Work Function Tuning in Hydrothermally Synthesized Vanadium-Doped MoO3 and Co3O4 Mesostructures for Energy Conversion Devices
Abstract
1. Introduction
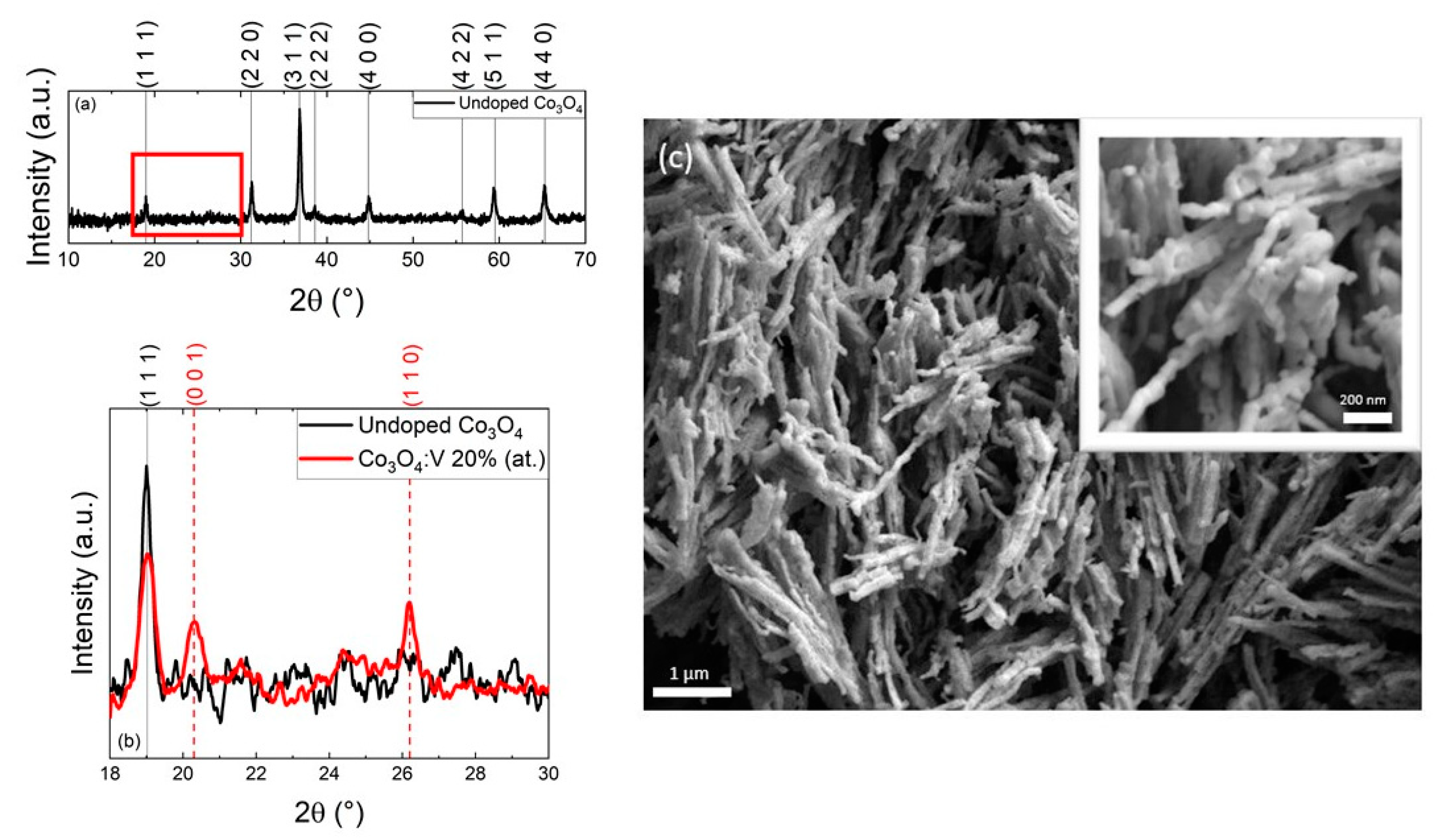
2. Results and Discussion
3. Conclusions
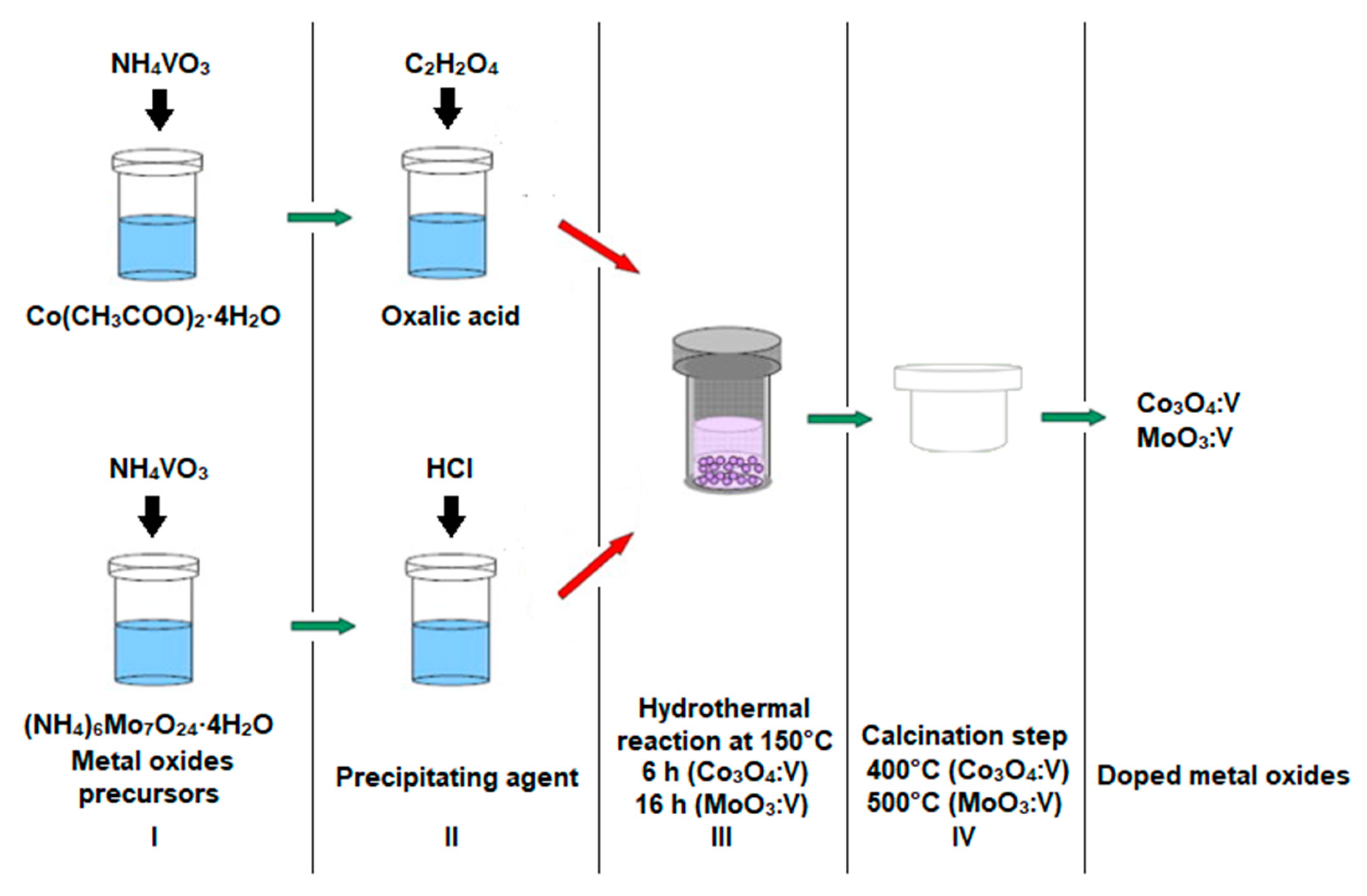
4. Experimental Section
Hydrothermal Synthesis of V-Doped Co3O4 and V-Doped MoO3
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cox, P.A. Transition Metal Oxides: An Introduction to Their Electronic Structure and Properties; International Series of Monographs on Chemistry; OUP: Oxford, UK, 2010; ISBN 9780199588947. [Google Scholar]
- Rao, C.N.R.; Raveau, B. Transition Metal Oxides: Structure, Properties, and Synthesis of Ceramic Oxides, 2nd ed.; Wiley VCH: Weinheim, Germany, 1998; ISBN 978-0-471-18971-8. [Google Scholar]
- Greenwood, N.N.; Earnshaw, A. Chemistry of the Elements; Elsevier: Amsterdam, The Netherlands, 1997; ISBN 9780750633659. [Google Scholar]
- Guo, T.; Yao, M.-S.; Lin, Y.-H.; Nan, C.-W. A comprehensive review on synthesis methods for transition-metal oxide nanostructures. CrystEngComm 2015, 17, 3551–3585. [Google Scholar] [CrossRef]
- Danish, M.S.S.; Bhattacharya, A.; Stepanova, D.; Mikhaylov, A.; Grilli, M.L.; Khosravy, M.; Senjyu, T. A Systematic Review of Metal Oxide Applications for Energy and Environmental Sustainability. Metals 2020, 10, 1604. [Google Scholar] [CrossRef]
- Bushell, M.; Beauchemin, S.; Kunc, F.; Gardner, D.; Ovens, J.; Toll, F.; Kennedy, D.; Nguyen, K.; Vladisavljevic, D.; Rasmussen, P.E.; et al. Characterization of Commercial Metal Oxide Nanomaterials: Crystalline Phase, Particle Size and Specific Surface Area. Nanomaterials 2020, 10, 1812. [Google Scholar] [CrossRef] [PubMed]
- Della Giustina, G.; Zambon, A.; Lamberti, F.; Elvassore, N.; Brusatin, G. Straightforward Micropatterning of Oligonucleotides in Microfluidics by Novel Spin-On ZrO2 Surfaces. ACS Appl. Mater. Interfaces 2015, 7, 13280–13288. [Google Scholar] [CrossRef]
- Lamberti, F.; Brigo, L.; Favaro, M.; Luni, C.; Zoso, A.; Cattelan, M.; Agnoli, S.; Brusatin, G.; Granozzi, G.; Giomo, M.; et al. Optoelectrochemical Biorecognition by Optically Transparent Highly Conductive Graphene-Modified Fluorine-Doped Tin Oxide Substrates. ACS Appl. Mater. Interfaces 2014, 6, 22769–22777. [Google Scholar] [CrossRef] [PubMed]
- Chavali, M.S.; Nikolova, M.P. Metal oxide nanoparticles and their applications in nanotechnology. SN Appl. Sci. 2019, 1, 607. [Google Scholar] [CrossRef]
- Pérez-Tomás, A.; Mingorance, A.; Tanenbaum, D.; Lira-Cantú, M. Chapter 8—Metal Oxides in Photovoltaics: All-Oxide, Ferroic, and Perovskite Solar Cells. In Metal Oxides; Lira-Cantu, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 267–356. ISBN 978-0-12-811165-9. [Google Scholar]
- Girardi, L.; Bardini, L.; Michieli, N.; Kalinic, B.; Maurizio, C.; Rizzi, G.; Mattei, G. Co3O4 Nanopetals on Si as Photoanodes for the Oxidation of Organics. Surfaces 2019, 2, 41–53. [Google Scholar] [CrossRef]
- Kim, J.; Iivonen, T.; Hämäläinen, J.; Kemell, M.; Meinander, K.; Mizohata, K.; Wang, L.; Räisänen, J.; Beranek, R.; Leskelä, M.; et al. Low-Temperature Atomic Layer Deposition of Cobalt Oxide as an Effective Catalyst for Photoelectrochemical Water-Splitting Devices. Chem. Mater. 2017, 29, 5796–5805. [Google Scholar] [CrossRef]
- Mai, L.; Yang, F.; Zhao, Y.; Xu, X.; Xu, L.; Hu, B.; Luo, Y.; Liu, H. Molybdenum oxide nanowires: Synthesis & properties. Mater. Today 2011, 14, 346–353. [Google Scholar]
- Haber, J.; Lalik, E. Catalytic properties of MoO3 revisited. Catal. Today 1997, 33, 119–137. [Google Scholar] [CrossRef]
- Mane, A.A.; Nikam, S.A.; Moholkar, A.V. NO2 gas sensing properties of sprayed composite porous MoO3-V2O5 thin films. Mater. Chem. Phys. 2018, 216, 294–304. [Google Scholar] [CrossRef]
- Bhatia, S.; Khanna, A. Structural and Optical Properties of Molybdenum Trioxide Thin Films. AIP Conf. Proc. 2015, 1665, 080057. [Google Scholar]
- Guo, Y.; Robertson, J. Origin of the high work function and high conductivity of MoO 3. Appl. Phys. Lett. 2014, 105, 222110. [Google Scholar] [CrossRef]
- Tao, C.; Ruan, S.; Zhang, X.; Xie, G.; Shen, L.; Kong, X.; Dong, W.; Liu, C.; Chen, W. Performance improvement of inverted polymer solar cells with different top electrodes by introducing a MoO3 buffer layer. Appl. Phys. Lett. 2008, 93, 193307. [Google Scholar] [CrossRef]
- De Melo, O.; Torres-Costa, V.; Climent-Font, A.; Galán, P.; Ruediger, A.; Sánchez, M.; Calvo-Mola, C.; Santana, G.; Torres-Costa, V. Optical and electrical properties of MoO2 and MoO3 thin films prepared from the chemically driven isothermal close space vapor transport technique. J. Phys. Condens. Matter 2019, 31, 295703. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.K.; Gouma, P.I.; Kubinski, D.J.; Visser, J.H.; Soltis, R.E.; Schmitz, P.J. Reactively sputtered MoO3 films for ammonia sensing. Thin Solid Films 2003, 436, 46–51. [Google Scholar] [CrossRef]
- Kupfer, B.; Majhi, K.; Keller, D.A.; Bouhadana, Y.; Rühle, S.; Barad, H.N.; Anderson, A.Y.; Zaban, A. Thin Film Co3O4/TiO2 Heterojunction Solar Cells. Adv. Energy Mater. 2015, 5, 1401007. [Google Scholar] [CrossRef]
- Rühle, S.; Anderson, A.Y.; Barad, H.-N.; Kupfer, B.; Bouhadana, Y.; Rosh-Hodesh, E.; Zaban, A. All-Oxide Photovoltaics. J. Phys. Chem. Lett. 2012, 3, 3755–3764. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, X.; Lu, X.; Gao, X.; Gao, J.; Shui, L.; Wu, S.; Liu, J. Promoting the Hole Extraction with Co3O4 Nanomaterials for Efficient Carbon-Based CsPbI 2 Br Perovskite Solar Cells. Sol. RRL 2019, 3, 1800315. [Google Scholar] [CrossRef]
- Bashir, A.; Shukla, S.; Lew, J.H.; Shukla, S.; Bruno, A.; Gupta, D.; Baikie, T.; Patidar, R.; Akhter, Z.; Priyadarshi, A.; et al. Spinel Co 3 O 4 nanomaterials for efficient and stable large area carbon-based printed perovskite solar cells. Nanoscale 2018, 10, 2341–2350. [Google Scholar] [CrossRef] [PubMed]
- Righetto, M.; Meggiolaro, D.; Rizzo, A.; Sorrentino, R.; He, Z.; Meneghesso, G.; Sum, T.C.; Gatti, T.; Lamberti, F. Coupling halide perovskites with different materials: From doping to nanocomposites, beyond photovoltaics. Prog. Mater. Sci. 2020, 110, 100639. [Google Scholar] [CrossRef]
- Yuhas, B.D.; Zitoun, D.O.; Pauzauskie, P.J.; He, R.; Yang, P. Transition-Metal Doped Zinc Oxide Nanowires. Angew. Chem. Int. Ed. 2006, 45, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Jansons, A.W.; Koskela, K.M.; Crockett, B.M.; Hutchison, J.E. Transition Metal-Doped Metal Oxide Nanocrystals: Efficient Substitutional Doping through a Continuous Growth Process. Chem. Mater. 2017, 29, 8167–8176. [Google Scholar] [CrossRef]
- Stavale, F.; Shao, X.; Nilius, N.; Freund, H.-J.; Prada, S.; Giordano, L.; Pacchioni, G. Donor Characteristics of Transition-Metal-Doped Oxides: Cr-Doped MgO versus Mo-Doped CaO. J. Am. Chem. Soc. 2012, 134, 11380–11383. [Google Scholar] [CrossRef] [PubMed]
- Hermann, K.; Witko, M.; Druzinic, R.; Chakrabarti, A.; Tepper, B.; Elsner, M.; Gorschlüter, A.; Kuhlenbeck, H.; Freund, H.J. Properties and identification of oxygen sites at the V2O5(010) surface: Theoretical cluster studies and photoemission experiments. J. Electron Spectrosc. Relat. Phenom. 1999, 98–99, 245–256. [Google Scholar] [CrossRef]
- Qu, G.; Wang, J.; Liu, G.; Tian, B.; Su, C.; Chen, Z.; Rueff, J.-P.; Wang, Z. Vanadium Doping Enhanced Electrochemical Performance of Molybdenum Oxide in Lithium-Ion Batteries. Adv. Funct. Mater. 2019, 29, 1805227. [Google Scholar] [CrossRef]
- Singh, P.K.; Singh, N.; Singh, M.; Tandon, P.; Singh, S.K. Preparation of Nanostructured Co3O4 and Ru-Doped Co3O4 and Their Applicability in Liquefied Petroleum Gas Sensing. J. Mater. Eng. Perform. 2019, 28, 7592–7601. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Z.; Wang, J.; Xiao, Z.; Huang, X.; Liu, Z.; Wang, S. N-doped nanoporous Co 3 O 4 nanosheets with oxygen vacancies as oxygen evolving electrocatalysts. Nanotechnology 2017, 28, 165402. [Google Scholar] [CrossRef] [PubMed]
- Shaban, M.; El Sayed, A.M. Influence of the spin deposition parameters and La/Sn double doping on the structural, optical, and photoelectrocatalytic properties of CoCo2O4 photoelectrodes. Sol. Energy Mater. Sol. Cells 2020, 217, 110705. [Google Scholar] [CrossRef]
- Venkatesh, R.; Dhas, C.R.; Sivakumar, R.; Dhandayuthapani, T.; Subramanian, B.; Sanjeeviraja, C.; Raj, A.M.E. Tailoring the physical properties and electrochromic performance of nebulizer spray coated Co3O4 films through copper doping. Solid State Ion. 2019, 334, 5–13. [Google Scholar] [CrossRef]
- Švegl, F.; Orel, B.; Grabec-Švegl, I.; Kaučič, V. Characterization of spinel Co3O4 and Li-doped Co3O4 thin film electrocatalysts prepared by the sol–gel route. Electrochim. Acta 2000, 45, 4359–4371. [Google Scholar] [CrossRef]
- Xing, Z.; Wu, H.; Wu, L.; Wang, X.; Zhong, H.; Li, F.; Shi, J.; Song, D.; Xiao, W.; Jiang, C.; et al. A multifunctional vanadium-doped cobalt oxide layer on silicon photoanodes for efficient and stable photoelectrochemical water oxidation. J. Mater. Chem. A 2018, 6, 21167–21177. [Google Scholar] [CrossRef]
- El-Shobaky, G.A.; Turky, A.E.-M.M. Catalytic decomposition of H2O2 on Co3O4 doped with MgO and V2O5. Colloids Surf. A Physicochem. Eng. Asp. 2000, 170, 161–172. [Google Scholar] [CrossRef]
- Ali, F.; Khalid, N.R. Effect of calcination temperature on structural, morphological and electrochemical properties of Sn doped Co3O4 nanorods. Ceram. Int. 2020, 46, 24137–24146. [Google Scholar] [CrossRef]
- Aadil, M.; Zulfiqar, S.; Shahid, M.; Haider, S.; Shakir, I.; Warsi, M.F. Binder free mesoporous Ag-doped Co3O4 nanosheets with outstanding cyclic stability and rate capability for advanced supercapacitor applications. J. Alloys Compd. 2020, 844, 156062. [Google Scholar] [CrossRef]
- Jiang, T.; Yin, N.; Bai, Z.; Dai, P.; Yu, X.; Wu, M.; Li, G. Wet chemical synthesis of S doped Co3O4 nanosheets/reduced graphene oxide and their application in dye sensitized solar cells. Appl. Surf. Sci. 2018, 450, 219–227. [Google Scholar] [CrossRef]
- Wei, R.; Bu, X.; Gao, W.; Villaos, R.A.B.; Macam, G.; Huang, Z.-Q.; Lan, C.; Chuang, F.-C.; Qu, Y.; Ho, J.C. Engineering Surface Structure of Spinel Oxides via High-Valent Vanadium Doping for Remarkably Enhanced Electrocatalytic Oxygen Evolution Reaction. ACS Appl. Mater. Interfaces 2019, 11, 33012–33021. [Google Scholar] [CrossRef]
- Peelaers, H.; Chabinyc, M.L.; Van de Walle, C.G. Controlling n -Type Doping in MoO 3. Chem. Mater. 2017, 29, 2563–2567. [Google Scholar] [CrossRef]
- Patil, M.K.; Gaikwad, S.H.; Mukherjee, S.P. Phase- and Morphology-Controlled Synthesis of Tunable Plasmonic MoO3–x Nanomaterials for Ultrasensitive Surface-Enhanced Raman Spectroscopy Detection. J. Phys. Chem. C 2020, 124, 21082–21093. [Google Scholar] [CrossRef]
- Shen, J.; Guo, S.; Chen, C.; Sun, L.; Wen, S.; Chen, Y.; Ruan, S. Synthesis of Ni-doped A-MoO3 nanolamella and their improved gas sensing properties. Sens. Actuators B Chem. 2017, 252, 757–763. [Google Scholar] [CrossRef]
- Rammal, M.B.; Omanovic, S. Synthesis and characterization of NiO, MoO3, and NiMoO4 nanostructures through a green, facile method and their potential use as electrocatalysts for water splitting. Mater. Chem. Phys. 2020, 255, 123570. [Google Scholar] [CrossRef]
- Ahmad, H.; Afzal, N.; Rafique, M.; Ahmed, A.A.; Ahmad, R.; Khaliq, Z. Post-deposition annealed MoO3 film based high performance MSM UV photodetector fabricated on Si (100). Ceram. Int. 2020, 46, 20477–20487. [Google Scholar] [CrossRef]
- Phuruangrat, A.; Chen, J.S.; Lou, X.W.; Yayapao, O.; Thongtem, S.; Thongtem, T. Hydrothermal synthesis and electrochemical properties of α-MoO 3 nanobelts used as cathode materials for Li-ion batteries. Appl. Phys. A Mater. Sci. Process. 2012, 107, 249–254. [Google Scholar] [CrossRef]
- Yang, X.; Ding, H.; Zhang, D.; Yan, X.; Lu, C.; Qin, J.; Zhang, R.; Tang, H.; Song, H. Hydrothermal synthesis of MoO3 nanobelt-graphene composites. Cryst. Res. Technol. 2011, 46, 1195–1201. [Google Scholar] [CrossRef]
- Qin, P.; Fang, G.; Cheng, F.; Ke, W.; Lei, H.; Wang, H.; Zhao, X. Sulfur-doped molybdenum oxide anode interface layer for organic solar cell application. ACS Appl. Mater. Interfaces 2014, 6, 2963–2973. [Google Scholar] [CrossRef]
- Layegh, M.; Ghodsi, F.E.; Hadipour, H. Experimental and theoretical study of Fe doping as a modifying factor in electrochemical behavior of mixed-phase molybdenum oxide thin films. Appl. Phys. A Mater. Sci. Process. 2020, 126, 1–14. [Google Scholar] [CrossRef]
- Li, Z.; Wang, W.; Zhao, Z.; Liu, X.; Song, P. Facile synthesis and enhanced trimethylamine sensing performances of W-doped MoO3 nanobelts. Mater. Sci. Semicond. Process. 2017, 66, 33–38. [Google Scholar] [CrossRef]
- Cheyns, D.; Kam, B.; Vasseur, K.; Heremans, P.; Rand, B.P. Structure induced conductivity enhancement in metal-doped molybdenum oxide thin films. J. Appl. Phys. 2013, 113, 043109. [Google Scholar] [CrossRef]
- Deori, K.; Deka, S. Morphology oriented surfactant dependent CoO and reaction time dependent Co3O4 nanocrystals from single synthesis method and their optical and magnetic properties. CrystEngComm 2013, 15, 8465. [Google Scholar] [CrossRef]
- Vijayakumar, Y.; Mani, G.K.; Reddy, M.V.R.; Rayappan, J.B.B. Nanostructured flower like V2O5 thin films and its room temperature sensing characteristics. Ceram. Int. 2015, 41, 2221–2227. [Google Scholar] [CrossRef]
- Lester, E.; Aksomaityte, G.; Li, J.; Gomez, S.; Gonzalez-Gonzalez, J.; Poliakoff, M. Controlled continuous hydrothermal synthesis of cobalt oxide (Co3O4) nanoparticles. Prog. Cryst. Growth Charact. Mater. 2012, 58, 3–13. [Google Scholar] [CrossRef]
- Yao, Y.; Yang, Z.; Sun, H.; Wang, S. Hydrothermal Synthesis of Co3O4–Graphene for Heterogeneous Activation of Peroxymonosulfate for Decomposition of Phenol. Ind. Eng. Chem. Res. 2012, 51, 14958–14965. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, J.; Cheng, C.; Li, H.; Zhang, J.; Gong, H.; Fan, H.J. Co3O4 Nanowire@MnO2 Ultrathin Nanosheet Core/Shell Arrays: A New Class of High-Performance Pseudocapacitive Materials. Adv. Mater. 2011, 23, 2076–2081. [Google Scholar] [CrossRef]
- Chen, Z.; Cummins, D.; Reinecke, B.N.; Clark, E.; Sunkara, M.K.; Jaramillo, T.F. Core–shell MoO3–MoS2 Nanowires for Hydrogen Evolution: A Functional Design for Electrocatalytic Materials. Nano Lett. 2011, 11, 4168–4175. [Google Scholar] [CrossRef]
- Chiang, T.; Yeh, H. The Synthesis of α-MoO3 by Ethylene Glycol. Materials 2013, 6, 4609–4625. [Google Scholar] [CrossRef]
- Barreca, D.; Massignan, C.; Daolio, S.; Fabrizio, M.; Piccirillo, C.; Armelao, L.; Tondello, E. Composition and Microstructure of Cobalt Oxide Thin Films Obtained from a Novel Cobalt(II) Precursor by Chemical Vapor Deposition. Chem. Mater. 2001, 13, 588–593. [Google Scholar] [CrossRef]
- Xiao, Z.; Wang, Y.; Huang, Y.-C.; Wei, Z.; Dong, C.-L.; Ma, J.; Shen, S.; Li, Y.; Wang, S. Filling the oxygen vacancies in Co3O4 with phosphorus: An ultra-efficient electrocatalyst for overall water splitting. Energy Environ. Sci. 2017, 10, 2563–2569. [Google Scholar] [CrossRef]
- Xiao, Z.; Huang, Y.-C.; Dong, C.-L.; Xie, C.; Liu, Z.; Du, S.; Chen, W.; Yan, D.; Tao, L.; Shu, Z.; et al. Operando Identification of the Dynamic Behavior of Oxygen Vacancy-Rich Co3O4 for Oxygen Evolution Reaction. J. Am. Chem. Soc. 2020, 142, 12087–12095. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Mao, H.; Xu, Y.; Liu, J. Oxygen vacancies confined in Co3O4 quantum dots for promoting oxygen evolution electrocatalysis. Inorg. Chem. Front. 2019, 6, 2055–2060. [Google Scholar] [CrossRef]
- Kim, M.; Alfano, A.; Perotto, G.; Serri, M.; Dengo, N.; Mezzetti, A.; Gross, S.; Prato, M.; Salerno, M.; Rizzo, A.; et al. Moisture resistance in perovskite solar cells attributed to a water-splitting layer. Commun. Mater. 2021, 2, 6. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How to Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV-Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [PubMed]
- George, G.; Anandhan, S. A comparative study on the physico–chemical properties of sol–gel electrospun cobalt oxide nanofibres from two different polymeric binders. RSC Adv. 2015, 5, 81429–81437. [Google Scholar] [CrossRef]
- Vogt, K.T.; Malmberg, C.E.; Buchanan, J.C.; Mattson, G.W.; Brandt, G.M.; Fast, D.B.; Cheong, P.H.-Y.; Wager, J.F.; Graham, M.W. Ultrabroadband density of states of amorphous In-Ga-Zn-O. Phys. Rev. Res. 2020, 2, 033358. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Gerling, L.; Mahato, S.; Voz, C.; Alcubilla, R.; Puigdollers, J. Characterization of Transition Metal Oxide/Silicon Heterojunctions for Solar Cell Applications. Appl. Sci. 2015, 5, 695–705. [Google Scholar] [CrossRef]
- DIFFRAC.EVA, Bruker. 2018. Available online: https://www.bruker.com/eva n.d (accessed on 2 October 2020).
- Coelho, A.A. TOPAS-Academic; Coelho Software: Brisbane, Australia, 2007. [Google Scholar]
- OriginPro, Version 2021; OriginLab Corporation: Northampton, MA, USA, 2021.
- Liu, X.; Gao, J.; Chen, Y.; Li, C.; Chen, J.; Qu, W.; Chen, X.; Ma, Z.; Tang, X. Rational Design of Alkali-Resistant NO Reduction Catalysts using a Stable Hexagonal V-Doped MoO 3 Support for Alkali Trapping. ChemCatChem 2018, 10, 3999–4003. [Google Scholar] [CrossRef]

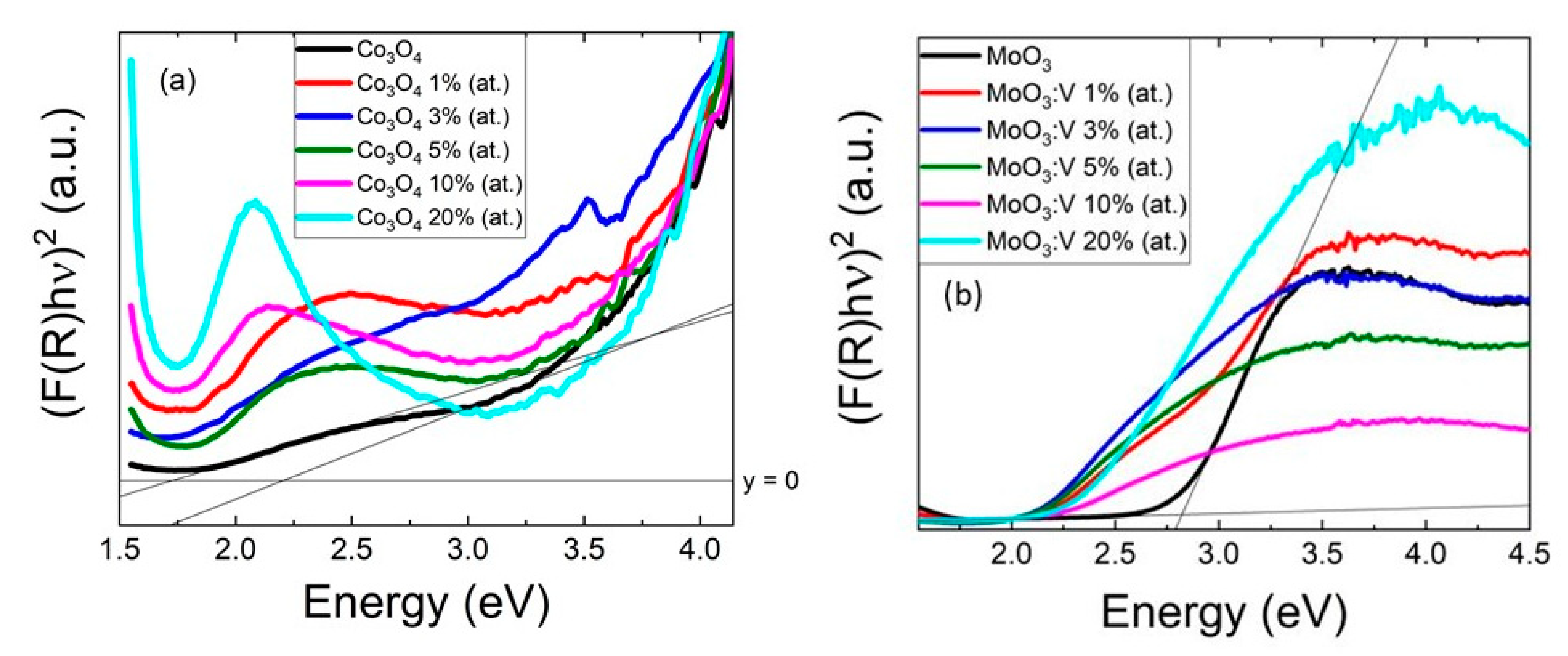
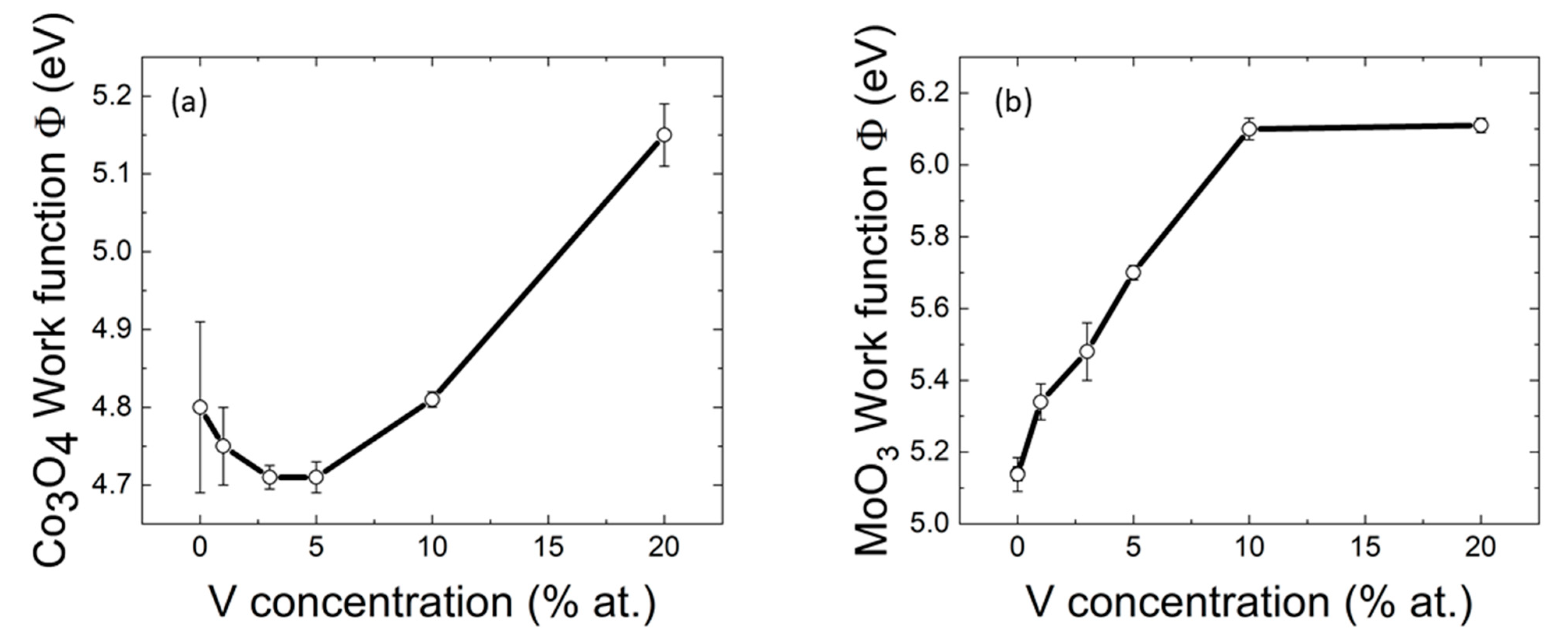
| Sample | V % 1 | Eg (eV) 2 (±4%) | WF (eV) 4 |
|---|---|---|---|
| Undoped Co3O4 | - | 1.74; 2.24 3 | 4.8 ± 0.11 |
| Co3O4:V (1% at) | <1 | 1.50; 2.20 3 | 4.75 ± 0.05 |
| Co3O4:V (3% at) | <1 | 1.50; 2.20 3 | 4.71 ± 0.02 |
| Co3O4:V (5% at) | <1 | 1.65; 2.14 3 | 4.71 ± 0.02 |
| Co3O4:V (10% at) | <1 | 1.48; 2.35 3 | 4.81 ± 0.01 |
| Co3O4:V (20% at) | 12 | 1.63; 2.62 3 | 5.15 ± 0.04 |
| Undoped MoO3 | - | 2.8 | 5.14 ± 0.05 |
| MoO3:V (1% at) | 1 | 2.3 | 5.34 ± 0.05 |
| MoO3:V (3% at) | 3 | 2.1 | 5.48 ± 0.08 |
| MoO3:V (5% at) | 4 | 2.1 | 5.70 ± 0.02 |
| MoO3:V (10% at) | 6 | 2.2 | 6.10 ± 0.03 |
| MoO3:V (20% at) | 13 | 2.3 | 6.11 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dalle Feste, P.; Crisci, M.; Barbon, F.; Tajoli, F.; Salerno, M.; Drago, F.; Prato, M.; Gross, S.; Gatti, T.; Lamberti, F. Work Function Tuning in Hydrothermally Synthesized Vanadium-Doped MoO3 and Co3O4 Mesostructures for Energy Conversion Devices. Appl. Sci. 2021, 11, 2016. https://doi.org/10.3390/app11052016
Dalle Feste P, Crisci M, Barbon F, Tajoli F, Salerno M, Drago F, Prato M, Gross S, Gatti T, Lamberti F. Work Function Tuning in Hydrothermally Synthesized Vanadium-Doped MoO3 and Co3O4 Mesostructures for Energy Conversion Devices. Applied Sciences. 2021; 11(5):2016. https://doi.org/10.3390/app11052016
Chicago/Turabian StyleDalle Feste, Pietro, Matteo Crisci, Federico Barbon, Francesca Tajoli, Marco Salerno, Filippo Drago, Mirko Prato, Silvia Gross, Teresa Gatti, and Francesco Lamberti. 2021. "Work Function Tuning in Hydrothermally Synthesized Vanadium-Doped MoO3 and Co3O4 Mesostructures for Energy Conversion Devices" Applied Sciences 11, no. 5: 2016. https://doi.org/10.3390/app11052016
APA StyleDalle Feste, P., Crisci, M., Barbon, F., Tajoli, F., Salerno, M., Drago, F., Prato, M., Gross, S., Gatti, T., & Lamberti, F. (2021). Work Function Tuning in Hydrothermally Synthesized Vanadium-Doped MoO3 and Co3O4 Mesostructures for Energy Conversion Devices. Applied Sciences, 11(5), 2016. https://doi.org/10.3390/app11052016










