Featured Application
The presented study opens the way for the inclusion of suPAR in the maxillofacial diagnostics. The study aims to predict the possibility of minor inflammatory complications after maxillofacial scheduled procedures to a population of potentially healthy patients as far as internal diseases are considered.
Abstract
Soluble urokinase-type plasminogen activator receptor (suPAR) is a marker of immune activation and reflects a more distinct aspect of inflammation than C-reactive protein (CRP) does. The study concerns a clinically silent state of the immune system expressed by the level of suPAR, which could affect the occurrence of complications (non-life threatening) after scheduled procedures. The purpose was the evaluation of suPAR predictive value in minor maxillofacial surgery complication incidents. Eighty patients were tested for suPAR, CRP and a series of basic laboratory serum tests on 1 day before surgery. Complications of orthognathic and minor injuries treatments were reported. The suPAR level, expressed as a measure independent of the patient’s age (Index of Body Inflammation, IBI), was analyzed. The protein level was also assessed on postoperative day 3. Basic statistical analysis did not reveal any relevant dependence between suPAR (or IBI) and occurrence of minor complications. The application of factor analysis, artificial neural network and inclusion of chlorides, glycaemia, alanine transaminase (ALT), albumin and hemoglobin levels allowed to indicate the suPAR/IBI ranges associated with an increased risk of minor postoperative complications. Concluding, it seems that, in the current state of the knowledge, the monitoring of pre-operational suPAR level solely does not include sufficient predictive information for the occurrence of minor complications after maxillofacial surgery. The suPAR/IBI level should be combined with other patient characteristics to predict healing complications.
1. Introduction
Soluble urokinase-type plasminogen activator receptor (suPAR) is a biomarker of activation of the inflammatory and immune systems [1]. Contrary to well-known inflammatory markers, such as the current gold standard, C-reactive protein (CRP), suPAR is not an acute-phase reactant, and its levels in the blood are less rapidly affected by surgical procedure and short-term influences [2,3]. Additionally, suPAR is more reliably associated even with early life risk factors, such as adverse childhood experiences and early life stress and violence, than CRP and interleukin-6, potentially because these more traditional biomarkers of inflammation as acute-phase reactants mix historical and acute effects [4,5]. This, along with its non-specific associations with pathologies in general, suggests that suPAR blood levels are an appropriate readout for low-grade inflammation which may influence post-operational course [6].
Having a prognostic marker revealing the possibility of developing a postoperative complication would help in proper and safe selection of patients for scheduled procedures within the facial part of the skull. Prognostic factors also are important as they can help improve risk stratification in clinical settings or provide guidance in treatment as well as in the design of randomized trials [7].
The aim of this study was to evaluate the suPAR predictive value in minor maxillofacial surgery complication incidents.
2. Materials and Methods
Eighty patients were enrolled in this study (49 females and 31 males). All patients provided written informed consent and the study was approved by the University Ethic Committee (RNN/646/13/KB). Inclusion criteria (Table 1) were: physical status classification lower than 3, i.e., no severe systemic disturbances (American Society of Anesthesiologists Physical Status Classification System, ASA) orthognathic and minor traumatologic scheduled procedure, completed laboratory tests: alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin, glycaemia, hemoglobin (Hb), chlorides (Cl), potassium (K), sodium (Na), white blood cells count (WBC), CRP. Exclusion criteria: cardiovascular disease (anamnesis), kidney disease, i.e., glomerular filtration rate estimated with the use of the Modification of Diet in Renal Disease (MDRD) formula of estimated glomerular filtration rate (GFR-MDRD) in normal range and oncological issues (anamnesis). Serum suPAR level was measured in included cases with the use of the suPARnostic ELISA, ViroGates, Birkerød, Denmark. The test was performed one day before surgical treatment. Laboratory parameters were analyzed in serum samples taken after fasting. After centrifugation, part of the serum was immediately aliquoted and frozen at −80 °C for up to 10 months. For the suPAR analysis, the samples were thawed, thoroughly mixed and centrifuged, and their suPAR concentrations were assessed.

Table 1.
Characteristics of patients included in these studies.
Next, Index of Body Inflammation (IBI) was calculated according to the equation below, to present the suPAR results in age-independent way [8]:
where age is in years and suPAR in ng/mL. Another suPAR, CRP test and IBI calculation were performed 3 days post-operationally, i.e., the day of hospital discharge. The healing process was monitored in patients for 6 months in an outpatient department. Postoperative complications were recorded.
Data was analyzed with the use of Statgraphics Centurion 18 (Statgraphics Technologies Inc. The Plains City, VA, USA). Paired samples t-test was used for data collected pre- and post-operation in the same patient. Categorical data relation was investigated by independence χ2 test. The relationship between the tested parameters and the occurrence of complications was studied with the use of one-way ANOVA. Relation between quantitative variable were analyzed with the use of linear regression. Level of significance was established as p < 0.05. Finally, factor analysis and an artificial neural network were used to determine the probability of any small complication to occur.
3. Results
There was no sex-related dependency of suPAR level and IBI value. No major complications or systemic involvement occurred. Minor post-operational complications were reported in 29 among 80 patients. There were: Herpes Simplex Virus infection (two cases), purulent fistula in operation site (four cases) wound dehiscence (eight cases), extensive swelling (15 cases). No complication was permanent. Markers of inflammation were presented in Table 2 and Figure 1.

Table 2.
Markers of inflammation in investigated patients.

Figure 1.
Detected levels of age independent soluble urokinase-type plasminogen activator receptor (suPAR) (value independent of the patient’s age (IBI)) before and after surgical procedures (density traces). No difference (paired samples t-test, p = 0.2162), i.e., serum suPAR level was stable throughout the study.
It was noted that the CRP level was not related with the suPAR level before treatment (correlation coefficient, CC = 0.21; p = 0.0587), while the increase in CRP level after treatment indicated a relatively weak relationship with suPAR level (CC = 0.34; p < 0.05). Nevertheless, when examining the relationship between CRP and suPAR level independently of the patient’s age, i.e., IBI, a relatively weak relationship between variables was found before surgery (CC = 0.24; p < 0.05) as well as after surgery (CC = 0.32; p < 0.05). Obviously, the suPAR level was strongly related to the IBI value: pre-operationally (CC = 0.98; p < 0.001) and post-operationally (CC = 0.98; p < 0.001). This relationship weakens slightly in the higher suPAR values because there is a dispersion of age-related results in the suPAR, which the IBI parameter does not reveal (Figure 2).
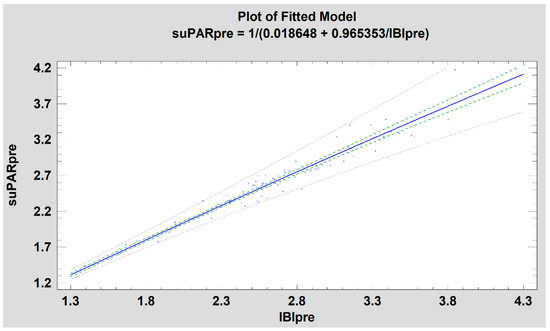
Figure 2.
Relation of suPAR and IBI (simple regression, R2 = 97%, blue line, p < 0.001). Green dash line–confidence limit. Gray dash line—prediction limit. Note: the expansion of confidence limits and especially prediction in the area of higher suPAR values due to its dependence on patient’s age.
No gender (p = 0.1296), ASA (p = 0.4199), age (p = 0.8099), BMI (p = 0.3560), GFR-MDRD (p = 0.6534), ALT (p = 0.5827), AST (p = 0.834), albumin (p = 0.2735), Hb (p = 0.7346), glycaemia (p = 0.7590), chloride (p = 0.8062), potassium (p = 0.4438), sodium (p = 0.1141), WBC (p = 0.8180), CRP measured before treatment (p = 0.9373), CRP increase (p = 0.9716), suPAR measured before treatment (p = 0.3303), suPAR increase (p = 0.9759), IBI calculated before treatment (p = 0.3322), IBI increase (p = 0.9759), surgical procedure (p = 0.0833), surgical duration (p = 0.4115) relation was found.
An association between persistence of post-treatment complications and serum suPAR and IBI levels determined before the treatment was statistically relevant (p < 0.05). However, this was a relatively weak relationship (correlation coefficient −0.24 and −0.23, respectively). Analogous association was not present for pre-treatment CRP levels (p = 0.4672). In addition, the regression analysis that revealed the association was only able to fit the proposed models (Figure 3) to a small extent to the experimental data: R2 = 5.9% for suPAR, R2 = 5.2 for IBI and R2 = 0.7% for CRP.
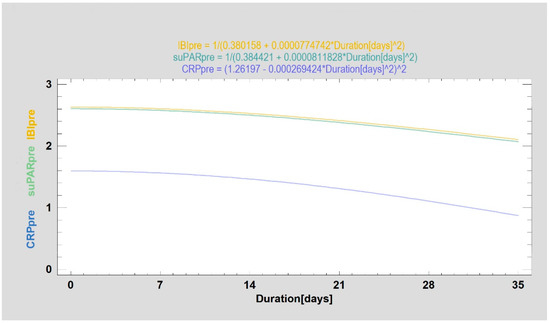
Figure 3.
Preoperative values of the examined markers (CRP—blue line, suPAR—green line and IBI—yellow line) according to the duration of minor complications expressed in days. CRP values are given in mg/L, suPAR values in ng/mL and IBI is an index without unit. Preoperative suPAr and IBI values are weakly related to the duration of postoperative complications.
Due to the fiasco in the search for the plain relationship between the examined parameters and the occurrence of small postoperative complications, the grouping of variables (factor analysis) and the mathematical fuzzy method, i.e., neural network, were applied.
By means of the Factor Analysis (FA) 3 variables (ALT, albumin and Hb) were combined together. The purpose of the FA was to obtain a small number of factors which account for most of the variability in those 3 variables. In this case, one factor has been extracted (eigenvalue = 1.78). It accounts for 59% of the variability in the original data. Since the principal components method was selected, the initial communality estimates have been set to assume that all of the variability in the data is due to the common factor. Next, rotation was performed in order to simplify the explanation of the factor meaning. The rotated factor (named ALT + Alb + Hb Factor) had the equation:
where the values of the variables in the equation are standardized by subtracting their means and dividing by their standard deviations. It also shows the estimated communalities, which can be interpreted as estimating the proportion of the variability in each variable attributable to the extracted factor (Figure 4).
ALT + Alb + Hb Factor = 0.777927 × ALT + 0.607865 × Albumin + 0.897814 × Hb
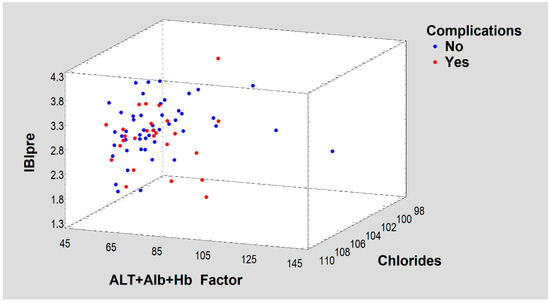
Figure 4.
A three-dimensional space defined by ALT + Alb + Hb Factor, IBIpre and Chlorides Chlorides showing a scattering of dichotomous treatment results (Complications).
Clinical complication incidents were established as the classification factor (Complications) in a probabilistic neural network (PNN). Input variables were: IBIpre, Chlorides, Glycaemia and ALT + Alb + Hb Factor (meta-variable obtained from the FA). Two neurons were built in summation hidden layer. Among the 74 complete cases in the training set, 74% were correctly classified by the PNN. Results of complication prediction are presented on Figure 5.
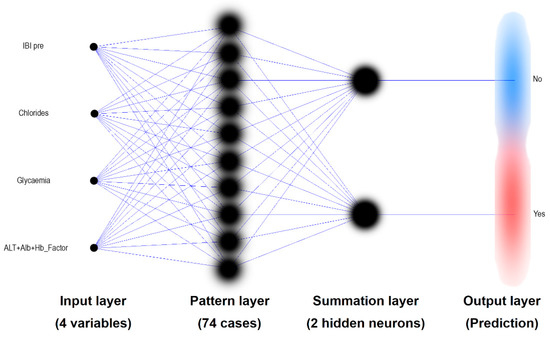
Figure 5.
Probabilistic Neural Network classifier (PNN) used for prediction of the minor complication after maxillo-facial surgery. The input data includes an age-independent suPAR level as IBI (among three other variables). The predictions of the PNN built in this way are 74% accurate.
The use of AF as a tool to create an input vector for PNN and the introduction of further input variables containing IBI made 74% of correct treatment results possible to predict (Figure 5). These results of maxillo-facial treatment are understood as an occurrence uneventful outcome or a minor complication. The ability to predict the outcome of treatment with such a method is slightly tilted towards excessive indication of uncomplicated surgical results.
Detailed results of PNN predictions indicate that complications should be expected (Figure 6d) in patients with lower preoperative IBI results (i.e., 1.8–2.7) in whom ALT + Alb + Hb_Factor is low (i.e., 65–90) simultaneously. Moreover, these two variables indicate the possibility of complications after surgery when the IBI takes average values (i.e., 2.2–3.4) and ALT + Alb + Hb_Factor is extremely low (i.e., 45–60) and when the IBI is extremely low (i.e., 1.3–1.7) and ALT + Alb + Hb_Factor takes moderately low values (i.e., 66–86). It can also be observed that the frequency of complications increases when the intermediate IBI pre (i.e., 2.3–3.1) in combination with intermediate Glycaemia level (i.e., 4.8–6.5 mmol/L) is present in the patient simultaneously (Figure 6c). The relationship between Chlorides and the occurrence of these minor complications is more complex. In a two-dimensional evaluation with IBI it can be seen a spread of complications from patients with extremely low both Chlorides values (i.e., below 102 mmol/L) and IBI (i.e., below 1.8), up to patients with high Chlorides values (i.e., 106–110 mmol/L) and intermediate IBI values (i.e., 2.3–3.5) (Figure 6b). When looking at the relationship between Glycaemia with Chlorides and the occurrence of complications, two local maximum probabilities of complications can be identified (Figure 6a): 1. medium-low Chlorides (i.e., 101–105 mmol/L) together with higher Glycaemia (i.e., 5.9–6.6 mmol/L); 2. more concentrated place with medium-high Chlorides (i.e., 101–105 mmol/L) together with intermediate Glycaemia (i.e., 4.5–5.9 mmol/L). In general, it can be observed that higher IBI values (i.e., 2.3–3.5) are generally related with postoperative complications (Figure 6b–d).
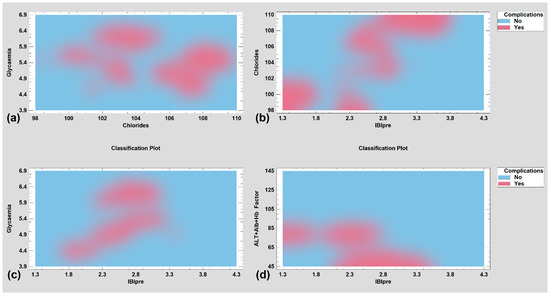
Figure 6.
Application of fuzzy mathematics for predicting minor complications after maxillofacial surgery procedures. Pre-operational IBI higher values (i.e., 3.5–4.3) protect patients from postoperative complications. (a) Prediction with the evaluation of Glycemia and Chlorides concentration assessment in blood serum; (b) based on IBIpre and Chlorides; (c) based on IBIpre and Glycaemia; (d) based on IBIpre an ALT + Alb + Hb Factor originated from Factor Analysis.
4. Discussion
Attempts to the use suPAR markings to predict the progress of severe diseases are made in general surgery [9,10] due to CRP, one of the most frequently assessed parameters, is nonspecific and its elevation is observed after 48 h [11] from the onset of symptoms, which can delay implementation of appropriate treatment. Therefore, there is a need for the detection of a new parameter of inflammation that could be applied as a rapid marker in the early prognosis of the course of severe complications [10].
In the pleural infection, suPAR has been shown to be potentially used in predicting of the need for tube thoracostomy in parapneumonic effusions [9,12]. Next, in renal injury [13] and other diseases [14] suPAR is known diagnostic marker. Intestinal inflammations in HIV-positive patients [15], acute pancreatitis and its progress prediction [10] also may by assessed with the use of suPAR. Additionally, in patients of advanced age [16], with renal insufficiency [17] and heart disease [18] the protein was studied. Its prognostic potential was also raised here. It is precisely these hidden deficiencies of the internal organs that may give rise to unexpected complications in patients after scheduled maxillofacial surgery.
The most frequent complication in maxillo-facial surgery is inflammation. The traditional anti-inflammatory and at the same time anti-infective treatment, i.e., antibiotic prophylaxis, is sometimes supplemented with topical anti-infective agents [19]. Nevertheless, inflammatory complications are still unavoidable and increasingly sophisticated methods such as neural networks are being used to predict complications [20,21], as was done in this study.
When considering the causes of complications, it is important to point out the significant shortcomings in their diagnosis in everyday hospital practice where markers of metalloproteinase activity and mitochondrial enzymes are not routinely used. The use of the fixative material itself and especially its effect on the tissue environment is debatable. On the one hand, negative effects in the form of oxidative and nitrosative stress are observed [22,23], while on the other hand, macrophage function is properly preserved [24]. Therefore, it seems appropriate to search for new predictive factors of successful treatment and/or indicating the risk of complications.
At the same time, general intrinsic factors, sex, place of residence, addictions and habits exert a influence on bone mineral content in the facial skeleton and its local immunity [25]. Thus, a great field of unknowns regarding the scheduled patient remains unexplained. Having a marker of overall immune system efficiency to predict even minor postoperative complications would be very valuable.
The use of the suPAR study to predict mild inflammatory complications, that are not life-threatening [26], requires a different approach than that published in the literature to date. This is related to the impossibility of obtaining a classical cut-off point, e.g., 4.75 ng/mL as in the case of patients entering into severe pancreatitis [10]. These authors also noted that predicting the development of moderate inflammatory process and its separation from mild process is already more difficult (although they give a limit of 3.65 ng/mL, it is less certain).
Multiorgan failure was predicted in severe inflammation at 5.2 ng/mL and the risk of death (already more difficult to predict and less certain) from 7.05 ng/mL. The suPAR level was also positively correlated with the time of hospitalization (p < 0.001) [6,10], which cannot be referred to in the present study as each patient was discharged on the third day after surgery. The highest levels of suPAR were 4.18 ng/mL pre-operationally and 4.19 ng/mL post-operationally. Fortunately, no complications were observed in both groups of patients. The question: why did they have such a high level of emerging biomarker without the features of inflammation and complications? remains open.
The authors of this text were even concerned with detecting a secret inflammation or some kind of dysfunction of the immune system [27,28]. As it turned out, it is necessary to add more laboratory results and use quite advanced mathematical tools (FA and PNN), which are unlikely to be used routinely in clinical trials. Another difficulty was the limited range of maxillofacial interventions, which does not lead to the increase of suPAR in the path of ischemia [29] and damage to organs [30]. Unfortunately, the use of fuzzy logic does not give accurate results, but only the density of the probability of a complication occurring (Figure 5 and Figure 6). This issue may result from a relatively young and healthy patient group. Such patients are typical for maxillofacial surgery.
It has recently been noted that suPAr may be a predictor in certain oral conditions. It has been observed that in patients with periodontal disease, the serum suPAR level rises to approximately 3.1 ng/mL [31]. The authors hypothesize that periodontitis, with its periodontal bacterial spectrum, may lead to increased plasma and salivary levels of suPAR and CRP. In that study, suPAR proved to be a valuable predictive biomarker of periodontitis. The results of the analysis of the suPAR concentration in patients with dental restorations, that reveal association of the protein level with intensification of inflammatory process in patients using acrylic dentures over 5 years and who have pathological lesions in the oral cavity, are also promising [32]. Therefore, it may be interesting to evaluate suPAR levels in other aspects of dentistry such as dental implant treatment [33], biomaterials appliocation [34,35,36] and immunocompromised patient therapy [37,38].
There are no studies available on seemingly healthy groups of patients with mild complications [6] In fact, the importance of diagnosing a life-threatening condition in a seriously ill patient is greater [26]. Nevertheless, the occurrence of visible although minor facial complications may be very important for the patient and medical lawyers.
There are certain limitations of this study. The data came from single center and, therefore, require validation from external scientists. A prospective, multicenter study should be conducted to discover a role of suPAR in prediction of incidents of maxillofacial surgical complication.
5. Conclusions
Finally, it seems that, in the current state of the knowledge, the monitoring of suPAR level does not include sufficient predictive information for the occurrence of minor complications in maxillofacial surgery. The suPAR level should be combined with other patient characteristics to achieve prediction of the healing complication.
Author Contributions
Conceptualization, M.K. and R.N.W.; methodology, M.K. and R.N.W.; software, M.K.; validation, M.K. and R.N.W.; formal analysis, M.K. and R.N.W.; investigation, M.K.; resources, M.K.; data curation, M.K. and R.N.W.; writing—original draft preparation, M.K. and R.N.W.; writing—review and editing, M.K. and R.N.W.; visualization, M.K.; supervision, M.K. and R.N.W.; project administration, M.K.; funding acquisition, M.K. Both authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Medical University of Lodz grant number: 503-1-138-01-503-51-001-17, 503-1-138-01-503-51-001-18 and 503-1-138-01-503-51-001-19-00.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Ethics Committee of Medical University of Lodz (protocol code RNN/646/13/KB approval date: 24 September 2013).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zimmermann, H.W.; Koch, A.; Seidler, S.; Trautwein, C.; Tacke, F. Circulating soluble urokinase plasminogen activator is elevated in patients with chronic liver disease, discriminates stage and aetiology of cirrhosis and predicts prognosis. Liver Int. 2012, 32, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Lyngbæk, S.; Marott, J.L.; Møller, D.V.; Christiansen, M.; Iversen, K.K.; Clemmensen, P.M.; Eugen-Olsen, J.; Jeppesen, J.L.; Hansen, P.R. Usefulness of soluble urokinase plasminogen activator receptor to predict repeat myocardial infarction and mortality in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous intervention. Am. J. Cardiol. 2012, 110, 1756–1763. [Google Scholar] [CrossRef]
- Andersen, O.; Eugen-Olsen, J.; Kofoed, K.; Iversen, J.; Haugaard, S.B. Soluble urokinase plasminogen activator receptor is a marker of dysmetabolism in HIV-infected patients receiving highly active antiretroviral therapy. J. Med. Virol. 2008, 80, 209–216. [Google Scholar] [CrossRef]
- Rasmussen, L.J.H.; Moffitt, T.E.; Arseneault, L.; Danese, A.; Eugen-Olsen, J.; Fisher, H.L.; Harrington, H.; Houts, R.; Matthews, T.; Sugden, K.; et al. Association of adverse experiences and exposure to violence in childhood and adolescence with inflammatory burden in young people. JAMA Pediatr. 2019, 174, 1–11. [Google Scholar] [CrossRef]
- Rasmussen, L.J.H.; Moffitt, T.E.; Eugen-Olsen, J.; Belsky, D.W.; Danese, A.; Harrington, H.; Houts, R.M.; Poulton, R.; Sugden, K.; Williams, B.; et al. Cumulative childhood risk is associated with a new measure of chronic inflammation in adulthood. J. Child. Psychol. Psychiatry 2019, 60, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Petersen, J.E.V.; Kallemose, T.; Barton, K.D.; Caspi, A.; Rasmussen, L.J.H. Soluble urokinase plasminogen activator receptor (suPAR) as a prognostic marker of mortality in healthy, general and patient populations: Protocol for a systematic review and meta-analysis. BMJ Open 2020, 10, e036125. [Google Scholar] [CrossRef]
- Riley, R.D.; Moons, K.G.M.; Snell, K.I.E.; Ensor, J.; Hooft, L.; Altman, D.G.; Hayden, J.; Collins, G.S.; Debray, T.P.A. A guide to systematic review and meta-analysis of prognostic factor studies. BMJ 2019, 364, k4597. [Google Scholar] [CrossRef]
- Kozakiewicz, M.; Trzcińska-Kubik, M.; Wlazeł, R.N. Index of body inflammation for maxillofacial surgery purpose—To make the soluble urokinase-type plasminogen activator receptor serum level independent on patient age. Appl. Sci. 2021, 11, 1345. [Google Scholar] [CrossRef]
- Arnold, D.T.; Hamilton, F.W.; Elvers, K.T.; Frankland, S.W.; Zahan-Evans, N.; Patole, S.; Medford, A.; Bhatnagar, R.; Maskell, N.A. Pleural fluid suPAR levels predict the need for invasive management in parapneumonic effusions. Am. J. Respir. Crit. Care Med. 2020, 201, 1545–1553. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, M.; Rydzewska-Rosolowska, A.; Rydzewski, A.; Cicha, M.; Rydzewska, G. Soluble urokinase-type plasminogen activator receptor (suPAR) in patients with acute pancreatitis (AP)—Progress in prediction of AP severity. Pancreatology 2017, 17, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Staubli, S.M.; Oertli, D.; Nebiker, C.A. Laboratory markers predicting severity of acute pancreatitis. Crit. Rev. Clin. Lab. Sci. 2015, 52, 273–283. [Google Scholar] [CrossRef]
- Sundaralingam, A.; Bedawi, E.O.; Rahman, N.M. Diagnostics in pleural disease. Diagnostics 2020, 10, 1046. [Google Scholar] [CrossRef]
- Wei, C.; Sigdel, T.K.; Sarwal, M.M.; Reiser, J. Circulating CD40 autoantibody and suPAR synergy drives glomerular injury. Ann. Transl. Med. 2015, 3, 300. [Google Scholar] [CrossRef]
- Zhang, S.; Breidenbach, J.D.; Russell, B.H.; George, J.; Haller, S.T. CD40/CD40L signaling as a promising therapeutic target for the treatment of renal disease. J. Clin. Med. 2020, 9, 3653. [Google Scholar] [CrossRef]
- Vujkovic-Cvijin, I.; Sortino, O.; Verheij, E.; Sklar, J.; Wit, F.W.; Kootstra, N.A.; Sellers, B.; Brenchley, J.M.; Ananworanich, J.; Loeff, M.S.V.; et al. HIV-Associated gut dysbiosis is independent of sexual practice and correlates with noncommunicable diseases. Nat. Commun. 2020, 11, 2448. [Google Scholar] [CrossRef]
- Wlazel, R.N.; Szwabe, K.; Guligowska, A.; Kostka, T. Soluble urokinase plasminogen activator receptor level in individuals of advanced age. Sci. Rep. 2020, 10, 15426. [Google Scholar] [CrossRef]
- Wlazeł, R.N.; Szadkowska, I.; Bartncki, P.; Rośniak-Bąk, K.; Rysz, J. Clinical and prognostic usefulness of soluble urokinase plasminogen activator receptor in hemodialysis patients. Int. Urol. Nephrol. 2017, 50, 339–345. [Google Scholar] [CrossRef]
- Wlazeł, R.N.; Migała, M.; Zielińska, M.; Pawlicki, L.; Rośniak-Bąk, K.; Szadkowska, I. Soluble urokinase plasminogen activator receptor in one-year prediction of major adverse cardiac events in patients after first myocardial infarction treated with primary percutaneous coronary intervention. Arch. Med. Sci. 2016, 15, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Zawadzki, P.J.; Perkowski, K.; Kotlarski, M.; Pietruczuk-Padzik, A.; Chomicz, L. Comparative study on usefulness of gentamycin-containing collagen implants in the treatment of patients with osteitis and osteomyelitis of the craniofacial skeleton. Ann. Agric. Environ. Med. 2017, 24, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Jabłoński, S.; Brocki, M.; Kujawski, K.; Wawrzycki, M.; Santorek-Strumiłło, E.; Lobos, M.; Kozakiewicz, M. Evaluation of prognostic value of selected biochemical markers in surgically treated patients with acute mediastinitis. Med. Sci. Monit. 2012, 18, CR308–CR315. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jabłoński, S.; Brocki, M.; Kordiak, J.; Misiak, P.; Terlecki, A.; Kozakiewicz, M. Acute mediastinitis: Evaluation of clinical risk factors for death in surgically treated patients. ANZ J. Surg. 2012, 83, 657–663. [Google Scholar] [CrossRef]
- Borys, J.; Maciejczyk, M.; Antonowicz, B.; Kretowski, A.; Waszkiel, D.; Bortnik, P.; Czarniecka-Bargłowska, K.; Kocisz, M.; Szulimowska, J.; Czajkowski, M.; et al. Exposure to Ti4Al4V titanium alloy leads to redox abnormalities, oxidative stress, and oxidative damage in patients treated for mandible fractures. Oxidative Med. Cell. Longev. 2018, 3714725, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Borys, J.; Maciejczyk, M.; Antonowicz, B.; Kretowski, A.; Sidun, J.; Domel, E.; Dąbrowski, J.; Ładny, J.R.; Morawska, K.; Zalewska, A. Glutathione metabolism, mitochondria activity, and nitrosative stress in patients treated for mandible fractures. J. Clin. Med. 2019, 8, 127. [Google Scholar] [CrossRef] [PubMed]
- Sikora, M.; Baranowska-Bosiacka, I.; Łukomska, A.; Goschorska, M.; Chlubek, D. Expression of metalloproteinase 2 (MMP-2) and metalloproteinase 9 (MMP-9) in THP-1 macrophages cultured with three-dimensional titanium mini-plate systems used for surgical treatment of condylar fractures. Acta Biochem. Pol. 2019, 66, 291–298. [Google Scholar] [CrossRef]
- Sikora, M.; Baranowska-Bosiackab, I.; Rębacz-Maronc, E.; Olszowskid, T.; Chlubek, D. The influence of the place of residence, smoking and alcohol consumption on bone mineral content in the facial skeleton. J. Trace Elements Med. Biol. 2019, 51, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Donadello, K.; Scolletta, S.; Taccone, F.S.; Covajes, C.; Santonocito, C.; Cortes, D.O.; Grazulyte, D.; Gottin, L.; Vincent, J.L. Soluble urokinase-type plasminogen activator receptor as a prognostic biomarker in critically ill patients. J. Crit. Care 2014, 29, 144–149. [Google Scholar] [CrossRef]
- Eugen-Olsen, J. suPAR—A future risk marker in bacteremia. J. Intern. Med. 2011, 270, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Thunø, M.; Macho, B.; Eugen-Olsen, J. suPAR: The molecular crystal ball. Dis. Markers 2009, 27, 157–172. [Google Scholar] [CrossRef]
- Armstead, W.M.; Cines, D.B.; Bdeir, K.; Kulikovskaya, I.; Stein, S.C.; Higazi, A.A. uPA impairs cerebrovasodilation after hypoxia/ischemia through LRP and ERK MAPK. Brain Res. 2008, 1231, 121–131. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Enocsson, H.; Wetterö, J.; Skogh, T.; Sjöwall, C. Soluble urokinase plasminogen activator receptor levels reflect organ damage in systemic lupus erythematosus. Transl Res. 2013, 162, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Isola, G.; Polizzi, A.; Alibrandi, A.; Williams, R.C.; Leonardi, R. Independent impact of periodontitis and cardiovascular disease on elevated soluble urokinase-type plasminogen activator receptor levels. J. Periodontol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Mastalerz, R.; Paradowski, M.; Rychlik, U.; Wlazeł, R.N. The analysis of the soluble urokinase plasminogen activator receptor (suPAR) concentrations in the patients with dental restorations. Diagn. Lab. 2018, 54, 73–80. [Google Scholar] [CrossRef]
- Crespi, R.; Capparè, P.; Gherlone, E. Immediate loading of dental implants placed in periodontally infected and non-infected sites: A 4-year follow-up clinical study. J. Periodontol. 2010, 81, 1140–1146. [Google Scholar] [CrossRef]
- Crespi, R.; Capparè, P.; Gherlone, E. Comparison of magnesium-enriched hydroxyapatite and porcine bone in human extraction socket healing: A histologic and histomorphometric evaluation. Int. J. Oral Maxillofac. Implants 2011, 26, 1057–1062. [Google Scholar] [PubMed]
- Crespi, R.; Capparé, P.; Romanos, G.E.; Mariani, E.; Benasciutti, E.; Gherlone, E. Corticocancellous porcine bone in the healing of human extraction sockets: Combining histomorphometry with osteoblast gene expression profiles in vivo. Int. J. Oral Maxillofac. Implants 2011, 26, 866–872. [Google Scholar]
- Crespi, R.; Capparè, P.; Gherlone, E. Dental implants placed in extraction sites grafted with different bone substitutes: Radiographic evaluation at 24 months. J. Periodontol. 2009, 80, 1616–1621. [Google Scholar] [CrossRef]
- Gherlone, E.F.; Capparé, P.; Tecco, S.; Polizzi, E.; Pantaleo, G.; Gastaldi, G.; Grusovin, M.G. Implant prosthetic rehabilitation in controlled HIV-positive patients: A prospective longitudinal study with 1-year follow-up. Clin. Implant. Dent. Relat. Res. 2016, 18, 725–734. [Google Scholar] [CrossRef]
- Capparé, P.; Teté, G.; Romanos, G.E.; Nagni, M.; Sannino, G.; Gherlone, E.F. The ‘All-on-four’ protocol in HIV-positive patients: A prospective, longitudinal 7-year clinical study. Int. J. Oral Implantol. 2019, 12, 501–510. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).