A Review of the Synthesis, Properties, and Applications of Bulk and Two-Dimensional Tin (II) Sulfide (SnS)
Abstract
1. Introduction
2. Structures and Physical Properties of Tin(II) Sulfide
3. Synthetic Routes to SnS
4. Applications of Bulk SnS
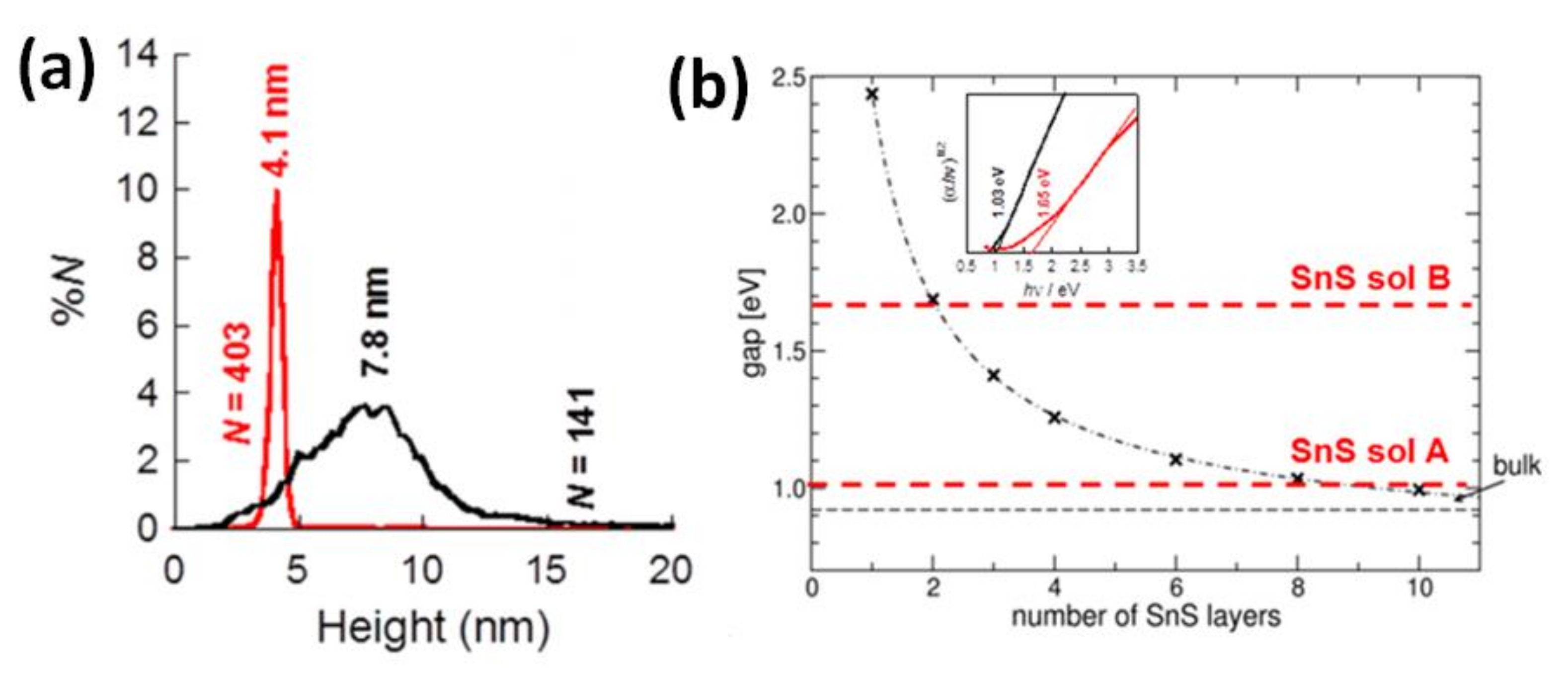
5. Transition from Bulk to Nanoscale
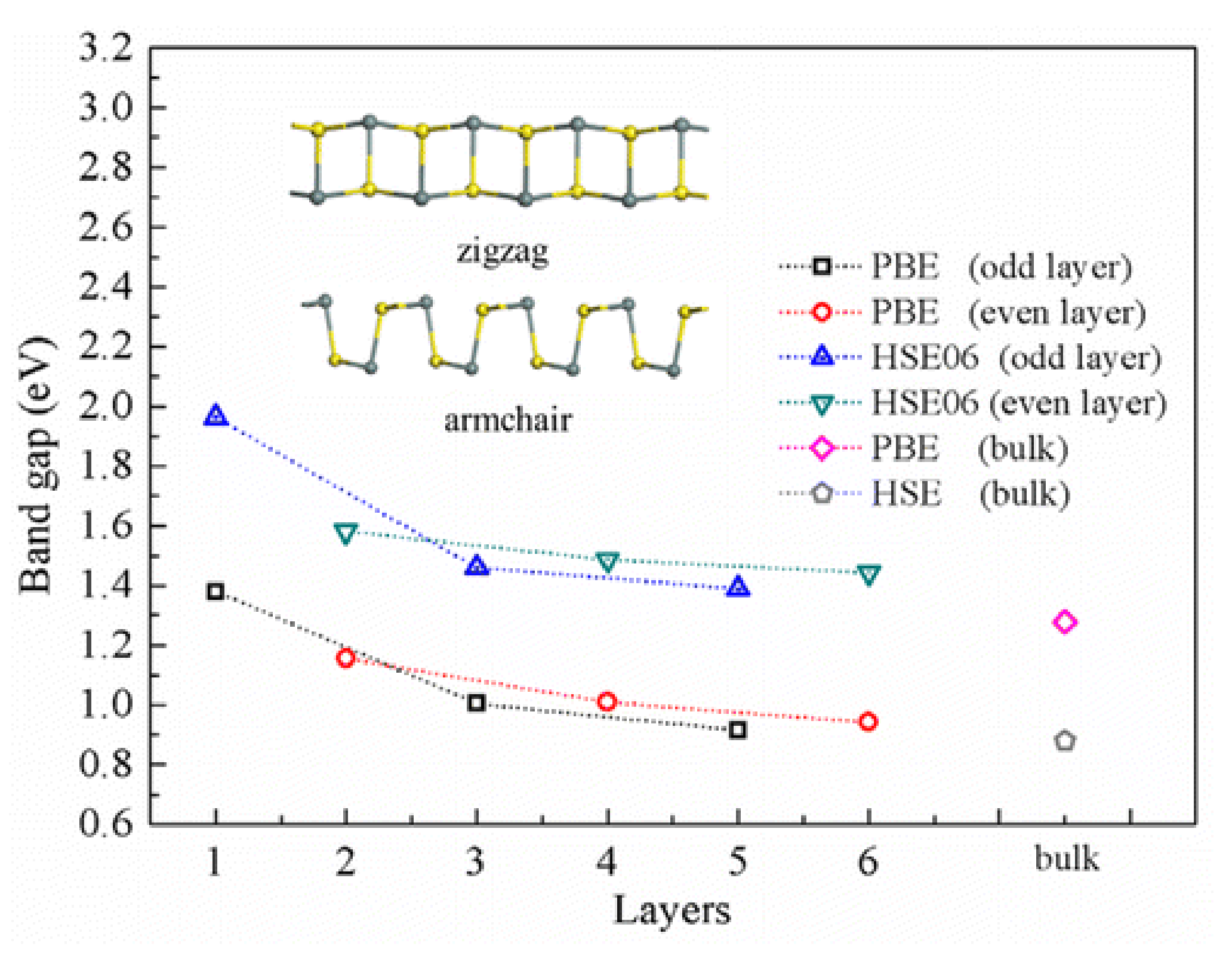
6. Properties of Two-Dimensional Tin(II) Sulfide
7. Synthesis of Two-Dimensional SnS
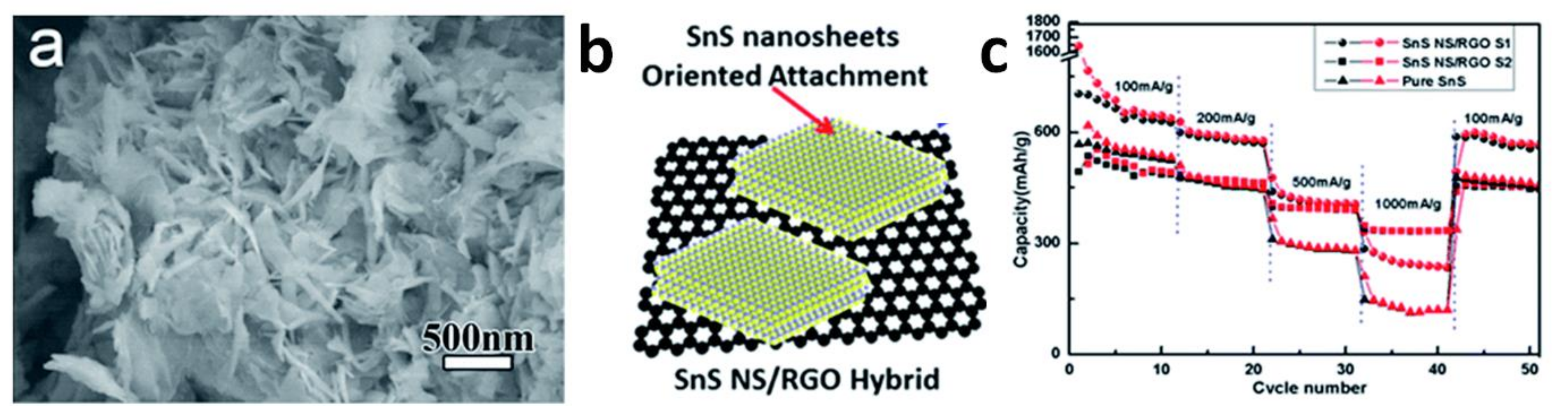
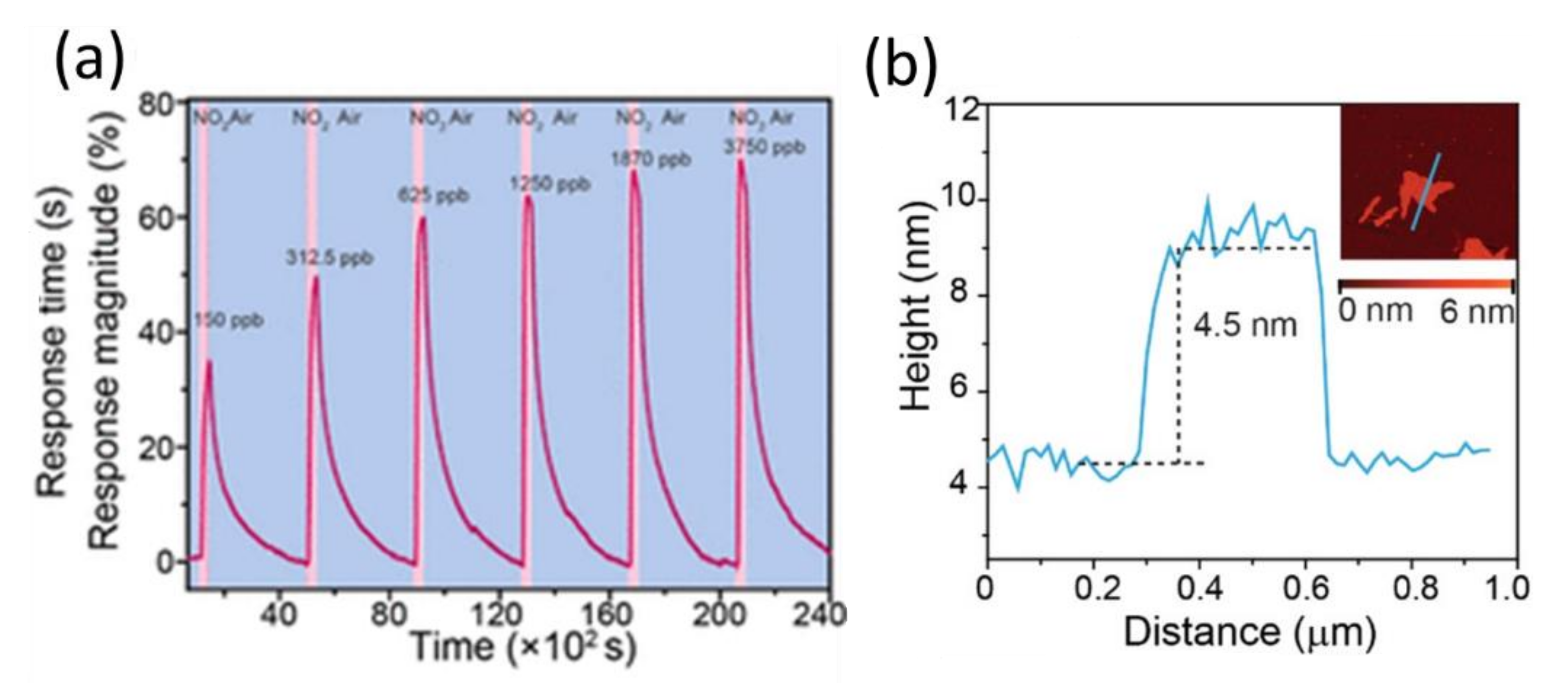
8. Applications of Two-Dimensional Nanoscale SnS
9. Future Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Brent, J.R.; Lewis, D.J.; Lorenz, T.; Lewis, E.A.; Savjani, N.; Haigh, S.J.; Seifert, G.; Derby, B.; O’Brien, P. Tin(II) Sulfide (SnS) Nanosheets by Liquid-Phase Exfoliation of Herzenbergite: IV-VI Main Group Two-Dimensional Atomic Crystals. J. Am. Chem. Soc. 2015, 137, 12689–12696. [Google Scholar] [CrossRef]
- Jiang, T.; Ozin, G.A. New directions in tin sulfide materials chemistry. J. Mater. Chem. 1998, 8, 1099–1108. [Google Scholar] [CrossRef]
- Hofmann, W.M. Ergebnisse der strukturbestimmung komplexer sulfide. Cryst. Mater. 1935, 92, 161–185. [Google Scholar] [CrossRef]
- Hickey, S.G.; Waurisch, C.; Rellinghaus, B.; Eychmüller, A. Size and shape control of colloidally synthesized IV–VI nanoparticulate tin (II) sulfide. J. Am. Chem. Soc. 2008, 130, 14978–14980. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Rath, T.; Lanzetta, L.; Marin-Beloqui, J.M.; Haque, S.A. Efficient Hybrid Solar Cells Based on Solution Processed Mesoporous TiO2/Tin (II) Sulfide Heterojunctions. ACS Appl. Energy Mater. 2018, 1, 3042–3047. [Google Scholar] [CrossRef]
- Noguchi, H.; Setiyadi, A.; Tanamura, H.; Nagatomo, T.; Omoto, O. Characterization of vacuum-evaporated tin sulfide film for solar cell materials. Sol. Energy Mater. Sol. Cells 1994, 35, 325–331. [Google Scholar] [CrossRef]
- Reddy, K.R.; Reddy, N.K.; Miles, R. Photovoltaic properties of SnS based solar cells. Sol. Energy Mater. Sol. Cells 2006, 90, 3041–3046. [Google Scholar] [CrossRef]
- Sinsermsuksakul, P.; Sun, L.; Lee, S.W.; Park, H.H.; Kim, S.B.; Yang, C.; Gordon, R.G. Overcoming efficiency limitations of SnS-based solar cells. Adv. Energy Mater. 2014, 4, 1400496. [Google Scholar] [CrossRef]
- Chen, X.; Hou, Y.; Zhang, B.; Yang, X.H.; Yang, H.G. Low-cost SnS x counter electrodes for dye-sensitized solar cells. Chem. Commun. 2013, 49, 5793–5795. [Google Scholar] [CrossRef]
- Alam, F.; Dutta, V. Tin sulfide (SnS) nanostructured films deposited by continuous spray pyrolysis (CoSP) technique for dye-sensitized solar cells applications. Appl. Surf. Sci. 2015, 358, 491–497. [Google Scholar] [CrossRef]
- O’regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar]
- Wang, M.; Anghel, A.M.; Marsan, B.; Ha, N.-L.C.; Pootrakulchote, N.; Zakeeruddin, S.M.; Grätzel, M. CoS supersedes Pt as efficient electrocatalyst for triiodide reduction in dye-sensitized solar cells. J. Am. Chem. Soc. 2009, 131, 15976–15977. [Google Scholar] [CrossRef] [PubMed]
- Yue, G.; Wu, J.; Xiao, Y.; Huang, M.; Lin, J.; Lin, J.-Y. High performance platinum-free counter electrode of molybdenum sulfide–carbon used in dye-sensitized solar cells. J. Mater. Chem. A 2013, 1, 1495–1501. [Google Scholar] [CrossRef]
- Wu, M.; Wang, Y.; Lin, X.; Yu, N.; Wang, L.; Wang, L.; Hagfeldt, A.; Ma, T. Economical and effective sulfide catalysts for dye-sensitized solar cells as counter electrodes. Phys. Chem. Chem. Phys. 2011, 13, 19298–19301. [Google Scholar] [CrossRef]
- Sun, H.; Qin, D.; Huang, S.; Guo, X.; Li, D.; Luo, Y.; Meng, Q. Dye-sensitized solar cells with NiS counter electrodes electrodeposited by a potential reversal technique. Energy Environ. Sci. 2011, 4, 2630–2637. [Google Scholar] [CrossRef]
- Gong, F.; Xu, X.; Li, Z.; Zhou, G.; Wang, Z.-S. NiSe2 as an efficient electrocatalyst for a Pt-free counter electrode of dye-sensitized solar cells. Chem. Commun. 2013, 49, 1437–1439. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Cao, D.; He, J.; Lin, S.; Lindsay, S.M.; Liu, Y. Solution synthesis of ultrathin single-crystalline SnS nanoribbons for photodetectors via phase transition and surface processing. ACS Nano 2012, 6, 6197–6207. [Google Scholar] [CrossRef]
- Chao, J.; Wang, Z.; Xu, X.; Xiang, Q.; Song, W.; Chen, G.; Hu, J.; Chen, D. Tin sulfide nanoribbons as high performance photoelectrochemical cells, flexible photodetectors and visible-light-driven photocatalysts. RSC Adv. 2013, 3, 2746–2753. [Google Scholar] [CrossRef]
- Tripathi, A.M.; Mitra, S. The influence of electrode structure on the performance of an SnS anode in Li-ion batteries: Effect of the electrode particle, conductive support shape and additive. RSC Adv. 2015, 5, 23671–23682. [Google Scholar] [CrossRef]
- Dutta, P.K.; Sen, U.K.; Mitra, S. Excellent electrochemical performance of tin monosulphide (SnS) as a sodium-ion battery anode. RSC Adv. 2014, 4, 43155–43159. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, L.; Zhuo, M.; Ni, W.; Wang, H.; Ma, J. Layered tin sulfide and selenide anode materials for Li-and Na-ion batteries. J. Mater. Chem. A 2018, 6, 12185–12214. [Google Scholar] [CrossRef]
- Vaughn, D.D.; Hentz, O.D.; Chen, S.; Wang, D.; Schaak, R.E. Formation of SnS nanoflowers for lithium ion batteries. Chem. Commun. 2012, 48, 5608–5610. [Google Scholar] [CrossRef] [PubMed]
- Sucharitakul, S.; Kumar, U.R.; Sankar, R.; Chou, F.-C.; Chen, Y.-T.; Wang, C.; He, C.; He, R.; Gao, X.P.A. Screening limited switching performance of multilayer 2D semiconductor FETs: The case for SnS. Nanoscale 2016, 8, 19050–19057. [Google Scholar] [CrossRef] [PubMed]
- Afsar, M.F.; Rafiq, M.A.; Tok, A.I.Y. Two-dimensional SnS nanoflakes: Synthesis and application to acetone and alcohol sensors. RSC Adv. 2017, 7, 21556–21566. [Google Scholar] [CrossRef]
- Tin(II) Sulfide, GESTIS Online Database. 2019. Available online: https://www.dguv.de/ifa/gestis/gestis-stoffdatenbank/index-2.jsp (accessed on 22 December 2020).
- Nassary, M.M. Temperature dependence of the electrical conductivity, Hall effect and thermoelectric power of SnS single crystals. J. Alloys Compd. 2005, 398, 21–25. [Google Scholar] [CrossRef]
- Brad, E.K.; Malone, D. Quasiparticle band structures and interface physics of SnS and GeS. Phys. Rev. 2013, 87, 245312. [Google Scholar]
- Tritsaris, G.A.; Brad, D.M.; Kaxiras, E. Optoelectronic properties of single-layer, double-layer, and bulk tin sulfide: A theoretical study. J. Appl. Phys. 2013, 113, 233507. [Google Scholar] [CrossRef]
- Li, Q.T.; Jing, F. Thermoelectric Properties of Sn-S Bulk Materials Prepared by Mechanical Alloying and Spark Plasma Sintering. J. Electron. Mater. 2014, 43, 2435–2439. [Google Scholar]
- Asfandiyar; Wei, T.-R.; Li, Z.; Sun, F.-H.; Pan, Y.; Wu, C.-F.; Farooq, M.U.; Tang, H.; Li, F.; Li, B.; et al. Thermoelectric SnS and SnS-SnSe solid solutions prepared by mechanical alloying and spark plasma sintering: Anisotropic thermoelectric properties. Sci. Rep. 2017, 7, 1–7. [Google Scholar] [CrossRef]
- Burton, L.A.; Walsh, A. Phase stability of the earth-abundant tin sulfides SnS, SnS2, and Sn2S3. J. Phys. Chem. C 2012, 116, 24262–24267. [Google Scholar] [CrossRef]
- Wiedemeier, H.; Csillag, F.J. The thermal expansion and high temperature transformation of SnS and SnSe. Z. Krist. 1979, 149, 17–29. [Google Scholar]
- Nasirov, V.; Adgezalova, K.A. Stabilization of low-temperature SnS by rare-earth doping. Inorg. Chem. 2001, 37, 1099–1100. [Google Scholar]
- Chattopadhyay, T.; Pannetier, J.; von Schnering, H. Neutron diffraction study of the structural phase transition in SnS and SnSe. J. Phys. Chem. Solids 1986, 47, 879–885. [Google Scholar] [CrossRef]
- Lewis, D.J.; Kevin, P.; Bakr, O.; Muryn, C.A.; Malik, M.A.; O’Brien, P. Routes to tin chalcogenide materials as thin films or nanoparticles: A potentially important class of semiconductor for sustainable solar energy conversion. Inorg. Chem. Front. 2014, 1, 577–598. [Google Scholar] [CrossRef]
- Rabkin, S.S.A.; Abutbul, R.E. New Nanocrystalline Materials: A Previously Unknown Simple Cubic Phase in the SnS Binary System. Nano Lett. 2015, 15, 2174–2179. [Google Scholar] [CrossRef]
- Abutbul, E.S.R.E.; Zeiri, L.; Ezersky, V. Synthesis and properties of nanocrystalline π-SnS—A new cubic phase of tin sulphide. RSC Adv. 2016, 6, 5848–5855. [Google Scholar] [CrossRef]
- Garcia-Angelmo, R.R.-T.A.R.; Campos-Álvarez, J. Thin film solar cell of SnS absorber with cubic crystalline structure. Phys. Status Solidi 2015, 212, 2332–2340. [Google Scholar] [CrossRef]
- Greyson, E.C.; Barton, J.E.; Odom, T.W. Tetrahedral Zinc Blende Tin Sulfide Nano-and Microcrystals. Small 2006, 2, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Bilenkii, B.; Mikolaichuk, A.; Freik, D. Struktur und optische Eigenschaften von epitaxialen SnTe-, SnSe-und SnS-Schichten. Phys. Status Solidi 1968, 28, K5–K7. [Google Scholar] [CrossRef]
- Burton, L.A.; Colombara, D.; Abellon, R.D.; Grozema, F.C.; Peter, L.M.; Savenije, T.J.; Dennler, G.; Walsh, A. Synthesis, characterization, and electronic structure of single-crystal SnS, Sn2S3, and SnS2. Chem. Mater. 2013, 25, 4908–4916. [Google Scholar] [CrossRef]
- Nitsche, R.; Bölsterli, H.; Lichtensteiger, M. Crystal growth by chemical transport reactions—I: Binary, ternary, and mixed-crystal chalcogenides. J. Phys. Chem. Solids 1961, 21, 199–205. [Google Scholar] [CrossRef]
- Sorgenfrei, T.; Hofherr, F.; Jauß, T.; Cröll, A. Synthesis and single crystal growth of SnS by the Bridgman-Stockbarger technique. Cryst. Res. Technol. 2013, 48, 193–199. [Google Scholar] [CrossRef]
- Ramasamy, K.; Kuznetsov, V.L.; Gopal, K.; Malik, M.A.; Raftery, J.; Edwards, P.P.; O’Brien, P. Organotin dithiocarbamates: Single-source precursors for tin sulfide thin films by aerosol-assisted chemical vapor deposition (AACVD). Chem. Mater. 2013, 25, 266–276. [Google Scholar] [CrossRef]
- Kevin, P.; Lewis, D.J.; Raftery, J.; Malik, M.A.; O’Brien, P. Thin films of tin (II) sulphide (SnS) by aerosol-assisted chemical vapour deposition (AACVD) using tin (II) dithiocarbamates as single-source precursors. J. Cryst. Growth 2015, 415, 93–99. [Google Scholar] [CrossRef]
- Al-Shakban, M.; Matthews, P.D.; Lewis, E.A.; Raftery, J.; Vitorica-Yrezabal, I.; Haigh, S.J.; Lewis, D.J.; O’Brien, P. Chemical vapor deposition of tin sulfide from diorganotin (IV) dixanthates. J. Mater. Sci. 2019, 54, 2315–2323. [Google Scholar] [CrossRef]
- Ahmet, I.Y.; Hill, M.S.; Johnson, A.L.; Peter, L.M. Polymorph-selective deposition of high purity SnS thin films from a single source precursor. Chem. Mater. 2015, 27, 7680–7688. [Google Scholar] [CrossRef]
- Price, L.S.; Parkin, I.P.; Hardy, A.M.E.; Clark, R.J.H.; Hibbert, T.G.; Molloy, K.C. Atmospheric Pressure Chemical Vapor Deposition of Tin Sulfides (SnS, Sn2S3, and SnS2) on Glass. Chem. Mater. 1999, 11, 1792–1799. [Google Scholar] [CrossRef]
- Price, L.S.; Parkin, I.P.; Field, M.N.; Hardy, A.M.; Clark, R.J.; Hibbert, T.G.; Molloy, K.C. Atmospheric pressure chemical vapour deposition of tin (II) sulfide films on glass substrates from Bun3SnO2CCF3 with hydrogen sulfide. J. Mater. Chem. 2000, 10, 527–530. [Google Scholar] [CrossRef]
- Hibbert, T.G.; Mahon, M.F.; Molloy, K.C.; Price, L.S.; Parkin, I.P. Deposition of tin sulfide thin films from novel, volatile (fluoroalkythiolato) tin (IV) precursors. J. Mater. Chem. 2001, 11, 469–473. [Google Scholar] [CrossRef]
- Kana, A.; Hibbert, T.; Mahon, M.; Molloy, K.; Parkin, I.; Price, L. Organotin unsymmetric dithiocarbamates: Synthesis, formation and characterisation of tin (II) sulfide films by atmospheric pressure chemical vapour deposition. Polyhedron 2001, 20, 2989–2995. [Google Scholar] [CrossRef]
- Price, L.S.; Parkin, I.P.; Hibbert, T.G.; Molloy, K.C. Atmospheric pressure CVD of SnS and SnS2 on glass. Chem. Vap. Depos. 1998, 4, 222–225. [Google Scholar] [CrossRef]
- Bade, B.P.; Garje, S.S.; Niwate, Y.S.; Afzaal, M.; O’Brien, P. Tribenzyltin (IV) chloride thiosemicarbazones: Novel single source precursors for growth of SnS thin films. Chem. Vap. Depos. 2008, 14, 292–295. [Google Scholar] [CrossRef]
- Tanusevski, A. Optical and photoelectric properties of SnS thin films prepared by chemical bath deposition. Semicond. Sci. Technol. 2003, 18, 501. [Google Scholar] [CrossRef]
- Ristov, G.S.M. Chemical deposition of TIN(II) sulphide thin films. Thin Solid Film. 1989, 173, 53–58. [Google Scholar] [CrossRef]
- Cheng, S.; Chen, G.; Chen, Y.; Huang, C. Effect of deposition potential and bath temperature on the electrodeposition of SnS film. Opt. Mater. 2006, 29, 439–444. [Google Scholar] [CrossRef]
- Sinsermsuksakul, P.; Heo, J.; Noh, W.; Hock, A.S.; Gordon, R.G. Atomic layer deposition of tin monosulfide thin films. Adv. Energy Mater. 2011, 1, 1116–1125. [Google Scholar] [CrossRef]
- Kim, J.Y.; George, S.M. Tin monosulfide thin films grown by atomic layer deposition using tin 2, 4-pentanedionate and hydrogen sulfide. J. Phys. Chem. C 2010, 114, 17597–17603. [Google Scholar] [CrossRef]
- Reddy, N.K.; Reddy, K.R. Growth of polycrystalline SnS films by spray pyrolysis. Thin Solid Film. 1998, 325, 4–6. [Google Scholar] [CrossRef]
- Calixto-Rodriguez, M.; Martinez, H.; Sanchez-Juarez, A.; Campos-Alvarez, J.; Tiburcio-Silver, A.; Calixto, M. Structural, optical, and electrical properties of tin sulfide thin films grown by spray pyrolysis. Thin Solid Film. 2009, 517, 2497–2499. [Google Scholar] [CrossRef]
- Ghosh, B.; Das, M.; Banerjee, P.; Das, S. Fabrication and optical properties of SnS thin films by SILAR method. Appl. Surf. Sci. 2008, 254, 6436–6440. [Google Scholar] [CrossRef]
- Banai, R.; Horn, M.W.; Brownson, J. A review of tin (II) monosulfide and its potential as a photovoltaic absorber. Sol. Energy Mater. Sol. Cells 2016, 150, 112–129. [Google Scholar] [CrossRef]
- Reddy, N.K.; Devika, M.; Gopal, E. Review on tin (II) sulfide (SnS) material: Synthesis, properties, and applications. Crit. Rev. Solid State Mater. Sci. 2015, 40, 359–398. [Google Scholar] [CrossRef]
- Balaz, T.O.P.; Bastl, Z. Properties and Reactivity of Mechanochemically Synthesized Tin Sulfides. J. Solid State Chem. 1999, 144, 1–7. [Google Scholar] [CrossRef]
- Dogrusoz, R.D.C.M. Mechanochemical synthesis of SnS anodes for sodium ion batteries. Int. J. Energy Res. 2020, 44, 10809–10820. [Google Scholar] [CrossRef]
- Park, B.I.; Jang, Y.H.; Lee, S.Y.; Lee, D.-K. Mechanochemically synthesized SnS nanocrystals: Impact of nonstoichiometry on phase purity and solar cell performance. ACS Sustain. Energy Eng. 2018, 6, 3002–3009. [Google Scholar] [CrossRef]
- Norton, K.; Kunstmann, J.; Ping, L.; Rakowski, A.; Wang, C.C.; Marsden, A.J.; Murtaza, G.; Zeng, N.T.; McAdams, S.G.; Bissett, M.A.; et al. Synthetic 2-D lead tin sulfide nanosheets with tuneable optoelectronic properties from a potentially scalable reaction pathway. Chem. Sci. 2019, 10, 2843. [Google Scholar] [CrossRef]
- Reiss, P.; Carriere, M.; Lincheneau, C.; Vaure, L.; Tamang, S. Synthesis of semiconductor nanocrystals, focusing on nontoxic and earth-abundant materials. Chem. Rev. 2016, 116, 10731–10819. [Google Scholar] [CrossRef]
- Steinmann, V.; Jaramillo, R.; Hartman, K.; Chakraborty, R.; Brandt, R.E.; Poindexter, J.R.; Lee, Y.S.; Sun, L.; Polizzotti, A.; Park, H.H.; et al. 3.88% Efficient Tin Sulfide Solar Cells using Congruent Thermal Evaporation. Adv. Mater. 2014, 26, 7488–7492. [Google Scholar] [CrossRef]
- Park, H.H.; Heasley, R.; Sun, L.; Steinmann, V.; Jaramillo, R.; Hartman, K.; Chakraborty, R.; Sinsermsuksakul, P.; Chua, D.; Buonassisi, T.; et al. Co-optimization of SnS absorber and Zn (O, S) buffer materials for improved solar cells. Prog. Photovolt. Res. Appl. 2015, 23, 901–908. [Google Scholar] [CrossRef]
- Guo, W.; Shen, Y.; Wu, M.; Ma, T. Highly efficient inorganic–organic heterojunction solar cells based on SnS-sensitized spherical TiO2 electrodes. Chem. Commun. 2012, 48, 6133–6135. [Google Scholar] [CrossRef]
- Rath, T.; Gury, L.; Sánchez-Molina, I.; Martinez, L.; Haque, S.A. Formation of porous SnS nanoplate networks from solution and their application in hybrid solar cells. Chem. Commun. 2015, 51, 10198–10201. [Google Scholar] [CrossRef]
- Nielsen, F.H.; Sandstead, H.H. Are nickel, vanadium, silicon, fluorine, and tin essential for man? A review. Am. J. Clin. Nutr. 1974, 27, 515–520. [Google Scholar] [CrossRef]
- Tang, H.; Dong, J.F.; Sun, F.H.; Shang, P.; Li, J.F. Adjusting Na doping via wet-chemical synthesis to enhance thermoelectric properties of polycrystalline SnS. Sci. China Mater. 2019, 62, 1005–1012. [Google Scholar] [CrossRef]
- Wang, Y.C.C. Improved thermoelectric properties of SnS synthesized by chemical precipitation. RSC Adv. 2017, 7, 16795–16800. [Google Scholar] [CrossRef]
- Feng, D.; Ge, Z.H.; Chen, Y.X.; Li, J.; He, J. Hydrothermal synthesis of SnQ (Q = Te, Se, S) and their thermoelectric properties. Nanotechnology 2017, 28, 455707. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Kaur, B.; Carrete, J.; Bera, C. A theoretical model of the thermoelectric properties of SnSxSe1−x and how to further enhance its thermoelectric performance. J. Appl. Phys. 2019, 126, 225105. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Backes, C.; Hanlon, D.; Szydlowska, B.M.; Harvey, A.; Smith, R.J.; Higgins, T.M.; Coleman, J.N. Preparation of Liquid-exfoliated Transition Metal Dichalcogenide Nanosheets with Controlled Size and Thickness: A State of the Art Protocol. J. Vis. Exp. 2016, 118, 54806. [Google Scholar] [CrossRef] [PubMed]
- Gomes, L.C.; Carvalho, A. Phosphorene analogues: Isoelectronic two-dimensional group-IV monochalcogenides with orthorhombic structure. Phys. Rev. B Condens. Matter Mater. Phys. 2015, 92, 085406. [Google Scholar] [CrossRef]
- Trainer, D.J.; Putilov, A.V.; di Giorgio, C.; Saari, T.; Wang, B.; Wolak, M.; Chandrasena, R.U.; Lane, C.; Chang, T.-R.; Jeng, H.-T.; et al. Inter-Layer Coupling Induced Valence Band Edge Shift in Mono- to Few-Layer MoS2. Sci. Rep. 2017, 7, 1–11. [Google Scholar]
- Graulis, S.; Chateigner, D.; Downs, R.T.; Yokochi, A.F.T.; Quirós, M.; Lutterotti, L.; Manakova, E.; Butkus, J.; Moeck, P.; le Bail, A. Crystallography Open Database - An open-access collection of crystal structures. J. Appl. Crystallogr. 2009, 42, 726–729. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Ghorannevis, Z.; Chu, L.; Toh, M.; Kloc, C.; Tan, P.H.; Eda, G. Evolution of electronic structure in atomically thin sheets of WS2 and WSe2. ACS Nano 2012, 7, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zheng, J.; Hu, Z.; Zuo, S.; Wu, Z.; Yan, P.; Pan, F. 3D-hierarchical SnS nanostructures: Controlled synthesis, formation mechanism and lithium-ion storage performance. RSC Adv. 2015, 5, 72857–72862. [Google Scholar] [CrossRef]
- Zhang, Y.; Shang, B.; Li, L.; Lei, J. Coupling effects of strain on structural transformation and bandgap engineering in SnS monolayer. RSC Adv. 2017, 7, 30327–30333. [Google Scholar] [CrossRef]
- Borisenko, V.E.; Gaponenko, S.V.; Gurin, V.S. Physics, Chemistry and Applications of Nanostructures: Proceedings of International Conference Nanomeeting, Minsk, Belarus, 26–29 May 2015; World Scientific Publishing Company: Singapore, 2015. [Google Scholar]
- Gashimzade, D.A.G.F.M. Second Order Phase Transition in a SnS Orthorhombic Crystal under Pressure. Lattice Dyn. 2015, 57, 378–380. [Google Scholar] [CrossRef]
- Higashitarumizu, N.; Kawamoto, H.; Nakamura, M.; Shimamura, K.; Ohashi, N.; Ueno, K.; Nagashio, K. Self-passivated ultra-thin SnS layers via mechanical exfoliation and post-oxidation. Nanoscale 2018, 10, 22474–22483. [Google Scholar] [CrossRef]
- Sarkar, A.S.; Mushtaq, A.; Kushavah, D.; Pal, S.K. Liquid exfoliation of electronic grade ultrathin tin(II) sulfide (SnS) with intriguing optical response. NPJ 2D Mater. Appl. 2020, 4, 1–9. [Google Scholar] [CrossRef]
- Yao, S.L.; Alexandra, C.; Shancheng, Y.; Roger, L.; Sujung, K.; Aleksandr, R.; Lídia, C.; Emory, M.C.; Xi, W.; Antonio, H.C.N.; et al. Accessing valley degree of freedom in bulk Tin(II) sulfide at room temperature. Nat. Comm. 2018, 9, 1–7. [Google Scholar]
- Guo, R.; Wang, X.; Kuang, Y.; Huang, B. First-principles study of anisotropic thermoelectric transport properties of IV–VI semiconductor compounds SnSe and SnS. Phys. Rev. B 2020, 92, 115202. [Google Scholar] [CrossRef]
- Dewandre, A.; Verstraete, M.J.; Grobert, N.; Zanolli, Z. Spectroscopic properties of few-layer tin chalcogenides. J. Phys. Mater. 2019, 2, 044005. [Google Scholar] [CrossRef]
- Xin, C.; Zheng, J.; Su, Y.; Li, S.; Zhang, B.; Feng, Y.; Pan, F. Few-layer tin sulfide: A new black-phosphorus-analogue 2D material with a sizeable band gap, odd-even quantum confinement effect, and high carrier mobility. J. Phys. Chem. C 2016, 120, 22663–22669. [Google Scholar] [CrossRef]
- Tian, Z.; Guo, C.; Zhao, M.; Li, R.; Xue, J. Two-Dimensional SnS: A Phosphorene Analogue with Strong In-Plane Electronic Anisotropy. ACS Nano 2017, 11, 2219–2226. [Google Scholar] [CrossRef]
- Qiao, J.; Kong, X.; Hu, Z.X.; Yang, F.; Ji, W. High-mobility transport anisotropy and linear dichroism in few-layer black phosphorus. Nat. Comm. 2014, 5, 4475. [Google Scholar] [CrossRef] [PubMed]
- Sandonas, L.M.; Teich, D.; Gutierrez, R.; Lorenz, T.; Pecchia, A.; Seifert, G.; Cuniberti, G. Anisotropic Thermoelectric Response in Two-Dimensional Puckered Structures. J. Phys. Chem. C 2016, 120, 18841–18849. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, H.J.; Cheng, L.; Wei, J.; Liang, J.H.; Fan, D.D.; Shi, J.; Tang, X.F.; Zhang, Q.J. Phosphorene nanoribbon as a promising candidate for thermoelectric applications. Sci. Rep. 2014, 4, 6452. [Google Scholar] [CrossRef]
- Yang, B.; Li, M.D.; Wang, J.Y.; Zhang, J.C.; Liao, D.M.; Yue, Y.N. Critical fracture properties of puckered and buckled arsenenes by molecular dynamics simulations. PCCP 2019, 21, 12372–12379. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Wang, L.; Wang, X.; Chen, G. Flexible films of poly(3,4-ethylenedioxythiophene):Poly(styrenesulfonate)/SnS nanobelt thermoelectric composites. Compos. Sci. Technol. 2018, 155, 247–251. [Google Scholar] [CrossRef]
- Zhou, H.Q.Y.; Yang, W.X.; Hong, W.; Bin, Z.; Dan, X.D.; Jin, C.Y.; Xu, L.; Dong, H.X.; Lei, M.; Xiao, Y. Sn vacancy engineering for enhancing the thermoelectric performance of two-dimensional SnS. J. Mater. Chem. C 2019, 7, 3351–3359. [Google Scholar]
- Kuriakose, S.; Ahmed, T.; Balendhran, S.; Bansal, V.; Sriram, S.; Bhaskaran, M.; Walia, S. Black phosphorus: Ambient degradation and strategies for protection. 2D Mater. 2018, 5, 032001. [Google Scholar] [CrossRef]
- Lam, D.; Chen, K.S.; Kang, J.; Liu, X.; Hersam, M.C. Anhydrous liquid-phase exfoliation of pristine electrochemically active GeS nanosheets. Chem. Mater. 2018, 30, 2245–2250. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, D.; Ji, Y.; Zhang, H.; Shen, X. Two-dimensional SnS nanosheets fabricated by a novel hydrothermal method. J. Mater. Sci. 2005, 40, 591–595. [Google Scholar] [CrossRef]
- Paton, K.R.; Varrla, E.; Backes, C.; Smith, R.J.; Khan, U.; O’Neill, A.; Boland, C.; Lotya, M.; Istrate, O.M.; King, P.; et al. Scalable production of large quantities of defect-free few-layer graphene by shear exfoliation in liquids. Nat. Mater. 2014, 13, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Backes, C.; Harvey, A.; Yuan, S.; Vega-Mayoral, V.; Davies, B.R.; Zhao, P.L.; Hanlon, D.; Santos, E.J.G.; Katsnelson, M.I.; Blau, W.J.; et al. Production of highly monolayer enriched dispersions of liquid-exfoliated nanosheets by liquid cascade centrifugation. ACS Nano 2016, 10, 1589–1601. [Google Scholar] [CrossRef]
- Huang, W.; Xie, Z.; Fan, T.; Li, J.; Wang, Y.; Wu, L.; Ma, D.; Li, Z.; Ge, Y.; Huang, Z.N.; et al. Black-phosphorus-analogue tin monosulfide: An emerging optoelectronic two-dimensional material for high-performance photodetection with improved stability under ambient/harsh conditions. J. Mater. Chem. C 2018, 10, 9582–9593. [Google Scholar] [CrossRef]
- Xie, Z.; Zhang, F.; Liang, Z.; Fan, T.; Li, Z.; Jiang, X.; Chen, H.; Li, J.; Zhang, H. Revealing of the ultrafast third-order nonlinear optical response and enabled photonic application in two-dimensional tin sulfide. Photon. Res. PRJ 2019, 7, 494–502. [Google Scholar] [CrossRef]
- Walia, H.; Lan, W.; Salvy, P.R.; Torben, D.; Yongxiang, L.; Kalantar-Zadeh, H.K.K.; Nasir, M.; Ali, Z.; Aaron, E.; Ataur, R.M.; et al. Liquid metal-based synthesis of high performance monolayer SnS piezoelectric nanogenerators. Nature Comm. 2020, 11, 1–8. [Google Scholar]
- Sarkar, A.S. Dispersion behavior of two dimensional monochalcogenides. arXiv 2020, arXiv:2012.04102. [Google Scholar]
- Eastman Solvent Selector Chart. Eastman Lit. Ctr. 2020. Online database. Available online: https://www.eastman.com/Brands/Eastman_Solvents/Pages/Resources.aspx. (accessed on 22 December 2020).
- Lin, S.; Chui, Y.; Li, Y.; Lau, S.P. Liquid-phase exfoliation of black phosphorus and its applications. FlatChem 2017, 2, 15–37. [Google Scholar] [CrossRef]
- Higashitarumizu, N.; Kawamoto, H.; Ueno, K.; Nagashio, K. Fabrication and surface engineering of two-dimensional SnS toward piezoelectric nanogenerator application. Fabrication and Surface Engineering of Two-Dimensional SnS Toward Piezoelectric Nanogenerator Application. MRS Adv. 2018, 3, 2809–2814. [Google Scholar] [CrossRef]
- Ju, H.; Park, D.; Kim, J. Fabrication of porous SnS nanosheets and their combination with conductive polymer for hybrid thermoelectric application. Chem. Eng. J. 2019, 356, 950–954. [Google Scholar] [CrossRef]
- Tian, H.; Fan, C.; Liu, G.; Yuan, S.; Zhang, Y.; Wang, M.; Li, E. Ultrafast broadband photodetector based on SnS synthesized by hydrothermal method. Ultrafast broadband photodetector based on SnS synthesized by hydrothermal method. Appl. Surf. Sci. 2019, 487, 1043–1048. [Google Scholar] [CrossRef]
- Meng, C.C. Chemical bath deposition of single crystal SnS nanobelts on glass substrates. Mater. Res. Bull. 2018, 104, 224–249. [Google Scholar]
- Jing, J.; Cao, M.; Wu, C.; Huang, J.; Lai, J.; Sun, Y.; Wang, L.; Shen, Y. Chemical bath deposition of SnS nanosheet thin films for FTO/SnS/CdS/Pt photocathode. J. Alloys Compd. 2017, 726, 720–728. [Google Scholar] [CrossRef]
- Shan, W.; Fu, Z.; Ma, M.; Liu, Z.; Xue, Z.; Xu, J.; Zhang, F.; Li, Y. Facile Chemical Bath Synthesis of SnS Nanosheets and Their Ethanol Sensing Properties. Sensors 2019, 19, 2581. [Google Scholar] [CrossRef]
- Choi, M.; William, W.; Hwang, J.; Yoon, D.; Kim, J. A supercritical ethanol route for one-pot synthesis of tin sulfide-reduced graphene oxides and their anode performance for lithium ion batteries. J. Ind. Eng. Chem. 2018, 59, 160–168. [Google Scholar] [CrossRef]
- Wu, Y.; Wei, T.; An, X.; Liu, L.-M. Colloidal synthesis of SnS nanocrystals with dimension-dependent photoelectrochemical properties. New J. Chem. 2019, 43, 7457–7462. [Google Scholar] [CrossRef]
- Biacchi, A.J.; Vaughn, D.D.; Schaak, R.E. Synthesis and crystallographic analysis of shape-controlled SnS nanocrystal photocatalysts: Evidence for a pseudotetragonal structural modification. J. Am. Chem. Soc. 2013, 135, 11634–11644. [Google Scholar] [CrossRef] [PubMed]
- De Kergommeaux, A.; Lopez-Haro, M.; Pouget, S.; Zuo, J.-M.; Lebrun, C.; Chandezon, F.; Aldakov, D.; Reiss, P. Synthesis, internal structure, and formation mechanism of monodisperse tin sulfide nanoplatelets. J. Am. Chem. Soc. 2015, 137, 9943–9952. [Google Scholar] [CrossRef]
- Li, F.; Moayed, M.M.R.; Gerdes, F.; Kull, S.; Klein, E.; Lesyuk, R.; Klinke, C. Colloidal tin sulfide nanosheets: Formation mechanism, ligand-mediated shape tuning and photo-detection. Colloidal tin sulfide nanosheets: Formation mechanism, ligand-mediated shape tuning and photo-detection. J. Mater. Chem. C 2018, 6, 9410–9419. [Google Scholar] [CrossRef]
- Ahmet, I.Y.; Guc, M.; Sánchez, Y.; Neuschitzer, M.; Izquierdo-Roca, V.; Saucedo, E.; Johnson, A.L. Evaluation of AA-CVD deposited phase pure polymorphs of SnS for thin films solar cells. RSC Adv. 2019, 9, 14899–14909. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Fal’Ko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef]
- Liu, G.Z.; Li, Y.; Li, B.; Tian, H.; Fan, C.; Zhang, Y.H.; Hua, Z.Q.; Wang, M.J.; Zheng, H.X.; Li, E.P. High-performance photodetectors based on two-dimensional tin(II) sulfide (SnS) nanoflakes. J. Mater. Chem. C 2018, 6, 10036–10041. [Google Scholar] [CrossRef]
- Mutlu, Z.; Wu, R.J.; Wickramaratne, D.; Shahrezaei, S.; Liu, C.; Temiz, S.; Patalano, A.; Ozkan, M.; Lake, R.K.; Mkhoyan, K.A.; et al. Phase Engineering of 2D Tin Sulfides. Small 2016, 12, 2998–3004. [Google Scholar] [CrossRef]
- Kawamoto, H.; Higashitarumizu, N.; Nagamura, N.; Nakamura, M.; Shimamura, K.; Ohashi, N.; Nagashio, K. Micrometer-scale monolayer SnS growth by physical vapor deposition. Nanoscale 2020, 12, 23274–23281. [Google Scholar] [CrossRef]
- Sutter, E.; Wang, J.; Sutter, P. Surface Passivation by Excess Sulfur for Controlled Synthesis of Large, Thin SnS Flakes. Chem. Mater. 2020, 32, 8034–8042. [Google Scholar] [CrossRef]
- Xia, J.; Li, X.; Huang, X.; Mao, N.; Zhu, D.D.; Wang, L.; Xu, H.; Meng, X.-M. Physical vapor deposition synthesis of two-dimensional orthorhombic SnS flakes with strong angle/temperature-dependent Raman responses. Nanoscale 2016, 8, 2063–2070. [Google Scholar] [CrossRef]
- Yuan, S.; Liu, G.; Tian, H.; Fan, C.; Wang, M.; Li, E. Facile synthesis of tin monosulfide nanosheets via physical vapour deposition and their near-infrared photoresponse. AIP Adv. 2019, 9, 095205. [Google Scholar] [CrossRef]
- Rath, J.K.; Prastani, C.; Nanu, D.E.; Nanu, M.; Schropp, R.E.I.; Vetushka, A.; Hyvl, M.; Fejfar, A. Fabrication of SnS quantum dots for solar-cell applications: Issues of capping and doping. Phys. Status Solidi B Basic Solid State Phys. 2014, 251, 1309–1321. [Google Scholar] [CrossRef]
- Deepa, K.G.; Nagaraju, J. Growth and photovoltaic performance of SnS quantum dots. Mater. Sci. Eng. B Adv. Funct. Solid State Mater. 2012, 177, 1023–1028. [Google Scholar] [CrossRef]
- Ngoi, K.K.; Jun, H.K. Study of fabrication of fully aqueous solution processed SnS quantum dot-sensitized solar cell. Green Process. Synth. 2019, 8, 443–450. [Google Scholar] [CrossRef]
- Rahaman, S.; Jagannatha, K.B.; Pradeep; Sriram, A.; Nitin, A. Synthesis and Characterization of SnS Quantum Dots material for Solar Cell. Mater. Today Proc. 2018, 5, 3117–3120. [Google Scholar] [CrossRef]
- Prastani, C.; Nanu, M.; Nanu, D.E.; Rath, J.K.; Schropp, R.E.I. Synthesis and conductivity mapping of SnS quantum dots for photovoltaic applications. Mater. Sci. Eng. B Adv. Funct. Solid State Mater. 2013, 178, 656–659. [Google Scholar] [CrossRef]
- Chowdhury, A.P.; Shambharkar, B.H.; Ghugal, S.G.; Umare, S.S.; Shende, A.G. Ethylene glycol mediated synthesis of SnS quantum dots and their application towards degradation of eosin yellow and brilliant green dyes under solar irradiation. RSC Adv. 2016, 6, 108290–108297. [Google Scholar] [CrossRef]
- Han, S.; Shih, W.Y.; Shih, W.H. Charge-Neutral, Stable, Non-Cytotoxic, Near-Infrared SnS Aqueous Quantum Dots for High Signal-to-Noise-Ratio Biomedical Imaging. ChemistrySelect 2017, 2, 7332–7339. [Google Scholar] [CrossRef]
- Ali, S.; Wang, F.; Iqbal, M.Z.; Zafar, S.; Shah, H.U.; Toufiq, A.M. Hydrothermal Synthesis of Chalcogenide SnS Nanorods: Lattice Vibrations and Optical Properties. Chalcogenide Lett. 2017, 14, 539–543. [Google Scholar]
- Su, H.L.; Xie, Y.; Xiong, Y.J.; Gao, P.; Qian, Y.T. Preparation and morphology control of rod-like nanocrystalline tin sulfides via a simple ethanol thermal route. J. Solid State Chem. 2001, 161, 190–196. [Google Scholar] [CrossRef]
- Biswas, S.; Kar, S.; Chaudhuri, S. Thioglycolic. acid (TGA) assisted hydrothermal synthesis of SnS nanorods and nanosheets. Appl. Surf. Sci. 2007, 253, 9259–9266. [Google Scholar] [CrossRef]
- Pan, J.; Li, J.Y.; Xiong, S.L.; Qian, Y.T. Ultrasonically Assisted Synthesis of Tin Sulfide Nanorods at Room Temperature. Adv. Mater. Res. 2009, 79–82, 313–316. [Google Scholar] [CrossRef]
- Panda, S.K.; Datta, A.; Dev, A.; Gorai, S.; Chaudhuri, S. Surfactant-assisted synthesis of SnS nanowires grown on tin foils. Cryst. Growth Des. 2006, 6, 2177–2181. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Wang, F.P.; Rafique, M.Y.; Ali, S.; Farooq, M.H.; Ellahi, M. Hydrothermal synthesis, characterization and hydrogen storage of SnS nanorods. Mater. Lett. 2013, 106, 33–36. [Google Scholar] [CrossRef]
- Zhu, J.S.; Wang, D.L.; Liu, T.F. One-pot synthesis of SnS nanorods and their lithium storage properties. Ionics 2014, 20, 141–144. [Google Scholar] [CrossRef]
- Tripathi, A.M.; Mitra, S. Tin sulfide (SnS) nanorods: Structural, optical and lithium storage property study. RSC Adv. 2014, 4, 10358–10366. [Google Scholar] [CrossRef]
- Baby, B.H.; Mohan, D.B. Phase optimization study of orthorhombic structured SnS nanorods from CTAB assisted polyol synthesis for higher efficiency thin film solar cells. Sol. Energy 2018, 174, 373–385. [Google Scholar] [CrossRef]
- Bhorde, A.; Pawbake, A.; Sharma, P.; Nair, S.; Funde, A.; Bankar, P.; More, M.; Jadkar, S. Solvothermal synthesis of tin sulfide (SnS) nanorods and investigation of its field emission properties. Appl. Phys. A Mater. Sci. Process. 2018, 124, 1–8. [Google Scholar] [CrossRef]
- Yang, D.L.; Chen, F.; He, S.L.; Shen, H.F.; Hu, Y.L.; Feng, N.; Wang, S.Y.; Weng, L.X.; Luo, Z.M.; Wang, L.H. One-pot growth of triangular SnS nanopyramids for photoacoustic imaging and photothermal ablation of tumors. New J. Chem. 2019, 43, 13256–13262. [Google Scholar] [CrossRef]
- Salavati-Niasari, M.; Ghanbari, D.; Davar, F. Shape selective hydrothermal synthesis of tin sulfide nanoflowers based on nanosheets in the presence of thioglycolic acid. J. Alloys Compd. 2010, 492, 570–575. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Kang, W.P.; Ma, P.; Cao, D.X.; Cao, D.W.; Kang, Z.X.; Sun, D.F. SnS@C nanospheres coated with few-layer MoS2 nanosheets and nitrogen, phosphorus-codoped carbon as robust sodium ion battery anodes. Mater. Chem. Front. 2020, 4, 1212–1221. [Google Scholar] [CrossRef]
- Truong, N.T.N.; Hoang, H.H.T.; Trinh, T.K.; Pham, V.T.H.; Smith, R.P.; Park, C. Effect of post-synthesis annealing on properties of SnS nanospheres and its solar cell performance. Korean J. Chem. Eng. 2017, 34, 1208–1213. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, J.; Zhang, K.; Chen, S.; Mei, F.; Shen, G. Anisotropic photoresponse of layered 2D SnS-based near infrared photodetectors. J. Mater. Chem. C 2017, 5, 11288–11293. [Google Scholar] [CrossRef]
- Yao, J.; Yang, G. Flexible and High-Performance All-2D Photodetector for Wearable Devices. Small 2018, 14, 1704524. [Google Scholar] [CrossRef]
- Kumar, M.; Kim, H.-S.; Park, D.Y.; Jeong, M.S.; Kim, J. Wide channel broadband CH3NH3PbI3/SnS hybrid photodetector: Breaking the limit of bandgap energy operation. RSC Adv. 2018, 8, 23206–23212. [Google Scholar] [CrossRef]
- Jamali-Sheini, F.; Cheraghizade, M.; Yousefi, R. SnS nanosheet films deposited via thermal evaporation: The effects of buffer layers on photovoltaic performance. Sol. Energy Mater. Sol. Cells 2016, 154, 49–56. [Google Scholar] [CrossRef]
- Guo, W.; Shen, Y.; Wu, M.; Wang, L.; Wang, L.; Ma, T. SnS-Quantum Dot Solar Cells Using Novel TiC Counter Electrode and Organic Redox Couples. Chem. A Eur. J. 2012, 18, 7862–7868. [Google Scholar] [CrossRef]
- Youn, N.K.; Jung, H.R.; Gwak, J.; Cho, A.; Ahn, S.J.; Ahn, S.K.; Kim, J.H.; Eo, Y.-J.; Kim, D.H. Fabrication of SnS solar cells via facile nanoparticle synthesis based on non-toxic solvents. Thin Solid Film. 2018, 660, 294–300. [Google Scholar] [CrossRef]
- Yue, G.; Lin, Y.; Wen, X.; Wang, L.; Peng, D. SnS homojunction nanowire-based solar cells. J. Mater. Chem. 2012, 22, 16437–16441. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, G.; Chen, S.; Guo, Z.; Yu, X.; Zhao, C.; Zhang, H.; Bao, Q.; Wen, S.; Tang, D. Mechanically exfoliated black phosphorus as a new saturable absorber for both Q-switching and Mode-locking laser operation. Opt. Express 2015, 23, 12823–12833. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Li, Y.; Pang, H. Applications of Tin Sulfide-Based Materials in Lithium-Ion Batteries and Sodium-Ion Batteries. Adv. Funct. Mater. 2020, 30, 2001298. [Google Scholar] [CrossRef]
- Kang, J.-G.; Park, J.-G.; Kim, D.-W. Superior rate capabilities of SnS nanosheet electrodes for Li ion batteries. Electrochem. Commun. 2010, 12, 307–310. [Google Scholar] [CrossRef]
- Hassan, F.M.; Hu, Q.; Fu, J.; Batmaz, R.; Li, J.; Yu, A.; Xiao, X.; Chen, Z. Hot-Chemistry Structural Phase Transformation in Single-Crystal Chalcogenides for Long-Life Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 20603–20612. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, G.; Yan, T.; Zhang, J.; Shi, L.; Zhang, D. Sandwich-Like C@SnS@TiO2 Anodes with High Power and Long Cycle for Li-Ion Storage. ACS Appl. Mater. Interfaces 2020, 12, 5857–5865. [Google Scholar] [CrossRef]
- Li, S.; Zheng, J.; Zuo, S.; Wu, Z.; Yan, P.; Pan, F. 2D hybrid anode based on SnS nanosheet bonded with graphene to enhance electrochemical performance for lithium-ion batteries. RSC Adv. 2015, 5, 46941–46946. [Google Scholar] [CrossRef]
- Cai, J.; Li, Z.; Shen, P.K. Porous SnS nanorods/carbon hybrid materials as highly stable and high capacity anode for Li-ion batteries. Appl. Mater. Interfaces 2012, 4, 4093–4098. [Google Scholar] [CrossRef]
- Ke, G.X.; Chen, H.H.; He, J.; Wu, X.C.; Gao, Y.; Li, Y.L.; Mi, H.W.; Zhang, Q.L.; He, C.X.; Ren, X.Z. Ultrathin MoS2 anchored on 3D carbon skeleton containing SnS quantum dots as a high-performance anode for advanced lithium ion batteries. Chem. Eng. J. 2021, 403, 126251. [Google Scholar] [CrossRef]
- Wang, H.; Huang, D.; Yan, Z.; Pan, Q.; Zheng, F.; Huang, Y.; Li, Q. Constructing 2D SnS@C nanosheets anchored on interconnected carbon nanotube networks as advanced anode materials for lithium ion and sodium ion batteries. J. Alloys Compd. 2020, 821, 153551. [Google Scholar] [CrossRef]
- Zhou, T.; Pang, W.K.; Zhang, C.; Yang, J.; Chen, Z.; Liu, H.K.; Guo, Z. Enhanced sodium-ion battery performance by structural phase transition from two-dimensional hexagonal-SnS2 to orthorhombic-SnS. ACS Nano 2014, 8, 8323–8333. [Google Scholar] [CrossRef]
- He, P.L.; Fang, Y.J.; Yu, X.Y.; Lou, X.W.D. Hierarchical Nanotubes Constructed by Carbon-Coated Ultrathin SnS Nanosheets for Fast Capacitive Sodium Storage. Angew. Chem. 2017, 56, 12202–12205. [Google Scholar] [CrossRef]
- Xiong, X.H.; Yang, C.H.; Wang, G.H.; Lin, Y.W.; Ou, X.; Wang, J.H.; Zhao, B.T.; Liu, M.L.; Lin, Z.; Huang, K. SnS nanoparticles electrostatically anchored on three-dimensional N-doped graphene as an active and durable anode for sodium-ion batteries. Energy Environ. Sci. 2017, 10, 1757–1763. [Google Scholar] [CrossRef]
- Hu, R.; Zhu, K.; Ye, K.; Yan, J.; Wang, Q.; Cao, D.X.; Wang, G.L. Influence of potential range selection on the SnS@C/rGO anodes in potassium ion battery. Appl. Surf. Sci. 2021, 536, 147832. [Google Scholar] [CrossRef]
- Li, Y.; Xie, H.; Wang, J. Preparation and electrochemical performances of carbon-coated nanoscale SnS for supercapacitors. J. Solid State Electrochem. 2011, 15, 1115–1119. [Google Scholar] [CrossRef]
- Ravuri, S.; Pandey, C.A.; Ramchandran, R.; Jeon, S.K.; Grace, A.N. Wet Chemical Synthesis of SnS/Graphene Nanocomposites for High Performance Supercapacitor Electrodes. Int. J. Nanosci. 2017, 17, 1760022. [Google Scholar] [CrossRef]
- Obreja, V.; Dinescu, A.; Obreja, A.C. Activated carbon based electrodes in commercial supercapacitors and their performance. Int. Rev. Electr. Eng. 2010, 5, 272–282. [Google Scholar]
- Purkait, T.; Singh, G.; Kumar, D.; Singh, M.; Dey, R.S. High-performance flexible supercapacitors based on electrochemically tailored three-dimensional reduced graphene oxide networks. Sci. Rep. 2018, 8, 640. [Google Scholar] [CrossRef]
- Ren, Q.; Li, B.; Peng, Z.; He, G.; Zhang, W.; Guan, G.; Huang, X.; Xiao, Z.; Liao, L.; Pan, Y.; et al. SnS nanosheets for efficient photothermal therapy. New J. Chem. 2016, 40, 4464–4467. [Google Scholar] [CrossRef]
- Robinson, J.T.; Tabakman, S.M.; Liang, Y.; Wang, H.; Sanchez Casalongue, H.; Vinh, D.; Dai, H. Ultrasmall reduced graphene oxide with high near-infrared absorbance for photothermal therapy. J. Am. Chem. Soc. 2011, 133, 6825–6831. [Google Scholar] [CrossRef]
- Cheng, L.; Liu, J.; Gu, X.; Gong, H.; Shi, X.; Liu, T.; Wang, C.; Wang, X.; Liu, G.; Xing, H.; et al. PEGylated WS2 Nanosheets as a Multifunctional Theranostic Agent for in vivo Dual-Modal CT/Photoacoustic Imaging Guided Photothermal Therapy. Adv. Mater. 2013, 26, 1886–1893. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Xie, H.; Tang, S.; Yu, X.-F.; Guo, Z.; Shao, J.; Zhang, H.; Huang, H.; Wang, H.; Chu, P.K. Ultrasmall Black Phosphorus Quantum Dots: Synthesis and Use as Photothermal Agents. Angew. Chem. 2015, 54, 11526–11530. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Hu, W.; Zhang, H.; Chen, L.; Wang, J.; Wang, Q. Cu5FeS4 nanoparticles with tunable plasmon resonances for efficient photothermal therapy of cancers. Front. Bioeng. Biotechnol. 2020, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Wang, D.; Fan, T.; Xing, C.; Li, Z.; Tao, W.; Liu, L.; Bao, S.; Fan, D.; Zhang, H. Black phosphorus analogue tin sulfide nanosheets: Synthesis and application as near-infrared photothermal agents and drug delivery platforms for cancer therapy. J. Mater. Chem. B 2018, 6, 4747–4755. [Google Scholar] [CrossRef] [PubMed]
- Sebők, D.; Janovák, L.; Kovács, D.; Sápi, A.; Dobó, D.G.; Kukovecz, Á.; Kónya, Z.; Dékány, I. Room temperature ethanol sensor with sub-ppm detection limit: Improving the optical response by using mesoporous silica foam. Sens. Actuators B Chem. 2017, 243, 1205–1213. [Google Scholar] [CrossRef]
- Yan, W.; Chen, Y.; Zeng, X.; Wu, G.; Jiang, W.; Wei, D.; Ling, M.; Ng, K.W.; Qin, Y. Ultrasensitive ethanol sensor based on segregated ZnO-In2O3 porous nanosheets. Appl. Surf. Sci. 2021, 535, 147697. [Google Scholar] [CrossRef]
- Donarelli, M.; Ottaviano, L.; Giancaterini, L.; Fioravanti, G.; Perrozzi, F.; Cantalini, C. Exfoliated black phosphorus gas sensing properties at room temperature. 2D Mater. 2016, 3, 025002. [Google Scholar] [CrossRef]
- Jannat, A.; Haque, F.; Xu, K.; Zhou, C.; Zhang, B.Y.; Syed, N.; Mohiuddin, M.; Messalea, K.A.; Li, X.; Gras, S.L.; et al. Exciton-Driven Chemical Sensors Based on Excitation-Dependent Photoluminescent Two-Dimensional SnS. Appl. Mater. Interfaces 2019, 11, 42462–42468. [Google Scholar] [CrossRef]
- Tang, H.Y.; Li, Y.T.; Ye, H.Y.; Hu, F.F.; Gao, C.S.; Tao, L.Q.; Tu, T.; Gou, G.Y.; Chen, X.P.; Fan, X.J.; et al. High-performance humidity sensor using Schottky-contacted SnS nanoflakes for noncontact healthcare monitoring. Nanotechnology 2020, 31, 055501. [Google Scholar] [CrossRef] [PubMed]
- Awais, M.; Khan, M.U.; Hassan, A.; Bae, J.; Chattha, T.E. Printable Highly Stable and Superfast Humidity Sensor Based on Two Dimensional Molybdenum Diselenide. Sci. Rep. 2020, 10, 5509. [Google Scholar] [CrossRef]
- Karami, H.; Babaei, S. Application of Tin Sulfide-Tin Dioxide Nanocomposite as Oxygen Gas-Sensing Agent. Int. J. Electrochem. Sci. 2013, 8, 12078–12087. [Google Scholar]
- Ellis, J.E.; Sorescu, D.C.; Burkert, S.C.; White, D.L.; Star, A. Uncondensed graphitic carbon nitride on reduced graphene oxide for oxygen sensing via a photoredox mechanism. Appl. Mater. Interfaces 2017, 9, 27142–27151. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, M.; Kan, H.; Li, C.; Quan, A.J.; Fu, C.; Luo, J.T.; Liu, X.L.; Wang, W.; Yang, Z.B.; et al. Surface acoustic wave NO2 sensors utilizing colloidal SnS quantum dot thin films. Surf. Coat. Technol. 2019, 362, 78–83. [Google Scholar] [CrossRef]
- Zhang, Q.X.; Ma, S.Y.; Zhang, R.; Tie, Y.; Pei, S.T. Optimization ethanol detection performance manifested by SnS/SnS2 nanoparticles. Mater. Lett. 2020, 258, 126783. [Google Scholar] [CrossRef]
- Muthuvinayagam, A.; Viswanathan, B. Hydrothermal synthesis and LPG sensing ability of SnS nanomaterial. Indian J. Chem. Sect. A Inorg. Bio Inorg. Phys. Theor. Anal. Chem. 2015, 54, 155–160. [Google Scholar]
- Ma, Y.T.; Ma, S.Y.; Tang, J.; Wu, Z.G.; Shi, J.; Zhao, Y.; Pei, S.T.; Cao, P.F. Hydrothermal-synthesis flower-like SnS microspheres gas sensors bonded physically by PVDF for detecting ethanol. Vacuum 2020, 181, 109657. [Google Scholar] [CrossRef]
- Murugan, E.; Kumar, K. Fabrication of SnS/TiO2@GO Composite Coated Glassy Carbon Electrode for Concomitant Determination of Paracetamol, Tryptophan, and Caffeine in Pharmaceutical Formulations. Anal. Chem. 2019, 91, 5667–5676. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Yu, X.; Kim, Y.K.; Kim, J. Facile Formation of Nanodisk-Shaped Orthorhombic SnS Layers from SnS2 Particles for Photoelectrocatalytic Hydrogen Production. Chem. Nanomater. 2017, 3, 591–600. [Google Scholar] [CrossRef]
- Popczun, E.J.; McKone, J.R.; Read, C.G.; Biacchi, A.J.; Wiltrout, A.M.; Lewis, N.S.; Schaak, R.E. Nanostructured nickel phosphide as an electrocatalyst for the hydrogen evolution reaction. J. Am. Chem. Soc. 2013, 135, 9267–9270. [Google Scholar] [CrossRef]
- Shinde, S.S.; Sami, A.; Kim, D.H.; Lee, J.H. Nanostructured SnS-N-doped graphene as an advanced electrocatalyst for the hydrogen evolution reaction. Chem. Commun. 2015, 51, 15716–15719. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, S.M.; Wu, A.M.; Huang, H.; Zhou, L.M. SnS2 and SnS/SnS2 heterojunction nanosheets prepared by in-situ one-step sulfurization and visible light-assisted electrochemical water splitting properties. J. Alloys Compd. 2020, 834, 155174. [Google Scholar] [CrossRef]
- Roberto, J.C.P.; de Souza, F. Electrochemical hydrogen production from water electrolysis using ionic liquid as electrolytes: Towards the best device. J. Power Sources 2007, 164, 792–798. [Google Scholar]
- Li, K.; Li, Y.; Wang, Y.; Ge, J.; Liu, C.; Xing, W. Enhanced electrocatalytic performance for the hydrogen evolution reaction through surface enrichment of platinum nanoclusters alloying with ruthenium in situ embedded in carbon. Energy Environ. Sci. 2018, 11, 1232–1239. [Google Scholar] [CrossRef]
- Zheng, X.; de Luna, P.; de Arquer, F.P.G.; Zhang, B.; Becknell, N.; Ross, M.B.; Li, Y.; Banis, M.N.; Li, Y.; Liu, M.; et al. Sulfur-Modulated Tin Sites Enable Highly Selective Electrochemical Reduction of CO2 to Formate. Joule 2017, 1, 794–805. [Google Scholar] [CrossRef]
- Huang, X.; Woo, H.; Wu, P.; Hong, H.J.; Jung, W.G.; Kim, B.-J.; Vanel, J.-C.; Choi, J. Simple eco-friendly synthesis of the surfactant free SnS nanocrystal toward the photoelectrochemical cell application W. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jamali-Sheini, F.; Cheraghizade, M.; Yousefi, R. Ultrasonic synthesis of In-doped SnS nanoparticles and their physical properties. Solid State Sci. 2018, 79, 30–37. [Google Scholar] [CrossRef]
- Jamali-Sheini, F.; Yousefi, R.; Bakr, N.A.; Cheraghizade, M.; Sookhakian, M.; Huang, N.M. Highly efficient photo-degradation of methyl blue and band gap shift of SnS nanoparticles under different sonication frequencies. Mater. Sci. Semicond. Process. 2015, 32, 172–178. [Google Scholar] [CrossRef]
- Tang, R.; Su, H.; Sun, Y.; Zhang, X.; Li, L.; Liu, C.; Zeng, S.; Sun, D. Enhanced photocatalytic performance in Bi2WO6/SnS heterostructures: Facile synthesis, influencing factors and mechanism of the photocatalytic process. J. Colloid Interface Sci. 2016, 466, 388–399. [Google Scholar] [CrossRef]
- Kalpana, K.; Selvaraj, V. Development of ZnS/SnS/A-FA nanorods at ambient temperature: Binary catalyst for the removal of congo red dye and pathogenic bacteria from wastewater. J. Ind. Eng. Chem. 2016, 41, 105–113. [Google Scholar] [CrossRef]
- Pomar, C.D.; Souza, A.T.; Sombrio, G.; Souza, F.L.; Bonvent, J.J.; Souza, J.A. Synthesis of SnS and ZnS Hollow Microarchitectures Decorated with Nanostructures and Their Photocatalytic Behavior for Dye Degradation. ChemistrySelect 2018, 3, 3774–3780. [Google Scholar] [CrossRef]
- Yu, W.K.; Shu, F.H.; Huang, Y.F.; Yang, F.Q.; Meng, Q.G.; Zou, Z.; Wang, J.; Zeng, Z.L.; Zou, G.F.; Deng, S.G. Enhanced electrocatalytic nitrogen reduction activity by incorporation of a carbon layer on SnS microflowers. J. Mater. Chem. A 2020, 8, 20677–20686. [Google Scholar] [CrossRef]
- Jiang, M.W.; Han, T.; Zhang, X.J. Hollow C@SnS2/SnS nanocomposites: High efficient oxygen evolution reaction catalysts. J. Colloid Interface Sci. 2021, 583, 149–156. [Google Scholar] [CrossRef]
- Kadam, S.R.; Ghosh, S.; Bar-Ziv, R.; Bar-Sadan, M. Structural Transformation of SnS2 to SnS by Mo Doping Produces Electro/Photocatalyst for Hydrogen Production. Chem. Eur. J. 2020, 26, 6679–6685. [Google Scholar] [CrossRef]
- Sun, H.; Park, S.J. Phosphorus-doped g-C3N4/SnS nanocomposite for efficient photocatalytic reduction of aqueous Cr(VI) under visible light. Appl. Surf. Sci. 2020, 531, 147325. [Google Scholar] [CrossRef]
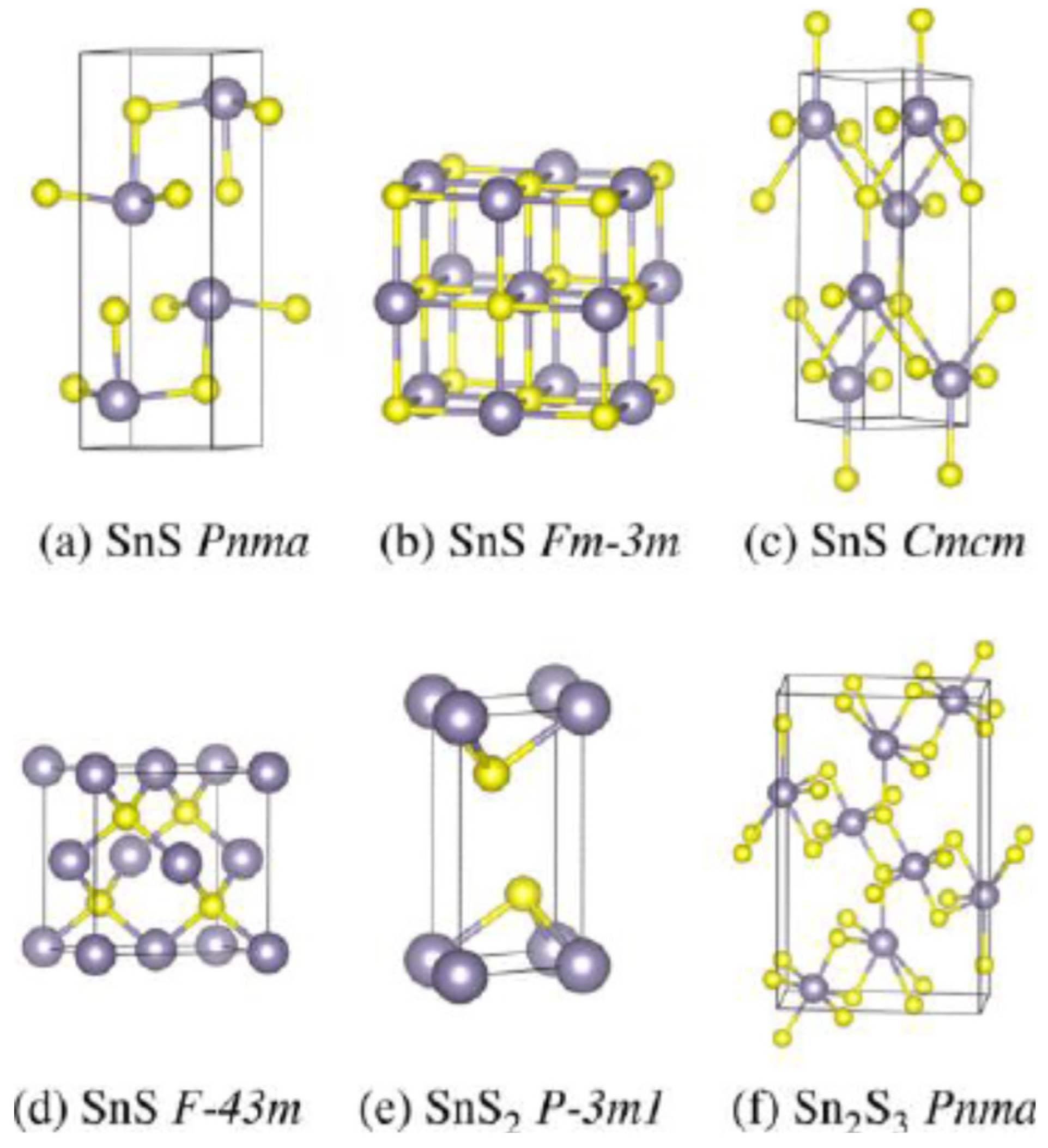




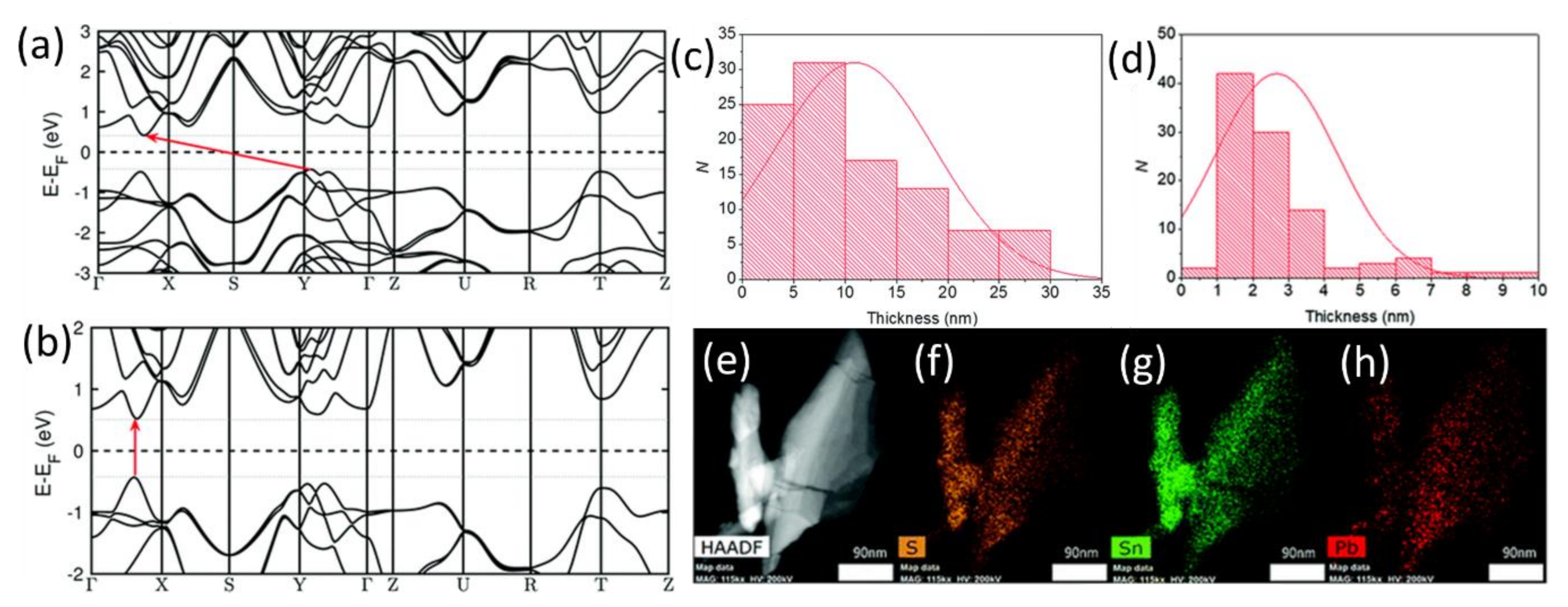


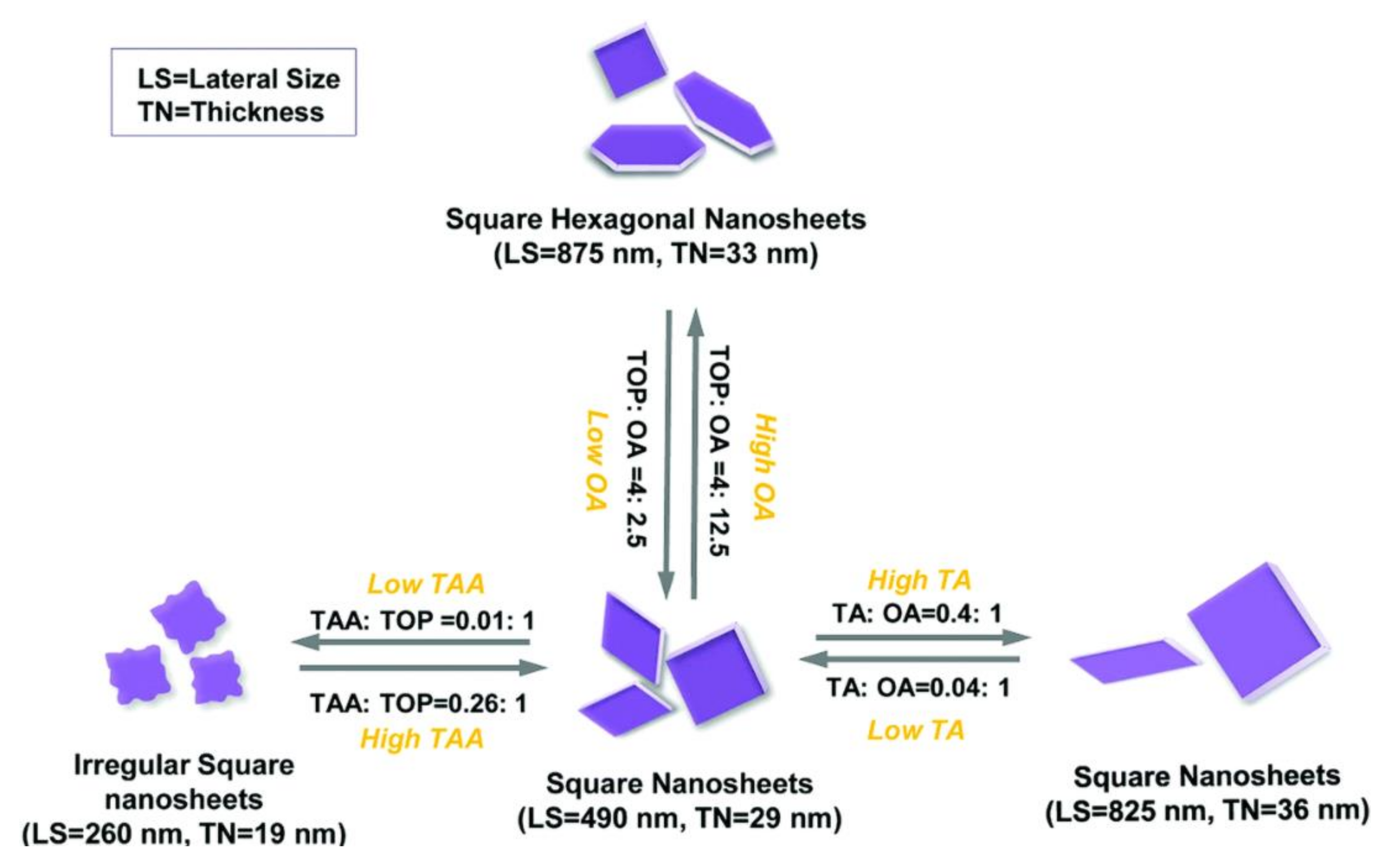
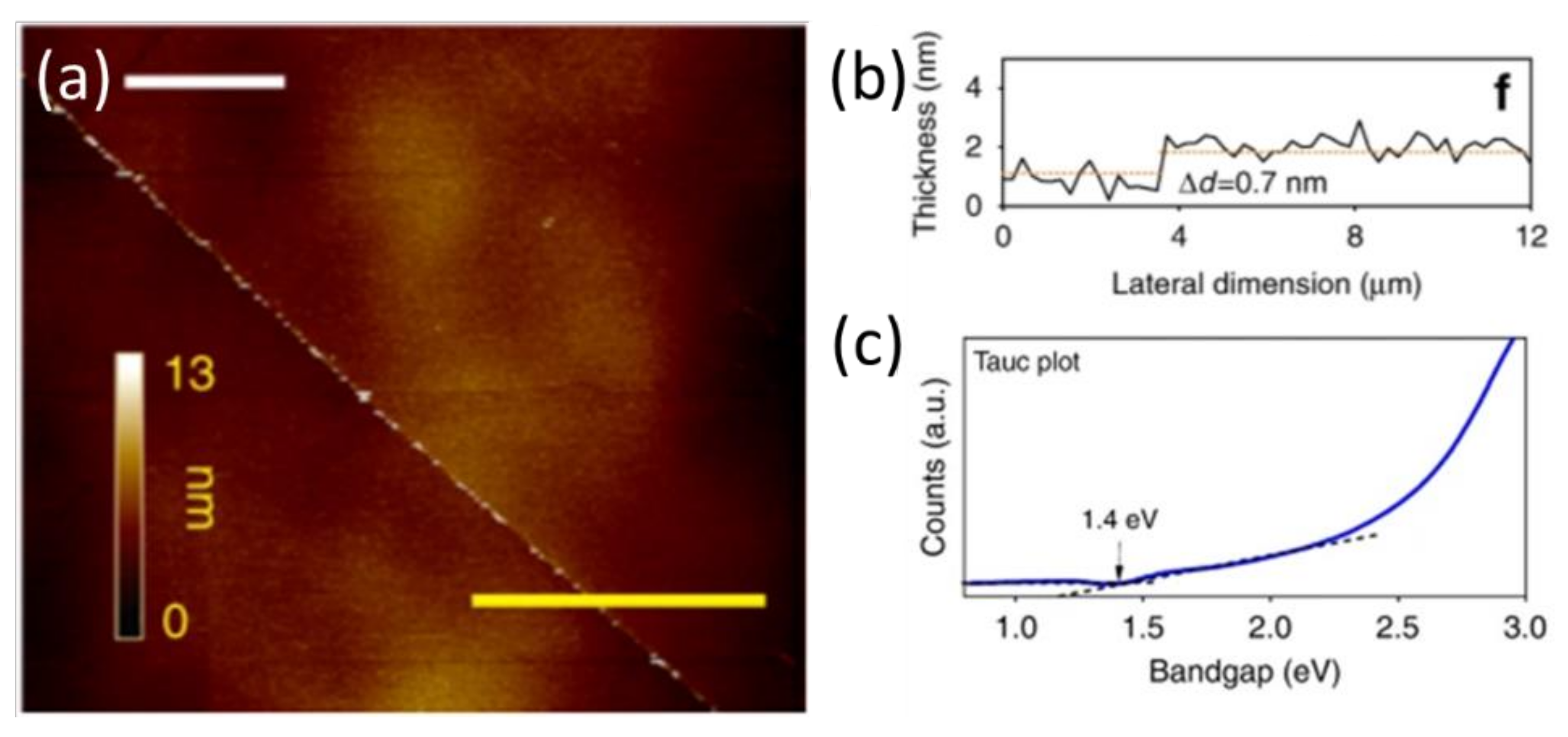
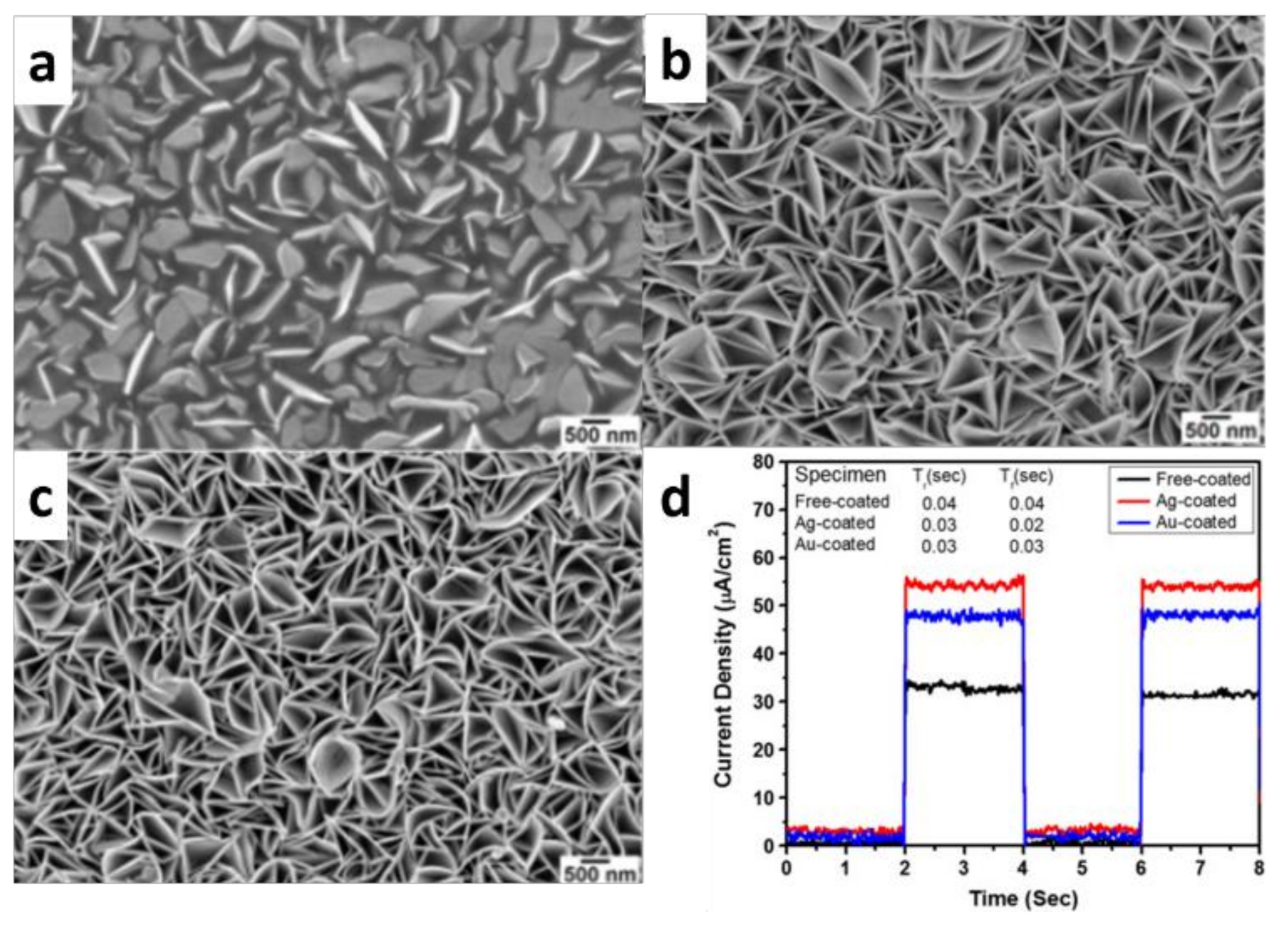
| Phase | Space Group | Experimental Parameters, Å | Relaxed Unit Cell Parameters, Å (% Error) | ΔHf DFT (kJ mol−1) | ΔHf Exp (kJ mol−1) | References |
|---|---|---|---|---|---|---|
| SnS | Pnma | a (11.32) | 11.11 (1.89) | −99.35 | −100 to −108 | [31,34] |
| b (4.05) | 3.99 (1.52) | |||||
| c (4.24) | 4.24 (0.10) | |||||
| Fm3m | a (5.8) | 5.75 (0.87) | −91.66 | Not available | [31,40] | |
| b (5.8) | 5.75 (0.87) | |||||
| c (5.8) | 5.75 (0.87) | |||||
| F43m | a (5.845) | 6.43 (10.0) | −27.80 | Not available | [31,39] | |
| b (5.845) | 6.43 (10.0) | |||||
| c (5.845) | 6.43 (10.0) |
| Phase | Temperature Gradient (°C) | Stoichiometric Ratio Sn:S | Time (Days) |
|---|---|---|---|
| SnS | 850–950 | 0.90:1 | 10 |
| SnS2 | 600–850 | 0.51:1 | 12 |
| Sn2S3 | 500–650 | 0.67:1 | 10 |
| Precursor | Deposition Temperature (°C) | Carrier Concentration, p (× 1018 cm−3) | Resistivity, ρ (Ω cm) | Mobility, µ (cm2 V−1 s−1) |
|---|---|---|---|---|
| 1 | 500 | 4.13 × 1017 | 10.8 | 1.4 |
| 2 | 530 | 3.60 × 1018 | 1.58 | 1.1 |
| 3 | 450 | 8.64 × 1018 | 0.83 | 0.87 |
| Role of SnS Layer | SnS Growth Method | ɳ (%) | VOC (V) | JSC (mA/cm2) | FF (%) | Device Area (cm2) | Reference |
|---|---|---|---|---|---|---|---|
| Absorber | ALD | 4.36 | 0.37 | 20.2 | 58 | 0.24 | [8] |
| Absorber | TE | 3.88 | 0.33 | 20.6 | 56.28 | 0.25 | [69] |
| Absorber | PCVD | 2.90 | 0.26 | 24.9 | 44.4 | 0.25 | [70] |
| CE | TD | 6.56 | 0.74 | 17.7 | 50 | 0.25 | [9] |
| CE | CoSP | 2.00 | 0.46 | 10.8 | 40 | 0.25 | [10] |
| Sensitizer | CBD | 2.81 | 0.85 | 7.4 | 45 | 0.04 | [71] |
| Sensitizer | Spin Coat | 1.20 | 0.27 | 11.6 | 38 | NA | [72] |
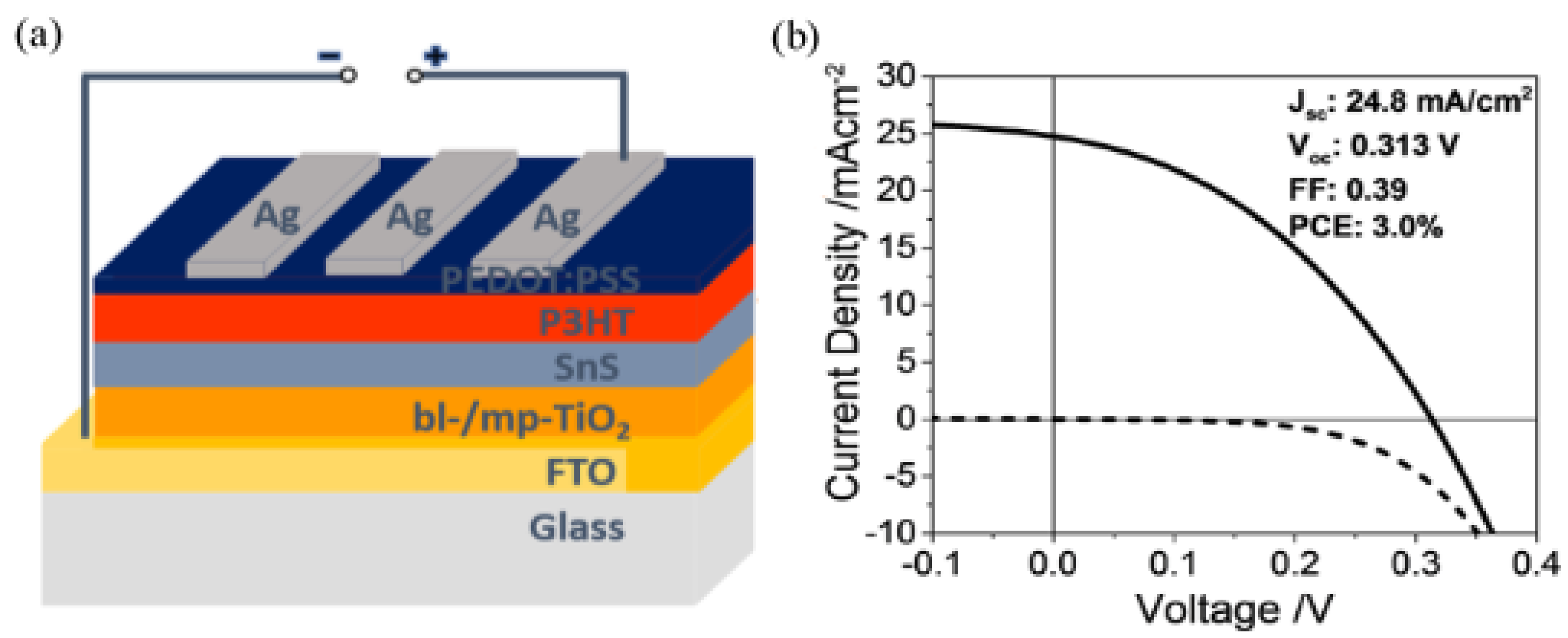
| Sensitizer | Spin Coat | 3.00 | 0.31 | 24.8 | 39 | 0.1 | [5] |
| EKS | Δxc | Eg(i) | Eg(d) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Single layer | 1.78 | 0.79 | 2.57 | 2.72 | 0.23 | 0.2 | 1.11 | 0.77 | 0.31 | 0.27 |
| Double layer | 1.11 | 0.47 | 1.57 | 1.98 | 0.19 | 0.23 | 1.06 | 1.13 | 0.23 | 0.55 |
| Bulk | 0.75 | 0.32 | 1.07 | 1.32 | 0.16 | 0.2 | 0.91 | 1.36 | 0.19 | 0.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Norton, K.J.; Alam, F.; Lewis, D.J. A Review of the Synthesis, Properties, and Applications of Bulk and Two-Dimensional Tin (II) Sulfide (SnS). Appl. Sci. 2021, 11, 2062. https://doi.org/10.3390/app11052062
Norton KJ, Alam F, Lewis DJ. A Review of the Synthesis, Properties, and Applications of Bulk and Two-Dimensional Tin (II) Sulfide (SnS). Applied Sciences. 2021; 11(5):2062. https://doi.org/10.3390/app11052062
Chicago/Turabian StyleNorton, Kane J., Firoz Alam, and David J. Lewis. 2021. "A Review of the Synthesis, Properties, and Applications of Bulk and Two-Dimensional Tin (II) Sulfide (SnS)" Applied Sciences 11, no. 5: 2062. https://doi.org/10.3390/app11052062
APA StyleNorton, K. J., Alam, F., & Lewis, D. J. (2021). A Review of the Synthesis, Properties, and Applications of Bulk and Two-Dimensional Tin (II) Sulfide (SnS). Applied Sciences, 11(5), 2062. https://doi.org/10.3390/app11052062






