In Vitro Model for Lumbar Disc Herniation to Investigate Regenerative Tissue Repair Approaches
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimens
2.2. New Physiological Loading Protocol
2.3. Defects
2.4. Flexibility Tests
2.5. Statistical Analysis
3. Results
3.1. Provocation of Disc Herniation
3.1.1. Influence of Shape and Size of the Annular Defect
3.1.2. Influence of Daily-Life Activities
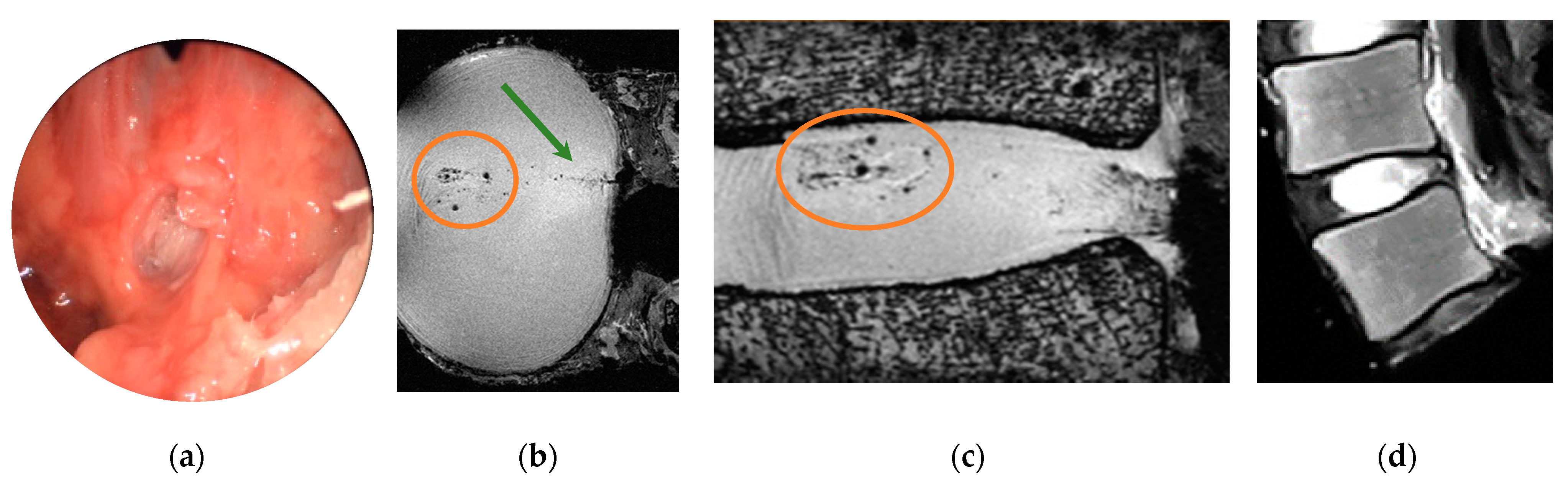
3.2. Ultra-High Field MR Imaging
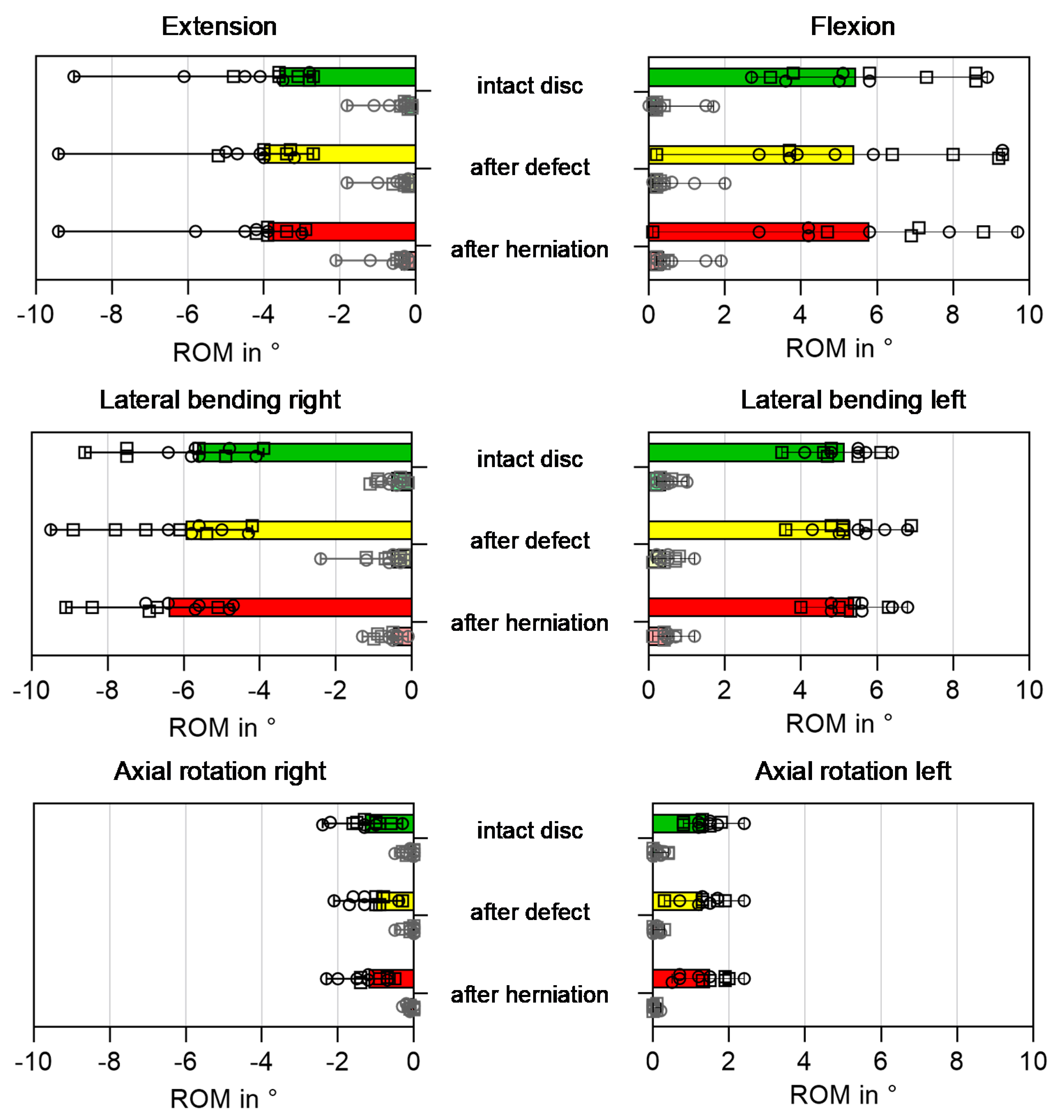
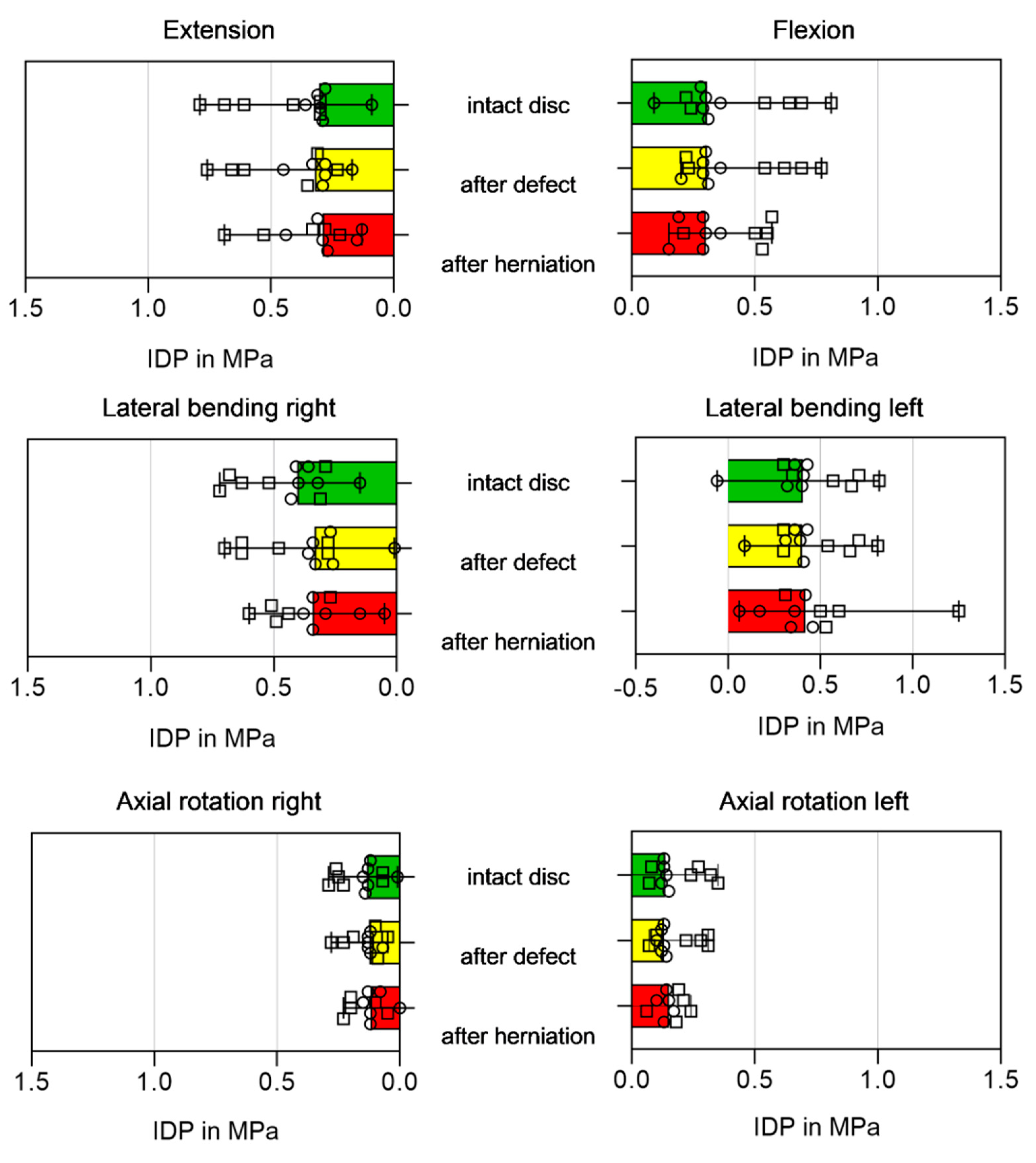
3.3. Biomechanical Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Evans, W.; Jobe, W.; Seibert, C. A cross-sectional prevalence study of lumbar disc degeneration in a working population. Spine 1989, 14, 60–64. [Google Scholar] [CrossRef]
- Adams, M.A.; Hutton, W.C. The mechanics of prolapsed intervertebral disc. Int. Orthop. 1982, 6, 249–253. [Google Scholar] [CrossRef]
- Spangfort, E.V. The lumbar disc herniation. A computer-aided analysis of 2504 operations. Acta Orthop. Scand. 1972, 142, 1–95. [Google Scholar] [CrossRef]
- White, A.A.; Panjabi, M.M.; Company, J.B.L. Clinical Biomechanics of the Spine; J. B. Lippincott Company: Philadelphia, PA, USA, 1978. [Google Scholar]
- Iatridis, J.C.; Nicoll, S.B.; Michalek, A.J.; Walter, B.A.; Gupta, M.S. Role of biomechanics in intervertebral disc degeneration and regenerative therapies: What needs repairing in the disc and what are promising biomaterials for its repair? Spine J. 2013, 13, 243–262. [Google Scholar] [CrossRef] [PubMed]
- Sloan, S.R., Jr.; Wipplinger, C.; Kirnaz, S.; Navarro-Ramirez, R.; Schmidt, F.; McCloskey, D.; Pannellini, T.; Schiavinato, A.; Härtl, R.; Bonassar, L.J. Combined nucleus pulposus augmentation and annulus fibrosus repair prevents acute intervertebral disc degeneration after discectomy. Sci. Transl. Med. 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Zengerle, L.; Köhler, A.; Debout, E.; Hackenbroch, C.; Wilke, H.-J. Nucleus replacement could get a new chance with annulus closure. Eur. Spine J. 2020, 29, 1733–1741. [Google Scholar] [CrossRef] [PubMed]
- Bowles, R.D.; Setton, L.A. Biomaterials for intervertebral disc regeneration and repair. Biomaterials 2017, 129, 54–67. [Google Scholar] [CrossRef]
- Huang, Y.C.; Hu, Y.; Li, Z.; Luk, K.D.K. Biomaterials for intervertebral disc regeneration: Current status and looming challenges. J. Tissue Eng. Regen. Med. 2018, 12, 2188–2202. [Google Scholar] [CrossRef]
- Huang, Y.C.; Urban, J.P.; Luk, K.D. Intervertebral disc regeneration: Do nutrients lead the way? Nat. Rev. Rheumatol. 2014, 10, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Sakai, D.; Andersson, G.B. Stem cell therapy for intervertebral disc regeneration: Obstacles and solutions. Nat. Rev. Rheumatol. 2015, 11, 243–256. [Google Scholar] [CrossRef]
- Campisi, M.; Shin, Y.; Osaki, T.; Hajal, C.; Chiono, V.; Kamm, R.D. 3D self-organized microvascular model of the human blood-brain barrier with endothelial cells, pericytes and astrocytes. Biomaterials 2018, 180, 117–129. [Google Scholar] [CrossRef]
- Foresti, R.; Rossi, S.; Pinelli, S.; Alinovi, R.; Barozzi, M.; Sciancalepore, C.; Galetti, M.; Caffarra, C.; Lagonegro, P.; Scavia, G. Highly-defined bioprinting of long-term vascularized scaffolds with Bio-Trap: Complex geometry functionalization and process parameters with computer aided tissue engineering. Materialia 2020, 9, 100560. [Google Scholar] [CrossRef]
- Foresti, R.; Rossi, S.; Pinelli, S.; Alinovi, R.; Sciancalepore, C.; Delmonte, N.; Selleri, S.; Caffarra, C.; Raposio, E.; Macaluso, G.; et al. In-vivo vascular application via ultra-fast bioprinting for future 5D personalised nanomedicine. Sci. Rep. 2020, 10, 3205. [Google Scholar] [CrossRef] [PubMed]
- Berger-Roscher, N.; Casaroli, G.; Rasche, V.; Villa, T.; Galbusera, F.; Wilke, H.J. Influence of Complex Loading Conditions on Intervertebral Disc Failure. Spine 2017, 42, E78–E85. [Google Scholar] [CrossRef]
- Rajasekaran, S.; Bajaj, N.; Tubaki, V.; Kanna, R.M.; Shetty, A.P. ISSLS Prize winner: The anatomy of failure in lumbar disc herniation: An in vivo, multimodal, prospective study of 181 subjects. Spine 2013, 38, 1491–1500. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, M.K.; Matin, T.; Ali, M.I.; Ali, M.Y.; Awwal, M.A.; Sakeb, N. Relationship between physical work load and lumbar disc herniation. Mymensingh Med. J. 2013, 22, 533–540. [Google Scholar] [PubMed]
- Kelsey, J.L.; Githens, P.B.; Walter, S.D.; Southwick, W.O.; Weil, U.; Holford, T.R.; Ostfeld, A.M.; Calogero, J.A.; O’Connor, T.; White, A.A. An epidemiological study of acute prolapsed cervical intervertebral disc. J. Bone Joint Surg. Am. 1984, 66, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Mundt, D.J.; Kelsey, J.L.; Golden, A.L.; Panjabi, M.M.; Pastides, H.; Berg, A.T.; Sklar, J.; Hosea, T. An epidemiologic study of sports and weight lifting as possible risk factors for herniated lumbar and cervical discs. The Northeast Collaborative Group on Low Back Pain. Am. J. Sports Med. 1993, 21, 854–860. [Google Scholar] [CrossRef]
- Pietila, T.A.; Stendel, R.; Kombos, T.; Ramsbacher, J.; Schulte, T.; Brock, M. Lumbar disc herniation in patients up to 25 years of age. Neurol. Med. Chir. 2001, 41, 340–344. [Google Scholar] [CrossRef]
- Hutton, W.C.; Adams, M.A. Can the lumbar spine be crushed in heavy lifting? Spine 1982, 7, 586–590. [Google Scholar]
- Adams, M.A.; Freeman, B.J.; Morrison, H.P.; Nelson, I.W.; Dolan, P. Mechanical initiation of intervertebral disc degeneration. Spine 2000, 25, 1625–1636. [Google Scholar] [CrossRef]
- Gordon, S.J.; Yang, K.H.; Mayer, P.J.; Mace, A.H., Jr.; Kish, V.L.; Radin, E.L. Mechanism of disc rupture. A preliminary report. Spine 1991, 16, 450–456. [Google Scholar] [CrossRef]
- Lu, Y.M.; Hutton, W.C.; Gharpuray, V.M. Do bending, twisting, and diurnal fluid changes in the disc affect the propensity to prolapse? A viscoelastic finite element model. Spine 1996, 21, 2570–2579. [Google Scholar] [CrossRef] [PubMed]
- McNally, D.S.; Adams, M.A.; Goodship, A.E. Can intervertebral disc prolapse be predicted by disc mechanics? Spine 1993, 18, 1525–1530. [Google Scholar] [CrossRef] [PubMed]
- Wade, K.R.; Robertson, P.A.; Thambyah, A.; Broom, N.D. How healthy discs herniate: A biomechanical and microstructural study investigating the combined effects of compression rate and flexion. Spine 2014, 39, 1018–1028. [Google Scholar] [CrossRef]
- Wade, K.R.; Robertson, P.A.; Thambyah, A.; Broom, N.D. “Surprise” Loading in Flexion Increases the Risk of Disc Herniation Due to Annulus-Endplate Junction Failure: A Mechanical and Microstructural Investigation. Spine 2015, 40, 891–901. [Google Scholar] [CrossRef]
- Wade, K.R.; Schollum, M.L.; Robertson, P.A.; Thambyah, A.; Broom, N.D. ISSLS Prize Winner: Vibration Really Does Disrupt the Disc: A Microanatomical Investigation. Spine 2016, 41, 1185–1198. [Google Scholar] [CrossRef] [PubMed]
- Edwards, W.T.; Ordway, N.R.; Zheng, Y.; McCullen, G.; Han, Z.; Yuan, H.A. Peak stresses observed in the posterior lateral anulus. Spine 2001, 26, 1753–1759. [Google Scholar] [CrossRef]
- Wade, K.; Berger-Roscher, N.; Rasche, V.; Wilke, H. Disc wall structural abnormalities can act as initiation sites for herniation. Eur. Cell Mater. 2020, 40, 227–238. [Google Scholar] [CrossRef]
- Behjati, M.; Arjmand, N. Biomechanical Assessment of the NIOSH Lifting Equation in Asymmetric Load-Handling Activities Using a Detailed Musculoskeletal Model. Hum. Factors 2018, 61, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Gunzburg, R.; Parkinson, R.; Moore, R.; Cantraine, F.; Hutton, W.; Vernon-Roberts, B.; Fraser, R. A cadaveric study comparing discography, magnetic resonance imaging, histology, and mechanical behavior of the human lumbar disc. Spine 1992, 17, 417–426. [Google Scholar] [CrossRef]
- Andersson, G.B. Epidemiologic aspects on low-back pain in industry. Spine 1981, 6, 53–60. [Google Scholar] [CrossRef]
- Pflaster, D.S.; Krag, M.H.; Johnson, C.C.; Haugh, L.D.; Pope, M.H. Effect of test environment on intervertebral disc hydration. Spine 1997, 22, 133–139. [Google Scholar] [CrossRef]
- Wilke, H.J.; Jungkunz, B.; Wenger, K.; Claes, L.E. Spinal segment range of motion as a function of in vitro test conditions: Effects of exposure period, accumulated cycles, angular-deformation rate, and moisture condition. Anat. Rec. 1998, 251, 15–19. [Google Scholar] [CrossRef]
- Pfirrmann, C.W.; Metzdorf, A.; Zanetti, M.; Hodler, J.; Boos, N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine 2001, 26, 1873–1878. [Google Scholar] [CrossRef] [PubMed]
- Pearcy, M.J. Stereo radiography of lumbar spine motion. Acta Orthop. Scand. 1985, 212, 1–45. [Google Scholar] [CrossRef]
- Wilke, H.J.; Neef, P.; Caimi, M.; Hoogland, T.; Claes, L.E. New in vivo measurements of pressures in the intervertebral disc in daily life. Spine 1999, 24, 755–762. [Google Scholar] [CrossRef]
- Wilke, H.J.; Kienle, A.; Maile, S.; Rasche, V.; Berger-Roscher, N. A new dynamic six degrees of freedom disc-loading simulator allows to provoke disc damage and herniation. Eur. Spine J. 2016, 25, 1363–1372. [Google Scholar] [CrossRef] [PubMed]
- Wilke, H.J.; Claes, L.; Schmitt, H.; Wolf, S. A universal spine tester for in vitro experiments with muscle force simulation. Eur. Spine J. 1994, 3, 91–97. [Google Scholar] [CrossRef]
- Wilke, H.J.; Wenger, K.; Claes, L. Testing criteria for spinal implants: Recommendations for the standardization of in vitro stability testing of spinal implants. Eur. Spine J. 1998, 7, 148–154. [Google Scholar] [CrossRef]
- Wilke, H.J.; Ressel, L.; Heuer, F.; Graf, N.; Rath, S. Can prevention of a reherniation be investigated? Establishment of a herniation model and experiments with an anular closure device. Spine 2013, 38, E587–E593. [Google Scholar] [CrossRef] [PubMed]
- Belavy, D.L.; Adams, M.; Brisby, H.; Cagnie, B.; Danneels, L.; Fairbank, J.; Hargens, A.R.; Judex, S.; Scheuring, R.A.; Sovelius, R.; et al. Disc herniations in astronauts: What causes them, and what does it tell us about herniation on earth? Eur. Spine J. 2016, 25, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.; Kettler, A.; Heuer, F.; Simon, U.; Claes, L.; Wilke, H.J. Intradiscal pressure, shear strain, and fiber strain in the intervertebral disc under combined loading. Spine 2007, 32, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Heuer, F.; Ulrich, S.; Claes, L.; Wilke, H.J. Biomechanical evaluation of conventional anulus fibrosus closure methods required for nucleus replacement. Laboratory investigation. J. Neurosurg. Spine 2008, 9, 307–313. [Google Scholar] [CrossRef] [PubMed]
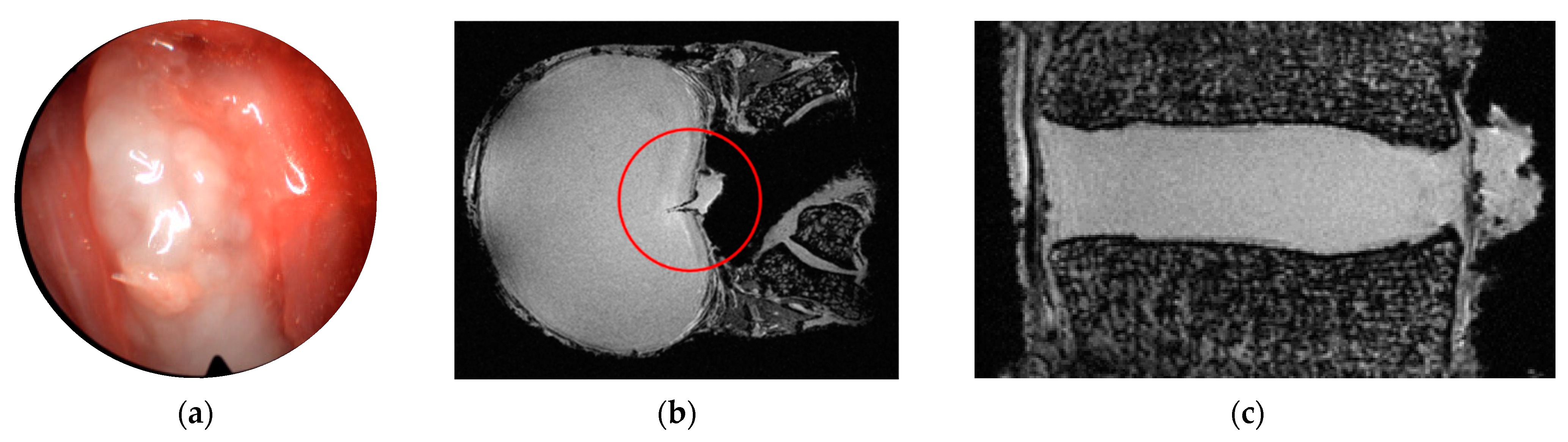
| Defect Group | Donor | Sex | Age in Years | Segment | Degeneration (acc. to Pfirrmann [36]) | Relevant Diagnoses |
|---|---|---|---|---|---|---|
| vertical cut | 2130 | male | 26 | L2-L3 | 2 | |
| L4-L5 | 3–4 | disc herniation 1 | ||||
| 2133 | female | 24 | L2-L3 | 1 | ||
| L4-L5 | 1 | |||||
| 2134 | male | 26 | L3-L4 | 1 | ||
| L5-S1 | 4 | disc herniation 1 | ||||
| 2138 | male | 33 | L2-L3 | 1 | ||
| L4-L5 | 2 | |||||
| median (range) | 26 (24, 33) | 1 (1, 2) 2 | ||||
| round hole | 2036 | male | 40 | L2-L3 | 1 | |
| L4-L5 | 1 | |||||
| 2072 | male | 53 | L3-L4 | 1–2 | ||
| L5-S1 | 1–2 | |||||
| 2129 | n.a. | 19 | L2-L3 | 1 | ||
| L4-L5 | 1 | |||||
| 2132 | male | 31 | L2-L3 | 1 | ||
| L4-L5 | 1 | |||||
| median (range) | 35.5 (19, 53) | 1 (1, 1–2) | ||||
| overall | median (range) | 35.5 (19, 53) | 1 (1, 2) |
| Activities | Standing | Tying Shoes | Sweeping Floor | Lifting Boxes | Lifting Boxes while Turning |
|---|---|---|---|---|---|
 |  |  |  |  | |
| bending directions (with ROM for L4-L5 [25]) | neutral (0°) | flexion 13° | flexion (13°) left lateral bending (2°) left axial rotation (2°) | flexion 13° | flexion (13°) left lateral bending (2°) left axial rotation (2°) |
| IDP in MPa [26] | 0.5 | 1.1 | n.a. | 2.3 | n.a. |
| Axial load in kN | 0.45 (0.24–0.54) | 0.97 (0.24–2.66) | 1.45 (0.23–2.63) | 2.13 (1.14–3.57) | 1.9 (1.13–3.58) |
| IDP peaks in MPa during dyn. loading | 0.5 (0.4–0.8) | 1.7 (1.4–3.5) | 1.9 (1.4–3.3) | 3.0 (2.7–3.6) | 2.8 (1.9–3.2) |
| Disc Condition | Defect Size | Number of Herniations | Physiological Activity (Cycle) When LDH Occurred | |
|---|---|---|---|---|
| intact disc | - | 0 | data | |
| vertical defect | 0.4 mm × 4.0 mm | 1 |  | (n = 1) |
| vertical defect | 1.0 mm × 5.5 mm | 1 |  | (n = 1) |
| vertical defect | 1.2 mm × 6.5 mm | 3 |  | (n = 1) |
 | (n = 2) | |||
| One specimen with vertical defect did NOT herniate | ||||
| round defect | Ø 4 mm | 6 | before dynamic loading | |
 | (n = 3) 1 (n = 3) | |||
| round defect | Ø 6 mm | no further LDH † | ||
| round defect | Ø 8 mm | no further LDH † | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zengerle, L.; Debout, E.; Kluger, B.; Zöllner, L.; Wilke, H.-J. In Vitro Model for Lumbar Disc Herniation to Investigate Regenerative Tissue Repair Approaches. Appl. Sci. 2021, 11, 2847. https://doi.org/10.3390/app11062847
Zengerle L, Debout E, Kluger B, Zöllner L, Wilke H-J. In Vitro Model for Lumbar Disc Herniation to Investigate Regenerative Tissue Repair Approaches. Applied Sciences. 2021; 11(6):2847. https://doi.org/10.3390/app11062847
Chicago/Turabian StyleZengerle, Laura, Elisabeth Debout, Bruno Kluger, Lena Zöllner, and Hans-Joachim Wilke. 2021. "In Vitro Model for Lumbar Disc Herniation to Investigate Regenerative Tissue Repair Approaches" Applied Sciences 11, no. 6: 2847. https://doi.org/10.3390/app11062847
APA StyleZengerle, L., Debout, E., Kluger, B., Zöllner, L., & Wilke, H.-J. (2021). In Vitro Model for Lumbar Disc Herniation to Investigate Regenerative Tissue Repair Approaches. Applied Sciences, 11(6), 2847. https://doi.org/10.3390/app11062847






