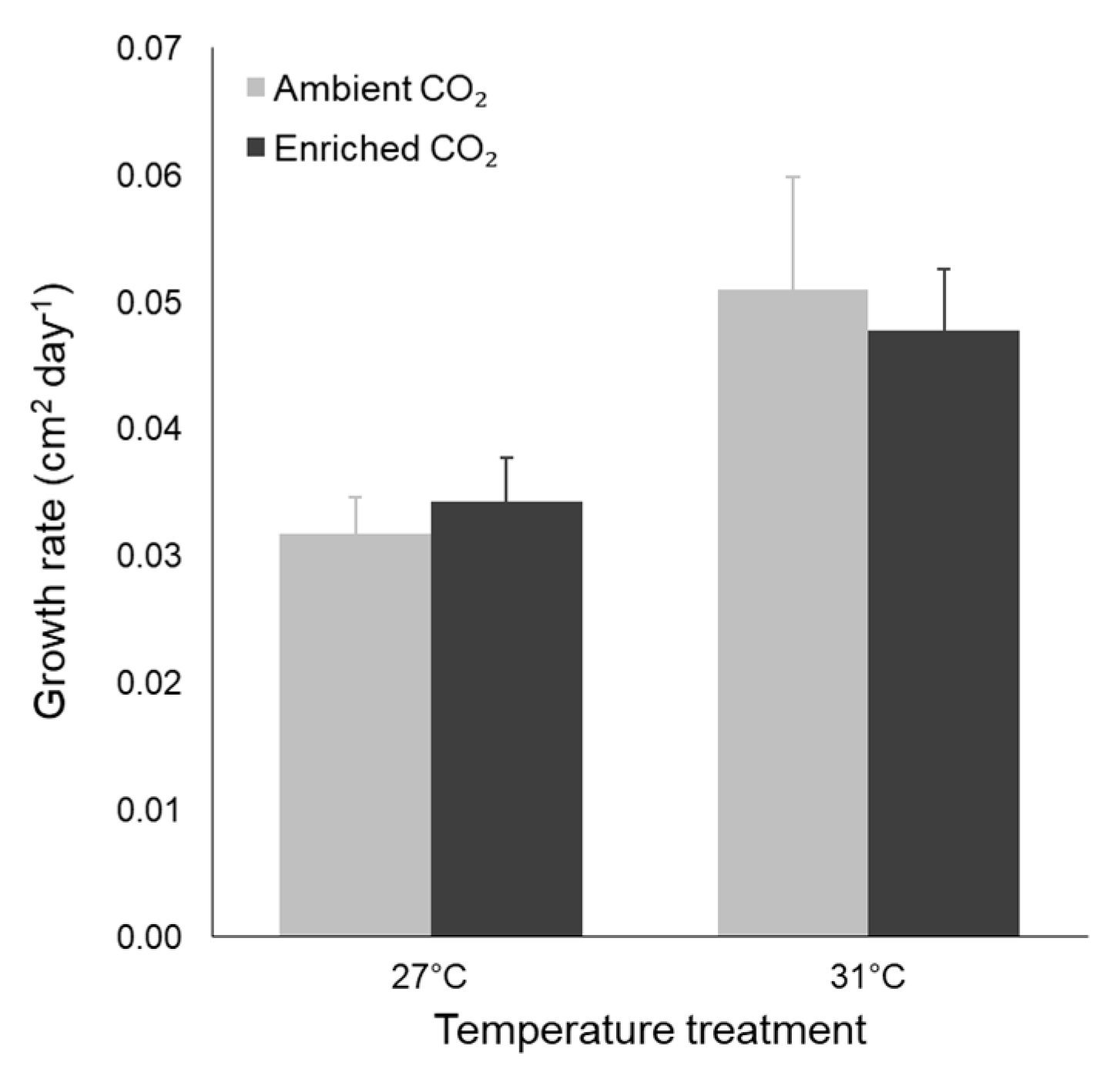
3.2. Morphological Traits
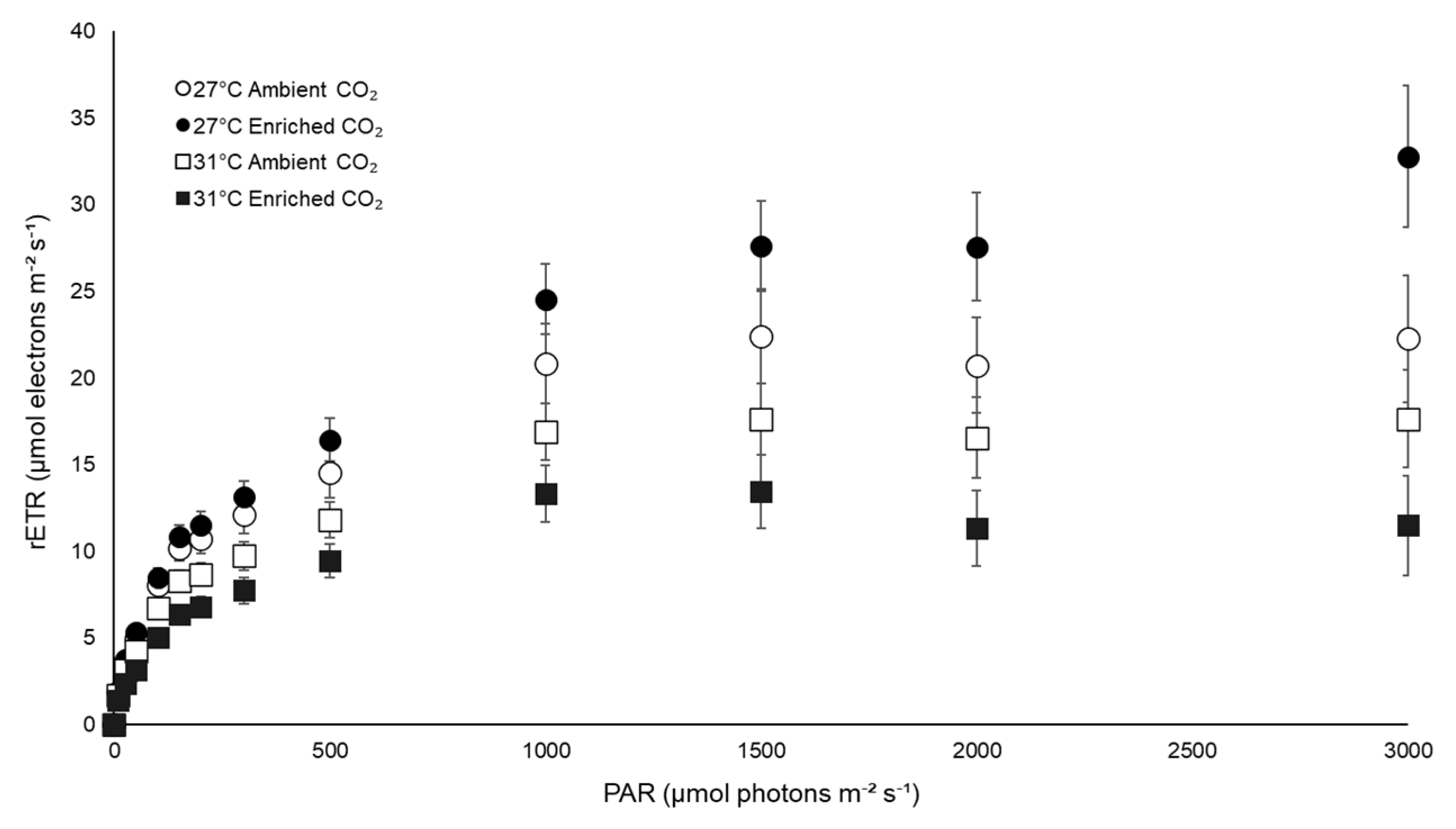
3.3. Physiological Traits
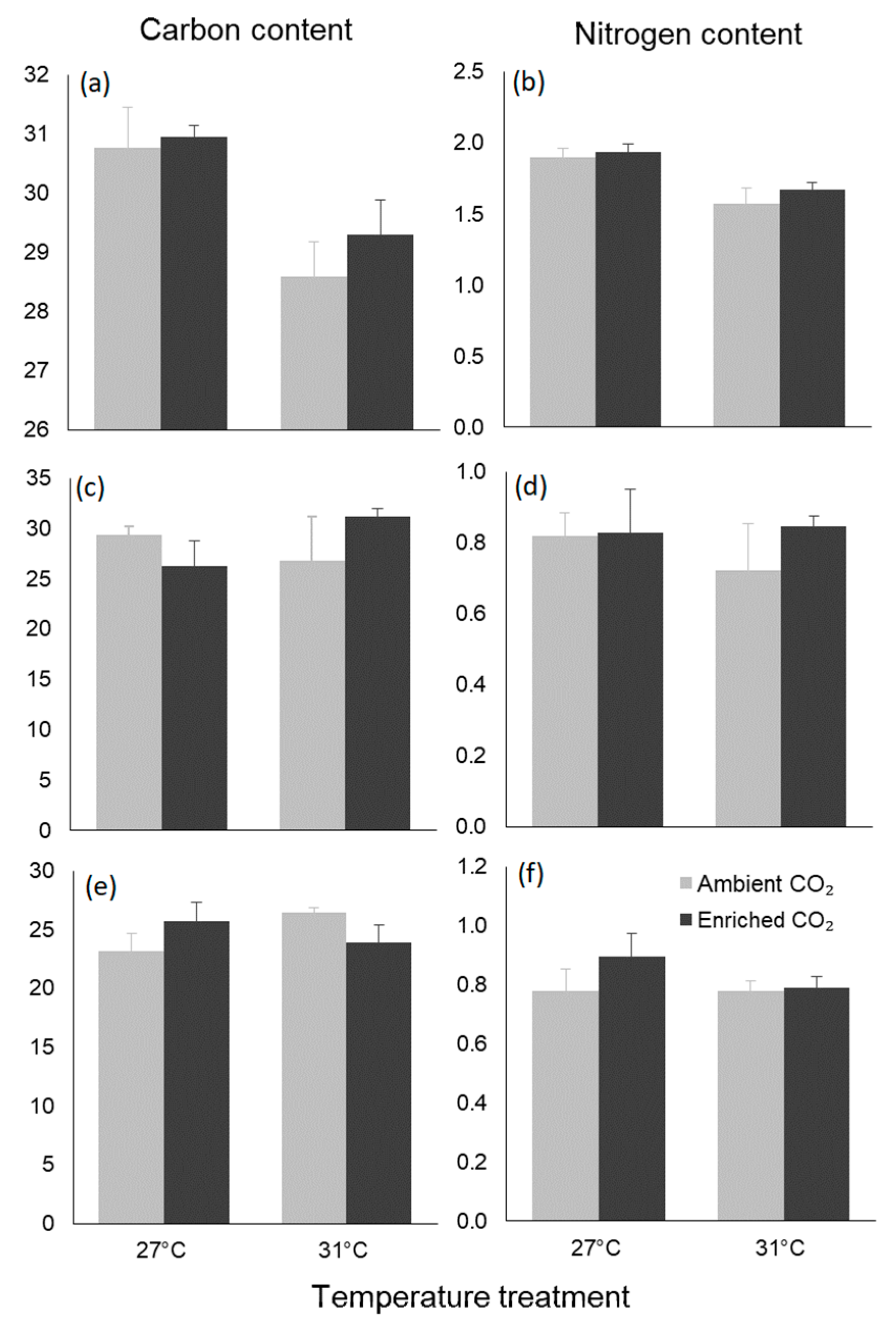
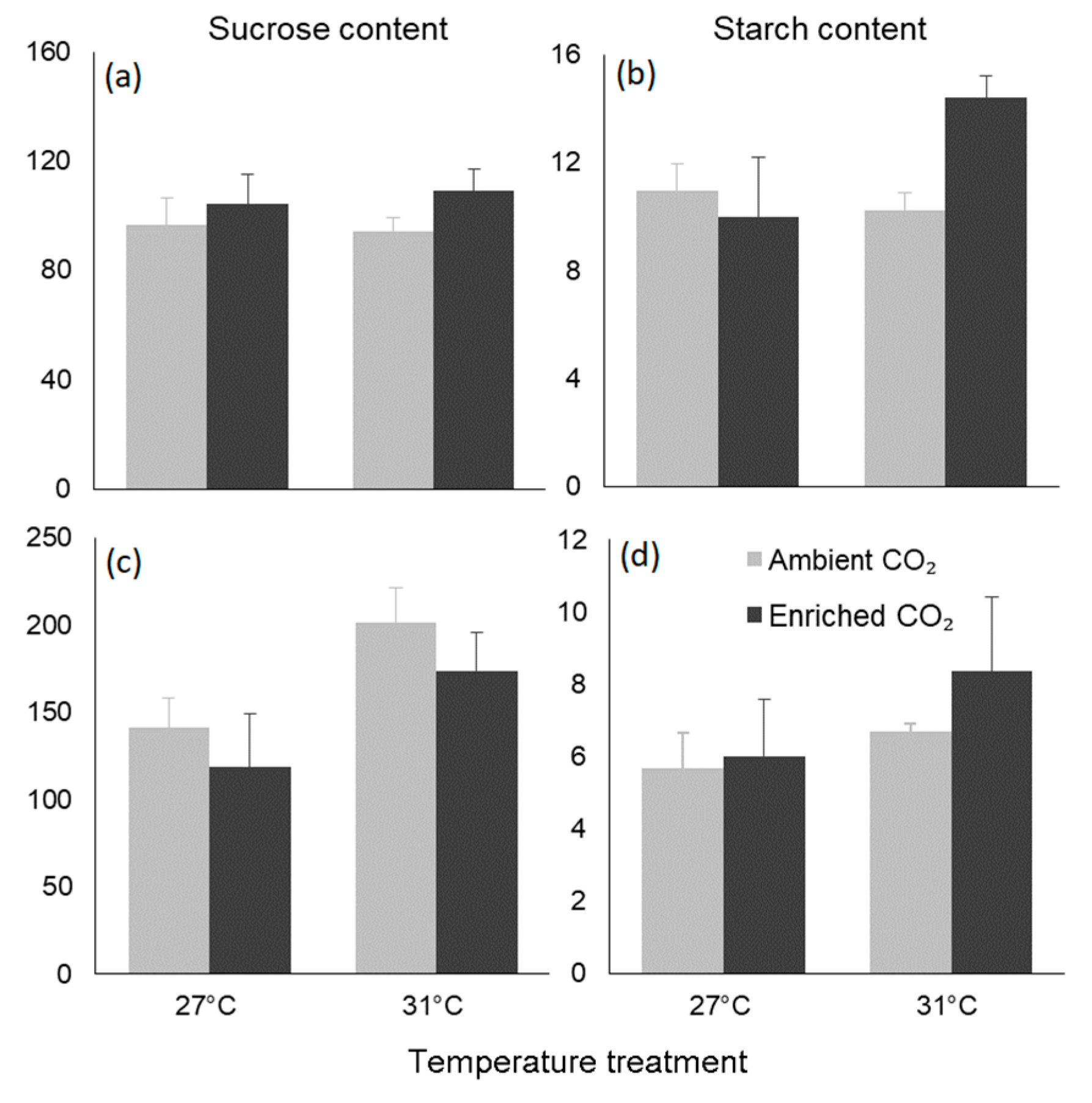
3.4. Biochemical Traits
4. Discussion
4.1. Temperature as the Main Driver of Seedling’s Trait Responses
4.2. Interactions of Temperature and CO2 Concentrations on Seedling’s Trait Responses
4.3. Different Trait Responses and Dependence on the Internal Reserves
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Short, F.; Carruthers, T.; Dennison, W.; Waycott, M. Global seagrass distribution and diversity: A bioregional model. J. Exp. Mar. Biol. Ecol. 2007, 350, 3–20. [Google Scholar] [CrossRef]
- Waycott, M.; Duarte, C.M.; Carruthers, T.J.B.; Orth, R.J.; Dennison, W.C.; Olyarnik, S.; Calladine, A.; Fourqurean, J.W.; Heck, K.L.; Hughes, A.R.; et al. Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proc. Natl. Acad. Sci. USA 2009, 106, 12377–12381. [Google Scholar] [CrossRef]
- De los Santos, C.B.; Krause-Jensen, D.; Alcoverro, T.; Marbà, N.; Duarte, C.M.; van Katwijk, M.M.; Pérez, M.; Romero, J.; Sánchez-Lizaso, J.L.; Roca, G.; et al. Recent trend reversal for declining European seagrass meadows. Nat. Commun. 2019, 10, 3356. [Google Scholar] [CrossRef]
- Schäfer, S.; Monteiro, J.; Castro, N.; Gizzi, F.; Henriques, F.; Ramalhosa, P.; Parente, M.I.; Rilov, G.; Gestoso, I.; Canning-Clode, J. Lost and found: A new hope for the seagrass Cymodocea nodosa in the marine ecosystem of a subtropical Atlantic island. Reg. Stud. Mar. Sci. 2021, 41, 101575. [Google Scholar] [CrossRef]
- Guerrero-Meseguer, L.; Veiga, P.; Sampaio, L.; Rubal, M. Resurgence of Zostera marina in the Ria de Aveiro Lagoon, Portugal. Aquat. Bot. 2021, 169, 103338. [Google Scholar] [CrossRef]
- Unsworth, R.K.F.; Ambo-Rappe, R.; Jones, B.L.; La Nafie, Y.A.; Irawan, A.; Hernawan, U.E.; Moore, A.M.; Cullen-Unsworth, L.C. Indonesia’s globally significant seagrass meadows are under widespread threat. Sci. Total Environ. 2018, 634, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Short, F.T.; Burdick, D.M. Development of a nutrient pollution indicator using the seagrass, Zostera marina, along nutrient gradients in three New England estuaries. Aquat. Bot. 2004, 78, 197–216. [Google Scholar] [CrossRef]
- Roca, G.; Alcoverro, T.; Krause-Jensen, D.; Balsby, T.J.S.; Van Katwijk, M.M.; Marbà, N.; Santos, R.; Arthur, R.; Mascaró, O.; Fernández-Torquemada, Y.; et al. Response of seagrass indicators to shifts in environmental stressors: A global review and management synthesis. Ecol. Indic. 2016, 63, 310–323. [Google Scholar] [CrossRef]
- Carruthers, T.J.B.; Dennison, W.C.; Longstaff, B.J.; Waycott, M.; Abal, E.G.; McKenzie, L.J.; Lee Long, W.J. Seagrass habitats of Northeast Australia: Models of key processes and controls. Bull. Mar. Sci. 2002, 71, 1153–1169. [Google Scholar]
- Egea, L.G.; Jiménez-Ramos, R.; Vergara, J.J.; Hernández, I.; Brun, F.G. Interactive effect of temperature, acidification and ammonium enrichment on the seagrass Cymodocea nodosa. Mar. Pollut. Bull. 2018, 134, 14–26. [Google Scholar] [CrossRef]
- Gunderson, A.R.; Armstrong, E.J.; Stillman, J.H. Multiple stressors in a changing world: The need for an improved perspective on physiological responses to the dynamic marine environment. Ann. Rev. Mar. Sci. 2016, 8, 357–378. [Google Scholar] [CrossRef] [PubMed]
- Ontoria, Y.; Gonzalez-Guedes, E.; Sanmartí, N.; Bernardeau-Esteller, J.; Ruiz, J.M.; Romero, J.; Pérez, M. Interactive effects of global warming and eutrophication on a fast-growing Mediterranean seagrass. Mar. Environ. Res. 2019, 145, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.; Bowes, G.; Ross, C.; Zhang, X.H. Climate change and ocean acidification effects on seagrasses and marine macroalgae. Glob. Change Biol. 2013, 19, 103–132. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Meseguer, L.; Marín, A.; Sanz-Lázaro, C. Future heat waves due to climate change threaten the survival of Posidonia oceanica seedlings. Environ. Pollut. 2017, 230, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Hernán, G.; Ortega, M.J.; Gándara, A.M.; Castejón, I.; Terrados, J.; Tomas, F. Future warmer seas: Increased stress and susceptibility to grazing in seedlings of a marine habitat-forming species. Glob. Chang. Biol. 2017, 23, 4530–4543. [Google Scholar] [CrossRef]
- Yue, S.; Zhou, Y.; Zhang, Y.; Xu, S.; Gu, R.; Xu, S.; Zhang, X.; Zhao, P. Effects of salinity and temperature on seed germination and seedling establishment in the endangered seagrass Zostera japonica Asch. & Graebn. in Northern China. Mar. Pollut. Bull. 2019, 146, 848–856. [Google Scholar] [CrossRef]
- Pereda-Briones, L.; Terrados, J.; Tomas, F. Negative effects of warming on seagrass seedlings are not exacerbated by invasive algae. Mar. Pollut. Bull. 2019, 141, 36–45. [Google Scholar] [CrossRef]
- Artika, S.R.; Ambo-Rappe, R.; Teichberg, M.; Moreira-Saporiti, A.; Viana, I.G. Morphological and physiological responses of Enhalus acoroides seedlings under varying temperature and nutrient treatment. Front. Mar. Sci. 2020, 7, 325. [Google Scholar] [CrossRef]
- Niu, S.; Zhang, P.; Liu, J.; Guo, D.; Zhang, X. The effect of temperature on the survival, growth, photosynthesis, and respiration of young seedlings of eelgrass Zostera marina L. Aquaculture 2012, 350–353, 98–108. [Google Scholar] [CrossRef]
- Hernán, G.; Ramajo, L.; Basso, L.; Delgado, A.; Terrados, J.; Duarte, C.M.; Tomas, F. Seagrass (Posidonia oceanica) seedlings in a high-CO2 world: From physiology to herbivory. Sci. Rep. 2016, 6, 38017. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O.; Mumby, P.J.; Hooten, A.J.; Steneck, R.S.; Greenfield, P.; Gomez, E.; Harvell, C.D.; Sale, P.F.; Edwards, A.J.; Caldeira, K.; et al. Coral reefs under rapid climate change and ocean acidification. Science 2007, 318, 1737–1742. [Google Scholar] [CrossRef]
- Collier, C.J.; Langlois, L.; Ow, Y.; Johansson, C.; Giammusso, M.; Adams, M.P.; O’Brien, K.R.; Uthicke, S. Losing a winner: Thermal stress and local pressures outweigh the positive effects of ocean acidification for tropical seagrasses. New Phytol. 2018, 219, 1005–1017. [Google Scholar] [CrossRef]
- Jiménez-Ramos, R.; Egea, L.G.; Ortega, M.J.; Hernández, I.; Vergara, J.J.; Brun, F.G. Global and local disturbances interact to modify seagrass palatability. PLoS ONE 2017, 12, e0183256. [Google Scholar] [CrossRef]
- Zimmerman, R.C.; Hill, V.J.; Jinuntuya, M.; Celebi, B.; Ruble, D.; Smith, M.; Cedeno, T.; Swingle, W.M. Experimental impacts of climate warming and ocean carbonation on eelgrass Zostera marina. Mar. Ecol. Prog. Ser. 2017, 566, 1–15. [Google Scholar] [CrossRef]
- Perry, D.; Staveley, T.; Deyanova, D.; Baden, S.; Dupont, S.; Hernroth, B.; Wood, H.; Björk, M.; Gullström, M. Global environmental changes negatively impact temperate seagrass ecosystems. Ecosphere 2019, 10, e02986. [Google Scholar] [CrossRef]
- Duarte, C.M. Allometric scaling of seagrass form and productivity. Mar. Ecol. Prog. Ser. 1991, 77, 289–300. [Google Scholar] [CrossRef]
- Rattanachot, E.; Prathep, A. Temporal variation in growth and reproduction of Enhalus acoroides (L.f.) Royle in a monospecific meadow in Haad Chao Mai National Park, Trang Province, Thailand. Bot. Mar. 2011, 54, 201–207. [Google Scholar] [CrossRef]
- Duarte, C.M.; Terrados, J.; Agawin, N.S.R.; Fortes, M.D.; Bach, S.; Kenworthy, W.J. Response of a mixed Philippine seagrass meadow to experimental burial. Mar. Ecol. Prog. Ser. 1997, 147, 285–294. [Google Scholar] [CrossRef]
- Rollon, R.N. Spatial variation and seasonality in growth and reproduction of Enhalus acoroides (L.f.) Royle populations in the coastal waters off Cape Bolinao, NW Philippines. Ph.D. Thesis, Wageningen Agricultural University, Wageningen, The Netherlands, 1998. [Google Scholar]
- Pedersen, O.; Colmer, T.D.; Borum, J.; Zavala-Perez, A.; Kendrick, G.A. Heat stress of two tropical seagrass species during low tides—Impact on underwater net photosynthesis, dark respiration and diel in situ internal aeration. New Phytol. 2016, 210, 1207–1218. [Google Scholar] [CrossRef]
- Björk, M.; Weil, A.; Beer, S.; Semesi, S. Photosynthetic utilisation of inorganic carbon by seagrasses from Zanzibar, East Africa. Mar. Biol. 1997, 129, 363–366. [Google Scholar] [CrossRef]
- Duarte, C.M. Seagrass depth limits. Aquat. Bot. 1991, 40, 363–377. [Google Scholar] [CrossRef]
- Schupp, E.W. Seed-seedling conflicts, habitat choice, and patterns of plant recruitment. Am. J. Bot. 1995, 82, 399–409. [Google Scholar] [CrossRef]
- Agawin, N.S.R.; Duarte, C.M.; Fortes, M.D. Nutrient limitation of Philippine seagrasses (Cape Bolinao, NW Philippines): In situ experimental evidence. Mar. Ecol. Prog. Ser. 1996, 138, 233–243. [Google Scholar] [CrossRef]
- Agawin, N.S.R.; Duarte, C.M.; Fortes, M.D.; Uri, J.S.; Vermaat, J.E. Temporal changes in the abundance, leaf growth and photosynthesis of three co-occurring Philippine seagrasses. J. Exp. Mar. Biol. Ecol. 2001, 260, 217–239. [Google Scholar] [CrossRef]
- Kongrueang, P.; Buapet, P.; Roongsattham, P. Physiological responses of Enhalus acoroides to osmotic stress. Bot. Mar. 2018, 61, 257–267. [Google Scholar] [CrossRef]
- McMillan, C. The distribution of tropical seagrasses with relation to their tolerance of high temperatures. Aquat. Bot. 1984, 19, 369–379. [Google Scholar] [CrossRef]
- Nakase, K.; Murakami, T.; Kohno, H.; Ukai, A.; Mizutani, A.; Shimokawa, S. Distribution of Enhalus acoroides according to waves and currents. In Geophysical Approach to Marine Coastal Ecology; Shimokawa, S., Murakami, T., Kohno, H., Eds.; Springer Oceanography, Springer: Singapore, 2020; pp. 197–215. [Google Scholar] [CrossRef]
- Terrados, J.; Agawin, N.S.R.; Duarte, C.M.; Fortes, M.D.; Kamp-Nielsen, L.; Borum, J. Nutrient limitation of the tropical seagrass Enhalus acoroides (L.) Royle in Cape Bolinao, NW Philippines. Aquat. Bot. 1999, 65, 123–139. [Google Scholar] [CrossRef]
- Teichberg, M.; Wild, C.; Bednarz, V.N.; Kegler, H.F.; Lukman, M.; Gärdes, A.A.; Heiden, J.P.; Weiand, L.; Abu, N.; Nasir, A.; et al. Spatio-temporal patterns in coral reef communities of the Spermonde archipelago, 2012–2014, I: Comprehensive reef monitoring of water and benthic indicators reflect changes in reef health. Front. Mar. Sci. 2018, 5, 33. [Google Scholar] [CrossRef]
- Erftemeijer, P.L.A.; Stapel, J. Primary production of deep-water Halophila ovalis meadows. Aquat. Bot. 1999, 65, 71–82. [Google Scholar] [CrossRef]
- Grasshoff, K.; Kremling, K.; Ehrhardt, M. Methods of Seawater Analysis; Verlag Chemie: Weinheim, Germany, 1999. [Google Scholar] [CrossRef]
- Dickson, A.G.; Sabine, C.L.; Christian, J.R. Guide to Best Practices for Ocean. CO2 Measurements; PICES Special Publication: Sidney, BC, Canada, 2007. [Google Scholar] [CrossRef]
- Pelletier, G.; Lewis, E.; Wallace, D. Xls: A Calculator for the CO2 System in Seawater for Microsoft Excel/VBA; Washington State Department of Ecology: Olympia, WA, USA; Brookhaven National Laboratory: Upton, NY, USA, 2005. [Google Scholar]
- Huber, S.C.; Israel, D.W. Biochemical basis for partitioning of photosynthetically fixed carbon between starch and sucrose in soybean (Glycine max Merr.) leaves. Plant. Physiol. 1982, 69, 691–696. [Google Scholar] [CrossRef]
- Yemm, E.W.; Willis, A.J. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Short, F.T.; Duarte, C.M. Methods for the measurement of seagrass growth and production. In Global Seagrass Research Methods; Short, F.T., Coles, R.G., Eds.; Elsevier, B.V.: Amsterdam, The Netherlands, 2001; pp. 141–153. [Google Scholar] [CrossRef]
- Winters, G.; Nelle, P.; Fricke, B.; Rauch, G.; Reusch, T.B.H. Effects of a simulated heat wave on photophysiology and gene expression of high- and low-latitude populations of Zostera marina. Mar. Ecol. Prog. Ser. 2011, 435, 83–95. [Google Scholar] [CrossRef]
- Silsbe, G.M.; Malkin, S.Y. Phytotools: Phytoplankton Production Tools. R Package Version 1.0. 2015. Available online: https://cran.r-project.org/package=phytotools (accessed on 30 March 2020).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Jassby, A.D.; Platt, T. Mathematical formulation of the relationship between photosynthesis and light for phytoplankton. Limnol. Oceanogr. 1976, 21, 540–547. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta Gen. Subj. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Sakshaug, E.; Bricaud, A.; Dandonneau, Y.; Falkowski, P.G.; Kiefer, D.A.; Legendre, L.; Morel, A.; Parslow, J.; Takahashi, M. Parameters of photosynthesis: Definitions, theory and interpretation of results. J. Plankton Res. 1997, 19, 1637–1670. [Google Scholar] [CrossRef]
- Invers, O.; Tomàs, F.; Pérez, M.; Romero, J. Potential effect of increased global CO2 availability on the depth distribution of the seagrass Posidonia oceanica (L.) Delile: A tentative assessment using a carbon balance model. Bull. Mar. Sci. 2002, 71, 1191–1198. [Google Scholar]
- Stapel, J.; Hemminga, M.A. Nutrient resorption from seagrass leaves. Mar. Biol. 1997, 128, 197–206. [Google Scholar] [CrossRef]
- Celdrán, D.; Marín, A. Seed photosynthesis enhances Posidonia oceanica seedling growth seed collection. Ecosphere 2013, 4, 149. [Google Scholar] [CrossRef]
- Kaldy, J.E.; Dunton, K.H. Ontogenetic photosynthetic changes, dispersal and survival of Thalassia testudinum (Turtle Grass) seedlings in a sub-tropical lagoon. J. Exp. Mar. Biol. Ecol. 1999, 240, 193–212. [Google Scholar] [CrossRef]
- Nielsen, S.L.; Pedersen, M.F. Growth, photosynthesis and nutrient content of seedlings and mature plants of Cymodocea nodosa—The importance of clonal integration. Aquat. Bot. 2000, 68, 265–271. [Google Scholar] [CrossRef]
- Statton, J.; Kendrick, G.A.; Dixon, K.W.; Cambridge, M.L. Inorganic nutrient supplements constrain restoration potential of seedlings of the seagrass, Posidonia australis. Restor. Ecol. 2014, 22, 196–203. [Google Scholar] [CrossRef]
- Alexandre, A.; Silva, J.; Buapet, P.; Björk, M.; Santos, R. Effects of CO2 enrichment on photosynthesis, growth, and nitrogen metabolism of the seagrass Zostera noltii. Ecol. Evol. 2012, 2, 2625–2635. [Google Scholar] [CrossRef] [PubMed]
- Ow, Y.X.; Collier, C.J.; Uthicke, S. Responses of three tropical seagrass species to CO2 enrichment. Mar. Biol. 2015, 162, 1005–1017. [Google Scholar] [CrossRef]
- Ow, Y.X.; Uthicke, S.; Collier, C.J. Light levels affect carbon utilisation in tropical seagrass under ocean acidification. PLoS ONE 2016, 11, e0150352. [Google Scholar] [CrossRef] [PubMed]
- Borum, J.; Pedersen, O.; Kotula, L.; Fraser, M.W.; Statton, J.; Colmer, T.D.; Kendrick, G.A. Photosynthetic response to globally increasing CO2 of co-occurring temperate seagrass species. Plant. Cell Environ. 2016, 39, 1240–1250. [Google Scholar] [CrossRef]
- Jiang, Z.J.; Huang, X.P.; Zhang, J.P. Effects of CO2 enrichment on photosynthesis, growth, and biochemical composition of seagrass Thalassia hemprichii (Ehrenb.) Aschers. J. Integr. Plant. Biol. 2010, 52, 904–913. [Google Scholar] [CrossRef]
- Thom, R.M. CO2-enrichment effects on eelgrass (Zostera marina L.) and bull kelp (Nereocystis luetkeana (Mert.) P. & R.). Water. Air. Soil Pollut. 1996, 88, 383–391. [Google Scholar] [CrossRef]
- Ralph, P.J.; Durako, M.J.; Enríquez, S.; Collier, C.J.; Doblin, M.A. Impact of light limitation on seagrasses. J. Exp. Mar. Biol. Ecol. 2007, 350, 176–193. [Google Scholar] [CrossRef]
- Zimmerman, R.C.; Kohrs, D.G.; Steller, D.L.; Alberte, R.S. Impacts of CO2 enrichment on productivity and light requirements of eelgrass. Plant. Physiol. 1997, 115, 599–607. [Google Scholar] [CrossRef]
- Ooi, J.L.S.; Kendrick, G.A.; Van Niel, K.P.; Affendi, Y.A. Knowledge gaps in tropical southeast Asian seagrass systems. Estuar. Coast. Shelf Sci. 2011, 92, 118–131. [Google Scholar] [CrossRef]
- Fortes, M.D.; Ooi, J.L.S.; Tan, Y.M.; Prathep, A.; Bujang, J.S.; Yaakub, S.M. Seagrass in Southeast Asia: A review of status and knowledge gaps, and a road map for conservation. Bot. Mar. 2018, 61, 269–288. [Google Scholar] [CrossRef]
- Stitt, M.; Krapp, A. The interaction between elevated carbon dioxide and nitrogen nutrition: The physiological and molecular background. Plant. Cell Environ. 1999, 22, 583–621. [Google Scholar] [CrossRef]
- Zayas-Santiago, C.C.; Rivas-Ubach, A.; Kuo, L.J.; Ward, N.D.; Zimmerman, R.C. Metabolic profiling reveals biochemical pathways responsible for eelgrass response to elevated CO2 and temperature. Sci. Rep. 2020, 10, 4693. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Meseguer, L.; Cox, T.E.; Sanz-Lázaro, C.; Schmid, S.; Enzor, L.A.; Major, K.; Gazeau, F.; Cebrian, J. Does ocean acidification benefit seagrasses in a mesohaline environment? A mesocosm experiment in the northern gulf of Mexico. Estuaries Coasts 2020, 43, 1377–1393. [Google Scholar] [CrossRef]
- Repolho, T.; Duarte, B.; Dionísio, G.; Paula, J.R.; Lopes, A.R.; Rosa, I.C.; Grilo, T.F.; Caçador, I.; Calado, R.; Rosa, R. Seagrass ecophysiological performance under ocean warming and acidification. Sci. Rep. 2017, 7, 41443. [Google Scholar] [CrossRef]
- Harvey, B.P.; Gwynn-Jones, D.; Moore, P.J. Meta-analysis reveals complex marine biological responses to the interactive effects of ocean acidification and warming. Ecol. Evol. 2013, 3, 1016–1030. [Google Scholar] [CrossRef]
- Leoni, V.; Vela, A.; Pasqualini, V.; Pergent-Martini, C.; Pergent, G. Effects of experimental reduction of light and nutrient enrichments (N and P) on seagrasses: A review. Aquat. Conserv. Mar. Freshw. Ecosyst. 2008, 18, 202–220. [Google Scholar] [CrossRef]
| Treatments | |||||
|---|---|---|---|---|---|
| n | 27 °C-Ambient CO2 | 27 °C-Enriched CO2 | 31 °C-Ambient CO2 | 31 °C-Enriched CO2 | |
| Temperature (°C) | 66 | 27.74 (±0.08) | 27.29 (±0.21) | 30.66 (±0.12) | 30.65 (±0.13) |
| Salinity | 66 | 34.43 (±0.25) | 34.42 (±0.46) | 35.07 (±0.31) | 34.25 (±0.5) |
| Conductivity (µS cm−1) | 60 | 61.35 (±0.25) | 61.83 (±0.34) | 62.01 (±0.73) | 60.91 (±0.6) |
| pH | 66 | 8.23 (±0.02) | 8.18 (±0.01) | 8.2 (±0.01) | 8.09 (±0.02) |
| Dissolved oxygen (mg L−1) | 48 | 6.92 (±0.06) | 7.08 (±0.06) | 6.52 (±0.03) | 6.54 (±0.03) |
| Alkalinity (µmol kg−1 SW) | 30 | 2369.67 (±8.2) | 2394.24 (±7.54) | 2374.08 (±14.71) | 2414.35 (±21.44) |
| pCO2 (µatm) * | 21 | 379.42 (±39.63) | 464.04 (±28.49) | 379.75 (±25.31) | 624.47 (±42.41) |
| PO4 (µmol kg−1 SW) | 21 | 0.07 (±0.01) | 0.07 (±0.01) | 0.06 (±0.01) | 0.07 (±0) |
| Si (µmol kg−1 SW) | 21 | 0.58 (±0.01) | 0.58 (±0.01) | 0.58 (±0.01) | 0.57 (±0.01) |
| Dependent Variables | Factor | TSS | F-Value | p-Value |
|---|---|---|---|---|
| Leaf number | Temperature | 0.375 | 1.800 | 0.195 |
| CO2 | 0.375 | 1.800 | 0.195 | |
| Interaction | 1.042 | 5.000 | 0.037 | |
| Maximum leaf length | Temperature | 46.482 | 15.381 | 0.001 |
| CO2 | 11.482 | 3.799 | 0.065 | |
| Interaction | 0.135 | 0.045 | 0.835 | |
| Leaf SA | Temperature | 2.059 | 14.429 | 0.001 |
| CO2 | 0.075 | 0.528 | 0.476 | |
| Interaction | 0.009 | 0.065 | 0.802 | |
| Leaf weight | Temperature | 0.058 | 5.542 | 0.029 |
| CO2 | 0.000 | 0.007 | 0.934 | |
| Interaction | 0.016 | 1.486 | 0.237 | |
| Leaf width | Temperature | 0.001 | 1.494 | 0.236 |
| CO2 | 0.000 | 0.477 | 0.498 | |
| Interaction | 0.000 | 0.017 | 0.898 | |
| Leaf SA growth rate | Temperature | 0.002 | 10.413 | 0.004 |
| CO2 | 0.000 | 0.002 | 0.961 | |
| Interaction | 0.000 | 0.292 | 0.595 |
| Treatments | |||
|---|---|---|---|
| Temp | Ambient CO2 | Enriched CO2 | |
| Fv/Fm | 27 | 0.75 (±0.01) | 0.76 (±0.01) |
| 31 | 0.74 (±0.01) | 0.75 (±0.005) | |
| rETRmax | 27 | 24.30 (±4.99) | 32.85 (±3.08) |
| 31 | 19.04 (±3.07) | 15.05 (±3.24) | |
| Alpha (α) | 27 | 0.69 (±0.02) | 0.69 (±0.02) |
| 31 | 0.70 (±0.01) | 0.69 (±0.03) | |
| Ek | 27 | 12.77 (±2.41) | 14.28 (±2.63) |
| 31 | 8.56 (±1.70) | 5.88 (±1.20) | |
| Dependent Variable | Factor | TSS | F-Value | p-Value |
|---|---|---|---|---|
| Fv/Fm | Temperature | 0.000 | 0.831 | 0.373 |
| CO2 | 0.000 | 1.062 | 0.315 | |
| Interaction | 0.000 | 0.039 | 0.845 | |
| rETRmax | Temperature | 797.634 | 11.731 | 0.003 |
| CO2 | 31.143 | 0.458 | 0.506 | |
| Interaction | 235.495 | 3.463 | 0.078 | |
| Alpha (α) | Temperature | 0.001 | 0.218 | 0.646 |
| CO2 | 0.000 | 0.074 | 0.788 | |
| Interaction | 0.000 | 0.123 | 0.730 | |
| Ek | Temperature | 238.285 | 11.174 | 0.003 |
| CO2 | 2.042 | 0.096 | 0.760 | |
| Interaction | 26.286 | 1.233 | 0.280 |
| Dependent Variable | Factor | TSS | F-Value | p-Value |
|---|---|---|---|---|
| Leaves | ||||
| Carbon content | Temperature | 21.045 | 14.638 | 0.001 |
| CO2 | 1.168 | 0.812 | 0.379 | |
| Interaction | 0.390 | 0.271 | 0.609 | |
| Nitrogen content | Temperature | 0.507 | 24.300 | <0.001 |
| CO2 | 0.028 | 1.333 | 0.263 | |
| Interaction | 0.006 | 0.276 | 0.605 | |
| Seeds | ||||
| Carbon content | Temperature | 8.339 | 0.248 | 0.624 |
| CO2 | 2.916 | 0.087 | 0.771 | |
| Interaction | 82.470 | 2.454 | 0.133 | |
| Nitrogen content | Temperature | 0.010 | 0.215 | 0.648 |
| CO2 | 0.026 | 0.577 | 0.456 | |
| Interaction | 0.019 | 0.416 | 0.526 | |
| Roots | ||||
| Carbon content | Temperature | 3.192 | 0.379 | 0.545 |
| CO2 | 0.009 | 0.001 | 0.974 | |
| Interaction | 37.233 | 4.425 | 0.049 | |
| Nitrogen content | Temperature | 0.016 | 1.027 | 0.324 |
| CO2 | 0.023 | 1.436 | 0.245 | |
| Interaction | 0.016 | 1.031 | 0.323 |
| Dependent Variable | Factor | TSS | F-Value | p-Value |
|---|---|---|---|---|
| Leaves | ||||
| Sucrose | Temperature | 0.000 | 0.051 | 0.87 |
| CO2 | 0.000 | 2.030 | 0.197 | |
| Interaction | 0.000 | 0.170 | 0.692 | |
| Starch | Temperature | 0.036 | 2.363 | 0.168 |
| CO2 | 0.027 | 1.785 | 0.224 | |
| Interaction | 0.069 | 4.558 | 0.069 | |
| Seeds | ||||
| Sucrose | Temperature | 0.012 | 7.464 | 0.013 |
| CO2 | 0.002 | 1.470 | 0.239 | |
| Interaction | 0.000 | 0.017 | 0.897 | |
| Starch | Temperature | 0.088 | 1.781 | 0.197 |
| CO2 | 0.031 | 0.633 | 0.436 | |
| Interaction | 0.013 | 0.270 | 0.609 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Artika, S.R.; Ambo-Rappe, R.; Samawi, M.F.; Teichberg, M.; Moreira-Saporiti, A.; Viana, I.G. Rising Temperature Is a More Important Driver Than Increasing Carbon Dioxide Concentrations in the Trait Responses of Enhalus acoroides Seedlings. Appl. Sci. 2021, 11, 2730. https://doi.org/10.3390/app11062730
Artika SR, Ambo-Rappe R, Samawi MF, Teichberg M, Moreira-Saporiti A, Viana IG. Rising Temperature Is a More Important Driver Than Increasing Carbon Dioxide Concentrations in the Trait Responses of Enhalus acoroides Seedlings. Applied Sciences. 2021; 11(6):2730. https://doi.org/10.3390/app11062730
Chicago/Turabian StyleArtika, Suci Rahmadani, Rohani Ambo-Rappe, Muhammad Farid Samawi, Mirta Teichberg, Agustín Moreira-Saporiti, and Inés G. Viana. 2021. "Rising Temperature Is a More Important Driver Than Increasing Carbon Dioxide Concentrations in the Trait Responses of Enhalus acoroides Seedlings" Applied Sciences 11, no. 6: 2730. https://doi.org/10.3390/app11062730
APA StyleArtika, S. R., Ambo-Rappe, R., Samawi, M. F., Teichberg, M., Moreira-Saporiti, A., & Viana, I. G. (2021). Rising Temperature Is a More Important Driver Than Increasing Carbon Dioxide Concentrations in the Trait Responses of Enhalus acoroides Seedlings. Applied Sciences, 11(6), 2730. https://doi.org/10.3390/app11062730








