Extraction, Identification, and Health Benefits of Anthocyanins in Blackcurrants (Ribes nigrum L.)
Abstract
Featured Application
Abstract
1. Introduction
2. Extraction Methods
2.1. Solvent Extraction for Blackcurrant Anthocyanins
2.2. Non-Conventional Extraction
2.2.1. Microwave-Assisted Solvent Extraction
2.2.2. Ultrasonic-Assisted Extraction
2.2.3. Enzyme-Assisted Extraction
2.2.4. Pulsed Electric Field
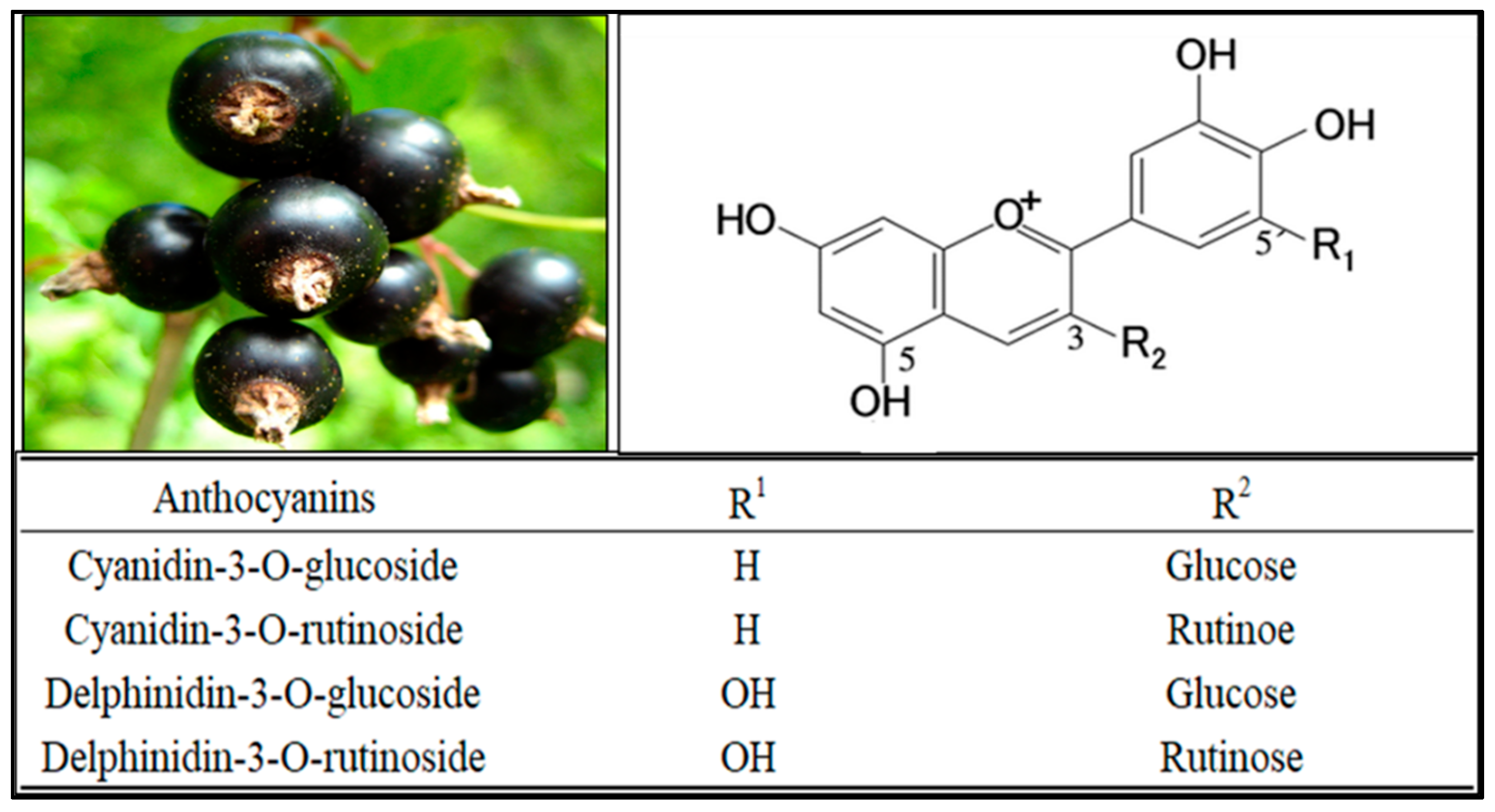
3. Identification of Blackcurrant Anthocyanins
4. Health Benefits of Blackcurrant Anthocyanins and Related Molecular Mechanisms
4.1. Antioxidant and Anti-Inflammatory Activities
4.2. Phytoestrogenic Activity
4.3. Anti-Postprandial Hyperglycemic and Anti-Diabetic Effect
4.4. Cardioprotective Effect
4.5. Neuroprotection and Cognitive Improvement
4.6. Chemoprevention
4.7. Vision and Eye Health
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Gopalan, A.; Reuben, S.C.; Ahmed, S.; Darvesh, A.S.; Hohmann, J.; Bishayee, A. The Health Benefits of Blackcurrants. Food Funct. 2012, 3, 795–809. [Google Scholar] [CrossRef] [PubMed]
- Rose, P.M.; Cantrill, V.; Benohoud, M.; Tidder, A.; Rayner, C.M.; Blackburn, R.S. Application of Anthocyanins from Blackcurrant (Ribes nigrum L.) Fruit Waste as Renewable Hair Dyes. J. Agric. Food Chem. 2018, 66, 6790–6798. [Google Scholar] [CrossRef] [PubMed]
- Brod, J.; Traitler, H.; Studer, A.; Lacharriere, O. Evolution of lipid composition in skin treated with blackcurrant seed oil. Int. J. Cosmet. Sci. 1988, 10, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Ligęza, M.; Wyglądacz, D.; Tobiasz, A.; Jaworecka, K.; Reich, A. Natural cold pressed oils as cosmetic products. Fam. Med. Prim. Care Rev. 2016, 443–447. [Google Scholar] [CrossRef]
- Bishayee, A.; Mbimba, T.; Thoppil, R.J.; Háznagy-Radnai, E.; Sipos, P.; Darvesh, A.S.; Folkesson, H.G.; Hohmann, J. Anthocyanin-Rich Black Currant (Ribes nigrum L.) Extract Affords Chemoprevention Against Diethylnitrosamine-Induced Hepatocellular Carcinogenesis in Rats. J. Nutr. Biochem. 2011, 22, 1035–1046. [Google Scholar] [CrossRef] [PubMed]
- Wichtl, M.; Anton, R.; Bernard, M.; Czygan, F.-C. Plantes Thérapeutiques: Tradition, Pratique Officinale, Science et Thérapeutique; Tec & Doc: Paris, French, 2003. [Google Scholar]
- Huo, J.; Li, Z.; Qin, D. Review of nutritional ingredients and health protectal function of black currant fruit and its prospect in industrial development. Dongbei Nongye Daxue Xuebao 2011, 42, 139–144. [Google Scholar]
- Nour, V.; Trandafir, I.; Ionica, M.E. Ascorbic acid, anthocyanins, organic acids and mineral content of some black and red currant cultivars. Fruits 2011, 66, 353–362. [Google Scholar] [CrossRef]
- Pieszka, M.; Migdał, W.; Gąsior, R.; Rudzińska, M.; Bederska-Łojewska, D.; Pieszka, M.; Szczurek, P. Native oils from apple, blackcurrant, raspberry, and strawberry seeds as a source of polyenoic fatty acids, tocochromanols, and phytosterols: A health implication. J. Chem. 2015, 2015. [Google Scholar] [CrossRef]
- Lu, Y.; Foo, L.Y. Polyphenolic constituents of blackcurrant seed residue. Food Chem. 2003, 80, 71–76. [Google Scholar] [CrossRef]
- Teleszko, M.; Wojdylo, A. Comparison of Phenolic Compounds and Antioxidant Potential between Selected Edible Fruits and Their Leaves. J. Funct. Foods 2015, 14, 736–746. [Google Scholar] [CrossRef]
- Zheng, J.; Yang, B.; Ruusunen, V.; Laaksonen, O.; Tahvonen, R.; Hellsten, J.; Kallio, H. Compositional differences of phenolic compounds between black currant (Ribes nigrum L.) cultivars and their response to latitude and weather conditions. J. Agric. Food Chem. 2012, 60, 6581–6593. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Ovando, A.; de Lourdes Pacheco-Hernández, M.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A.J.F.C. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Ichiyanagi, T.; Shida, Y.; Rahman, M.M.; Sekiya, M.; Hatano, Y.; Matsumoto, H.; Hirayama, M.; Konishi, T.; Ikeshiro, Y.J.F.C. Effect on both aglycone and sugar moiety towards Phase II metabolism of anthocyanins. Food Chem. 2008, 110, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, J.; Tanabe, S.; Bergeron, C.; Gafner, S.; Grenier, D. Anthocyanin-rich black currant extract and cyanidin-3-O-glucoside have cytoprotective and anti-inflammatory properties. J. Med. Food 2012, 15, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Cyboran, S.; Bonarska-Kujawa, D.; Pruchnik, H.; Żyłka, R.; Oszmiański, J.; Kleszczyńska, H. Phenolic Content and Biological Activity of Extracts of Blackcurrant Fruit and Leaves. Food Res. Int. 2014, 65, 47–58. [Google Scholar] [CrossRef]
- Rubinskiene, M.; Viskelis, P.; Jasutiene, I.; Viskeliene, R.; Bobinas, C.J.F.R.I. Impact of various factors on the composition and stability of black currant anthocyanins. Food Res. Int. 2005, 38, 867–871. [Google Scholar] [CrossRef]
- Kaufmann, B.; Christen, P. Recent extraction techniques for natural products: Microwave-assisted extraction and pressurised solvent extraction. Phytochem. Anal. 2002, 13, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Kahkonen, M.P.; Hopia, A.I.; Heinonen, M. Berry phenolics and their antioxidant activity. J. Agric. Food Chem. 2001, 49, 4076–4082. [Google Scholar] [CrossRef] [PubMed]
- Lapornik, B.; Prošek, M.; Wondra, A.G. Comparison of extracts prepared from plant by-products using different solvents and extraction time. J. Food Eng. 2005, 71, 214–222. [Google Scholar] [CrossRef]
- Laczko-Zold, E.; Komlosi, A.; Ulkei, T.; Fogarasi, E.; Croitoru, M.; Fulop, I.; Domokos, E.; Stefanescu, R.; Varga, E. Extractability of polyphenols from black currant, red currant and gooseberry and their antioxidant activity. Acta Biol. Hung. 2018, 69, 156–169. [Google Scholar] [CrossRef]
- Nour, V.; Stampar, F.; Veberic, R.; Jakopic, J. Anthocyanins Profile, Total Phenolics and Antioxidant Activity of Black Currant Ethanolic Extracts as Influenced by Genotype and Ethanol Concentration. Food Chem. 2013, 141, 961–966. [Google Scholar] [CrossRef] [PubMed]
- Harbourne, N.; Marete, E.; Jacquier, J.C.; O’Riordan, D. Stability of Phytochemicals as Sources of Anti-Inflammatory Nutraceuticals in Beverages—A Review. Food Res. Int. 2013, 50, 480–486. [Google Scholar] [CrossRef]
- Kapasakalidis, P.G.; Rastall, R.A.; Gordon, M.H. Extraction of polyphenols from processed black currant (Ribes nigrum L.) residues. J. Agric. Food Chem. 2006, 54, 4016–4021. [Google Scholar] [CrossRef]
- Azman, E.M.; Charalampopoulos, D.; Chatzifragkou, A. Acetic acid buffer as extraction medium for free and bound phenolics from dried blackcurrant (Ribes nigrum L.) skins. J. Food Sci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Van Sumere, C.; Vande Casteele, K.; De Loose, R.; Heursel, J. Reversed phase-HPLC analysis of flavonoids and the biochemical identification of cultivars of evergreen Azalea. Biochem. Plant Phenolics 1985, 17–43. [Google Scholar]
- Anthemidis, A.N.; Ioannou, K.-I.G. Recent Developments in Homogeneous and Dispersive Liquid-Liquid Extraction for Inorganic Elements Determination. A Review. Talanta 2009, 80, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Juedtz, K.; Aberdein, N.; Stewart, D.; McDougall, G.J. Sequential Extraction of Black Currant Residues Produces Anthocyanin-Rich Extracts Containig Anthocyanin Dimers. J. Berry Res. 2013, 3, 53–64. [Google Scholar] [CrossRef]
- Selvamuthukumaran, M.; Shi, J.J.F.Q. Recent advances in extraction of antioxidants from plant by-products processing industries. Food Qual. Saf. 2017, 1, 61–81. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.; Rahman, M.; Sharif, K.; Mohamed, A.; Sahena, F.; Jahurul, M.; Ghafoor, K.; Norulaini, N.; Omar, A. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, S.; Xu, Y.; Yu, Z. Optimization and Comparison of Three Methods on Anthocyanins Extraction from Blackcurrant (Ribes nigrum L.) Using RSM. Adv. Mater. Res. 2012, 361–363, 691–700. [Google Scholar] [CrossRef]
- Pap, N.; Beszédes, S.; Pongrácz, E.; Myllykoski, L.; Gábor, M.; Gyimes, E.; Hodúr, C.; Keiski, R.L. Microwave-Assisted Extraction of Anthocyanins from Black Currant Marc. Food Bioprocess Technol. 2012, 6, 2666–2674. [Google Scholar] [CrossRef]
- Oancea, S.; Ghincevici, D.; Ketney, O. The Effect of Ultrasonic Pretreatment and Sample Preparation on the Extraction Yield of Antioxidant Compounds and Activity of Black Currant Fruits. Acta Chim. Slov. 2015, 62, 242–248. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Holtung, L.; Grimmer, S.; Aaby, K. Effect of processing of black currant press-residue on polyphenol composition and cell proliferation. J. Agric. Food Chem. 2011, 59, 3632–3640. [Google Scholar] [CrossRef] [PubMed]
- Kou, P.; Kang, Y.-F.; Wang, L.-T.; Niu, L.-J.; Xiao, Y.; Guo, N.; Cui, Q.; Li, Y.-Y.; Fu, Y.-J. An integrated strategy for production of four anthocyanin compounds from Ribes nigrum L. by deep eutectic solvents and flash chromatography. J. Ind. Eng. Chem. 2019, 80, 614–625. [Google Scholar] [CrossRef]
- Landbo, A.-K.; Meyer, A.S. Effects of different enzymatic maceration treatments on enhancement of anthocyanins and other phenolics in black currant juice. Innov. Food Sci. Emerg. Technol. 2004, 5, 503–513. [Google Scholar] [CrossRef]
- Landbo, A.K.; Meyer, A.S. Enzyme-assisted extraction of antioxidative phenols from black currant juice press residues (Ribes nigrum). J. Agric. Food Chem. 2001, 49, 3169–3177. [Google Scholar] [CrossRef] [PubMed]
- Gagneten, M.; Leiva, G.; Salvatori, D.; Schebor, C.; Olaiz, N. Optimization of Pulsed Electric Field Treatment for the Extraction of Bioactive Compounds from Blackcurrant. Food Bioprocess Technol. 2019, 12, 1102–1109. [Google Scholar] [CrossRef]
- Tatke, P.; Jaiswal, Y. An overview of microwave assisted extraction and its applications in herbal drug research. Res. J. Med. Plant 2011, 5, 21–31. [Google Scholar] [CrossRef]
- Li, X.; Chen, F.; Li, S.; Jia, J.; Gu, H.; Yang, L. An Efficient Homogenate-Microwave-Assisted Extraction of Flavonols and Anthocyanins from Blackcurrant Marc: Optimization Using Combination of Plackett-Burman Design and Box-Behnken Design. Ind. Crops Prod. 2016, 94, 834–837. [Google Scholar] [CrossRef]
- Tiwari, B.K. Ultrasound: A Clean, Green Extraction Technology. Trends Anal. Chem. 2015, 71, 100–109. [Google Scholar] [CrossRef]
- Picó, Y. Ultrasound-Assisted Extraction for Food and Environmental Samples. Trends Anal. Chem. 2013, 43, 84–99. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound Assisted Extraction of Food and Natural Products. Mechanisms, Techniques, Combinations, Protocols and Applications. A Review. Ultrason. Sonochemistry 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Virot, M.; Tomao, V.; Le Bourvellec, C.; Renard, C.M.; Chemat, F. Towards the industrial production of antioxidants from food processing by-products with ultrasound-assisted extraction. Ultrason. Sonochemistry 2010, 17, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Jung, J.; Tomasino, E.; Zhao, Y. Optimization of solvent and ultrasound-assisted extraction for different anthocyanin rich fruit and their effects on anthocyanin compositions. LWT Food Sci. Technol. 2016, 72, 229–238. [Google Scholar] [CrossRef]
- Puri, M.; Sharma, D.; Barrow, C.J. Enzyme-assisted extraction of bioactives from plants. Trends Biotechnol. 2012, 30, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Toepfl, S.; Siemer, C.; Saldaña-Navarro, G.; Heinz, V. Overview of pulsed electric fields processing for food. In Emerging Technologies for Food Processing; Elsevier: Amsterdam, The Netherlands, 2014; pp. 93–114. [Google Scholar]
- Paunović, S.M.; Mašković, P.; Nikolić, M.; Miletić, R. Bioactive Compounds and Antimicrobial Activity of Black Currant (Ribes nigrum L.) Berries and Leaves Extract Obtained by Different Soil Management System. Sci. Hortic. 2017, 222, 69–75. [Google Scholar] [CrossRef]
- Mattila, P.H.; Hellström, J.; Karhu, S.; Pihlava, J.-M.; Veteläinen, M. High Variability in Flavonoid Contents and Composition between Different North-European Currant (Ribes spp.) varieties. Food Chem. 2016, 204, 14–20. [Google Scholar] [CrossRef]
- Vagiri, M.; Ekholm, A.; Andersson, S.C.; Johansson, E.; Rumpunen, K. An Optimized Method for Analysis of Phenolic Compounds in Buds, Leaves, and Fruits of Black Currant (Ribes nigrum L.). J. Agric. Food Chem. 2012, 60, 10501–10510. [Google Scholar] [CrossRef]
- Slimestad, R.; Solheim, H. Anthocyanins from black currants (Ribes nigrum L.). J. Agric. Food Chem. 2002, 50, 3228–3231. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Alimbetov, D.; George, T.; Gordon, M.H.; Lovegrove, J.A. A randomised trial to investigate the effects of acute consumption of a blackcurrant juice drink on markers of vascular reactivity and bioavailability of anthocyanins in human subjects. Eur. J. Clin. Nutr. 2011, 65, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Rohrig, T.; Kirsch, V.; Schipp, D.; Galan, J.; Richling, E. Absorption of Anthocyanin Rutinosides after Consumption of a Blackcurrant (Ribes nigrum L.) Extract. J. Agric. Food Chem. 2019, 67, 6792–6797. [Google Scholar] [CrossRef] [PubMed]
- Vepsäläinen, S.; Koivisto, H.; Pekkarinen, E.; Mäkinen, P.; Dobson, G.; McDougall, G.J.; Stewart, D.; Haapasalo, A.; .Karjalainen, R.O.; Tanila, H.; et al. Anthocyanin-Enriched Bilberry and Blackcurrant Extracts Modulate Amyloid Precursor Protein Processing and Alleviate Behavioral Abnormalities in the APP/PS1 Mouse Model of Alzheimer’s Disease. J. Nutr. Biochem. 2013, 24, 360–370. [Google Scholar] [CrossRef]
- Ghosh, D.; McGhie, T.K.; Zhang, J.; Adaim, A.; Skinner, M. Effects of anthocyanins and other phenolics of boysenberry and blackcurrant as inhibitors of oxidative stress and damage to cellular DNA in SH-SY5Y and HL-60 cells. J. Sci. Food Agric. 2006, 86, 678–686. [Google Scholar] [CrossRef]
- Hurst, R.D.; Lyall, K.A.; Roberts, J.M.; Perthaner, A.; Wells, R.W.; Cooney, J.M.; Jensen, D.J.; Burr, N.S.; Hurst, S.M. Consumption of an anthocyanin-Rich extract made from new zealand blackcurrants prior to exercise may assist recovery from oxidative stress and maintains circulating neutrophil function: A Pilot study. Front. Nutr. 2019, 6, 73. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [PubMed]
- J Thoppil, R.; Bhatia, D.; Barnes, F.K.; Haznagy-Radnai, E.; Hohmann, J.; S Darvesh, A.; Bishayee, A. Black currant anthocyanins abrogate oxidative stress through Nrf2-mediated antioxidant mechanisms in a rat model of hepatocellular carcinoma. Curr. Cancer Drug Targets 2012, 12, 1244–1257. [Google Scholar]
- Lee, S.G.; Kim, B.; Yang, Y.; Pham, T.X.; Park, Y.K.; Manatou, J.; Koo, S.I.; Chun, O.K.; Lee, J.Y. Berry anthocyanins suppress the expression and secretion of proinflammatory mediators in macrophages by inhibiting nuclear translocation of NF-kappaB independent of NRF2-mediated mechanism. J. Nutr. Biochem. 2014, 25, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Lyall, K.A.; Hurst, S.M.; Cooney, J.; Jensen, D.; Lo, K.; Hurst, R.D.; Stevenson, L.M. Short-term blackcurrant extract consumption modulates exercise-induced oxidative stress and lipopolysaccharide-stimulated inflammatory responses. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, R70–R81. [Google Scholar] [CrossRef]
- Shaw, O.M.; Nyanhanda, T.; McGhie, T.K.; Harper, J.L.; Hurst, R.D. Blackcurrant anthocyanins modulate CCL11 secretion and suppress allergic airway inflammation. Mol. Nutr. Food Res. 2017, 61, 1600868. [Google Scholar] [CrossRef] [PubMed]
- Benn, T.; Kim, B.; Park, Y.-K.; Wegner, C.J.; Harness, E.; Nam, T.-G.; Kim, D.-O.; Lee, J.S.; Lee, J.-Y. Polyphenol-Rich Blackcurrant Extract Prevents Inflammation in Diet-Induced Obese Mice. J. Nutr. Biochem. 2014, 25, 1019–1025. [Google Scholar] [CrossRef]
- Moynagh, P.N. The NF-kappaB pathway. J. Cell Sci. 2005, 118, 4589–4592. [Google Scholar] [CrossRef] [PubMed]
- Aboonabi, A.; Aboonabi, A. Anthocyanins reduce inflammation and improve glucose and lipid metabolism associated with inhibiting nuclear factor-kappaB activation and increasing PPAR-gamma gene expression in metabolic syndrome subjects. Free Radic Biol. Med. 2020, 150, 30–39. [Google Scholar] [CrossRef]
- Sirotkin, A.V.; Harrath, A.H. Phytoestrogens and their effects. Eur. J. Pharmacol. 2014, 741, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Nanashima, N.; Horie, K.; Maeda, H.; Tomisawa, T.; Kitajima, M.; Nakamura, T. Blackcurrant Anthocyanins Increase the Levels of Collagen, Elastin, and Hyaluronic Acid in Human Skin Fibroblasts and Ovariectomized Rats. Nutrients 2018, 10, 495. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Mun, S.; Lee, S.G.; Vance, T.M.; Hubert, P.; Koo, S.I.; Lee, S.K.; Chun, O.K. Anthocyanin-Rich Blackcurrant Extract Attenuates Ovariectomy-Induced Bone Loss in Mice. J. Med. Food 2016, 19, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Nanashima, N.; Horie, K. Blackcurrant Extract with Phytoestrogen Activity Alleviates Hair Loss in Ovariectomized Rats. Molecules 2019, 24, 1272. [Google Scholar] [CrossRef] [PubMed]
- Nanashima, N.; Horie, K.; Tomisawa, T.; Chiba, M.; Nakano, M.; Fujita, T.; Maeda, H.; Kitajima, M.; Takamagi, S.; Uchiyama, D. Phytoestrogenic activity of blackcurrant (Ribes nigrum) anthocyanins is mediated through estrogen receptor alpha. Mol. Nutr. Food Res. 2015, 59, 2419–2431. [Google Scholar] [CrossRef] [PubMed]
- Nanashima, N.; Horie, K.; Maeda, H.J.M. Phytoestrogenic activity of blackcurrant anthocyanins is partially mediated through estrogen receptor beta. Molecules 2018, 23, 74. [Google Scholar] [CrossRef] [PubMed]
- Castro-Acosta, M.L.; Smith, L.; Miller, R.J.; McCarthy, D.I.; Farrimond, J.A.; Hall, W.L. Drinks containing anthocyanin-rich blackcurrant extract decrease postprandial blood glucose, insulin and incretin concentrations. J. Nutr. Biochem. 2016, 38, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Nolan, A.; Brett, R.; Strauss, J.; Stewart, C.; Shepherd, S. Short-term, but not acute, intake of New Zealand blackcurrant extract improves insulin sensitivity and free-living postprandial glucose excursions in individuals with overweight or obesity. Eur. J. Nutr. 2020, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Barik, S.K.; Russell, W.R.; Moar, K.M.; Cruickshank, M.; Scobbie, L.; Duncan, G.; Hoggard, N. The anthocyanins in black currants regulate postprandial hyperglycaemia primarily by inhibiting alpha-glucosidase while other phenolics modulate salivary alpha-amylase, glucose uptake and sugar transporters. J. Nutr. Biochem. 2020, 78, 108325. [Google Scholar] [CrossRef] [PubMed]
- Hui, X.; Wu, G.; Han, D.; Stipkovits, L.; Wu, X.; Tang, S.; Brennan, M.A.; Brennan, C.S. The effects of bioactive compounds from blueberry and blackcurrant powders on the inhibitory activities of oat bran pastes against alpha-amylase and alpha-glucosidase linked to type 2 diabetes. Food Res. Int. 2020, 138, 109756. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Miao, M. Dietary polyphenols modulate starch digestion and glycaemic level: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 541–555. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, Y.; Ozeki, A.; Tani, T.; Tsuda, T. Blackcurrant extract ameliorates hyperglycemia in type 2 diabetic mice in association with increased basal secretion of glucagon-like peptide-1 and activation of AMP-activated protein kinase. J. Nutr. Sci. Vitaminol. 2018, 64, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Esposito, D.; Damsud, T.; Wilson, M.; Grace, M.H.; Strauch, R.; Li, X.; Lila, M.A.; Komarnytsky, S. Black Currant Anthocyanins Attenuate Weight Gain and Improve Glucose Metabolism in Diet-Induced Obese Mice with Intact, but Not Disrupted, Gut Microbiome. J. Agric. Food Chem. 2015, 63, 6172–6180. [Google Scholar] [CrossRef] [PubMed]
- Nanashima, N.; Horie, K.; Yamanouchi, K.; Tomisawa, T.; Kitajima, M.; Oey, I.; Maeda, H. Blackcurrant (Ribes nigrum) Extract Prevents Dyslipidemia and Hepatic Steatosis in Ovariectomized Rats. Nutrients 2020, 12, 1541. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Shen, T.; Jiang, X.; Xia, M.; Sun, X.; Guo, H.; Ling, W.J.J.; Chemistry, F. Purified anthocyanins from bilberry and black currant attenuate hepatic mitochondrial dysfunction and steatohepatitis in mice with methionine and choline deficiency. J. Agric. Food Chem. 2015, 63, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Kho, M.C.; Kim, H.Y.; Ahn, Y.M.; Lee, Y.J.; Kang, D.G.; Lee, H.S. Blackcurrant Suppresses Metabolic Syndrome Induced bt High-Fructose Diet in Rats. Evid. Based Complement. Altern. Med. 2015, 2015, 1–11. [Google Scholar]
- Tomisawa, T.; Nanashima, N.; Kitajima, M.; Mikami, K.; Takamagi, S.; Maeda, H.; Horie, K.; Lai, F.-C.; Osanai, T. Effects of Blackcurrant Anthocyanin on Endothelial Function and Peripheral Temperature in Young Smokers. Molecules 2019, 24, 4295. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Hashimoto, Y.; Kobayashi, R.; Nakazato, K.; Willems, M.E.T. Effects of blackcurrant extract on arterial functions in older adults: A randomized, double-blind, placebo-controlled, crossover trial. Clin. Exp. Hypertens. 2020. [Google Scholar] [CrossRef]
- Cook, M.D.; Myers, S.D.; Gault, M.L.; Edwards, V.C.; Willems, M.E.T. Cardiovascular function during supine rest in endurance-trained males with New Zealand blackcurrant: A dose–response study. Eur. J. Appl. Physiol. 2017, 117, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Ray, S.; Craigie, A.M.; Kennedy, G.; Hill, A.; Barton, K.L.; Broughton, J.; Belch, J.J. Lowering of oxidative stress improves endothelial function in healthy subjects with habitually low intake of fruit and vegetables: A randomized controlled trial of antioxidant- and polyphenol-rich blackcurrant juice. Free Radic. Biol. Med. 2014, 72, 232–237. [Google Scholar] [CrossRef]
- Insull, W., Jr. The pathology of atherosclerosis: Plaque development and plaque responses to medical treatment. Am. J. Med. 2009, 122, S3–S14. [Google Scholar] [CrossRef] [PubMed]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y.; Ali, F. Atherosclerotic cardiovascular disease: A review of initiators and protective factors. Inflammopharmacology 2016, 24, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Linton, M.R.F.; Yancey, P.G.; Davies, S.S.; Jerome, W.G.; Linton, E.F.; Song, W.L.; Doran, A.C.; Vickers, K.C. The Role of Lipids and Lipoproteins in Atherosclerosis. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dungan, K., Grossman, A., Hershman, J.M., Hofland, J., Kaltsas, G., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Qin, Y.; Xia, M.; Ma, J.; Hao, Y.; Liu, J.; Mou, H.; Cao, L.; Ling, W. Anthocyanin supplementation improves serum LDL- and HDL-cholesterol concentrations associated with the inhibition of cholesteryl ester transfer protein in dyslipidemic subjects. Am. J. Clin. Nutr. 2009, 90, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Aboonabi, A.; Meyer, R.R.; Gaiz, A.; Singh, I. Anthocyanins in berries exhibited anti-atherogenicity and antiplatelet activities in a metabolic syndrome population. Nutr. Res. 2020, 76, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Bae, M.; Park, Y.K.; Ma, H.; Yuan, T.; Seeram, N.P.; Lee, J.Y. Blackcurrant anthocyanins stimulated cholesterol transport via post-transcriptional induction of LDL receptor in Caco-2 cells. Eur. J. Nutr. 2018, 57, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Benn, T.; Kim, B.; Park, Y.-K.; Yang, Y.; Pham, T.X.; Ku, C.S.; Farruggia, C.; Harness, E.; Smyth, J.A.; Lee, J.-Y. Polyphenol-Rich Blackcurrant Extract Exerts Hypocholesterolaemic and Hypoglycaemic Effects in Mice Fed a Diet Containing High Fat and Cholesterol. Br. J. Nutr. 2015, 113, 1697–1703. [Google Scholar] [CrossRef] [PubMed]
- Naseem, K.M. The role of nitric oxide in cardiovascular diseases. Mol. Asp. Med. 2005, 26, 33–65. [Google Scholar] [CrossRef] [PubMed]
- Liu, V.W.; Huang, P.L. Cardiovascular roles of nitric oxide: A review of insights from nitric oxide synthase gene disrupted mice. Cardiovasc. Res. 2008, 77, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Edirisinghe, I.; Banaszewski, K.; Cappozzo, J.; McCarthy, D.; Burton-Freeman, B.M.J.J.; Chemistry, F. Effect of black currant anthocyanins on the activation of endothelial nitric oxide synthase (eNOS) in vitro in human endothelial cells. J. Agric. Food Chem. 2011, 59, 8616–8624. [Google Scholar] [CrossRef] [PubMed]
- Horie, K.; Nanashima, N.; Maeda, H.J.M. Phytoestrogenic effects of blackcurrant anthocyanins increased endothelial nitric oxide synthase (eNOS) expression in human endothelial cells and ovariectomized rats. Molecules 2019, 24, 1259. [Google Scholar] [CrossRef] [PubMed]
- Finne Nielsen, I.L.; Elbol Rasmussen, S.; Mortensen, A.; Ravn-Haren, G.; Ma, H.P.; Knuthsen, P.; Hansen, B.F.; McPhail, D.; Freese, R.; Breinholt, V.; et al. Anthocyanins increase low-density lipoprotein and plasma cholesterol and do not reduce atherosclerosis in Watanabe Heritable Hyperlipidemic rabbits. Mol. Nutr. Food Res. 2005, 49, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Strathearn, K.E.; Yousef, G.G.; Grace, M.H.; Roy, S.L.; Tambe, M.A.; Ferruzzi, M.G.; Wu, Q.-L.; Simon, J.E.; Lila, M.A.; Rochet, J.-C. Neuroprotective Effects of Anthocyanin- and Proanthocyanin-Rich Extracts in Cellular Models of Parkinson’s Disease. Brain Res. 2014, 1555, 60–77. [Google Scholar] [CrossRef]
- Watson, A.W.; Haskell-Ramsay, C.F.; Kennedy, D.O.; Cooney, J.M.; Trower, T.; Scheepens, A. Acute Supplementation with Blackcurrant Extracts Modulates Cognitive Functioning and Inhibits Monoamine Oxidase-B in Healthy Young Adults. J. Funct. Foods 2015, 17, 524–539. [Google Scholar] [CrossRef]
- Dos Santos, N.M.; Batista, P.B.; Batista, Â.G.; Júnior, M.R.M.J.C. Current evidence on cognitive improvement and neuroprotection promoted by anthocyanins. Curr. Opin. Food Sci. 2019, 26, 71–78. [Google Scholar] [CrossRef]
- Fan, D.; Alamri, Y.; Liu, K.; MacAskill, M.; Harris, P.; Brimble, M.; Dalrymple-Alford, J.; Prickett, T.; Menzies, O.; Laurenson, A.J.N. Supplementation of blackcurrant anthocyanins increased cyclic glycine-proline in the cerebrospinal fluid of parkinson patients: Potential treatment to improve insulin-like growth factor-1 function. Nutrients 2018, 10, 714. [Google Scholar] [CrossRef] [PubMed]
- Bishayee, A.; Haznagy-Radnai, E.; Mbimba, T.; Sipos, P.; Morazzoni, P.; Darvesh, A.S.; Bhatia, D.; Hohmann, J. Anthocyanin-rich black currant extract suppresses the growth of human hepatocellular carcinoma cells. Nat. Prod. Commun. 2010, 5, 1613–1618. [Google Scholar] [CrossRef] [PubMed]
- Bishayee, A.; Thoppil, R.J.; Mandal, A.; Darvesh, A.S.; Ohanyan, V.; Meszaros, J.G.; Haznagy-Radnai, E.; Hohmann, J.; Bhatia, D. Black currant phytoconstituents exert chemoprevention of diethylnitrosamine-initiated hepatocarcinogenesis by suppression of the inflammatory response. Mol. Carcinog. 2013, 52, 304–317. [Google Scholar] [CrossRef] [PubMed]
- Bellezza, I.; Mierla, A.L.; Minelli, A. Nrf2 and NF-kappaB and Their Concerted Modulation in Cancer Pathogenesis and Progression. Cancers 2010, 2, 483–497. [Google Scholar] [CrossRef] [PubMed]
- Iida, H.; Nakamura, Y.; Matsumoto, H.; Takeuchi, Y.; Harano, S.; Ishihara, M.; Katsumi, O.J.O. Effect of black-currant extract on negative lens-induced ocular growth in chicks. Ophthalmic Res. 2010, 44, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Iida, H.; Nakamura, Y.; Matsumoto, H.; Kawahata, K.; Koga, J.; Katsumi, O.J.J. Differential effects of black currant anthocyanins on diffuser-or negative lens-induced ocular elongation in chicks. J. Ocul. Pharmacol. Ther. 2013, 29, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Nakaishi, H.; Matsumoto, H.; Tominaga, S.; Hirayama, M. Effects of black currant anthocyanoside intake on dark adaptation and VDT work-induced transient refractive alteration in healthy humans. Altern. Med. Rev. 2000, 5, 553–562. [Google Scholar] [PubMed]
- Ohguro, H.; Ohguro, I.; Yagi, S. Effects of black currant anthocyanins on intraocular pressure in healthy volunteers and patients with glaucoma. J. Ocul. Pharmacol. Ther. 2013, 29, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Ohguro, H.; Ohguro, I.; Katai, M.; Tanaka, S. Two-year randomized, placebo-controlled study of black currant anthocyanins on visual field in glaucoma. Ophthalmologica 2012, 228, 26–35. [Google Scholar] [CrossRef]
- Yoshida, K.; Ohguro, I.; Ohguro, H. Black currant anthocyanins normalized abnormal levels of serum concentrations of endothelin-1 in patients with glaucoma. J. Ocul. Pharmacol. Ther. 2013, 29, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, R.; Fromm, M. Endothelin antagonism as an active principle for glaucoma therapy. Br. J. Pharmacol. 2011, 162, 806–816. [Google Scholar] [CrossRef] [PubMed]
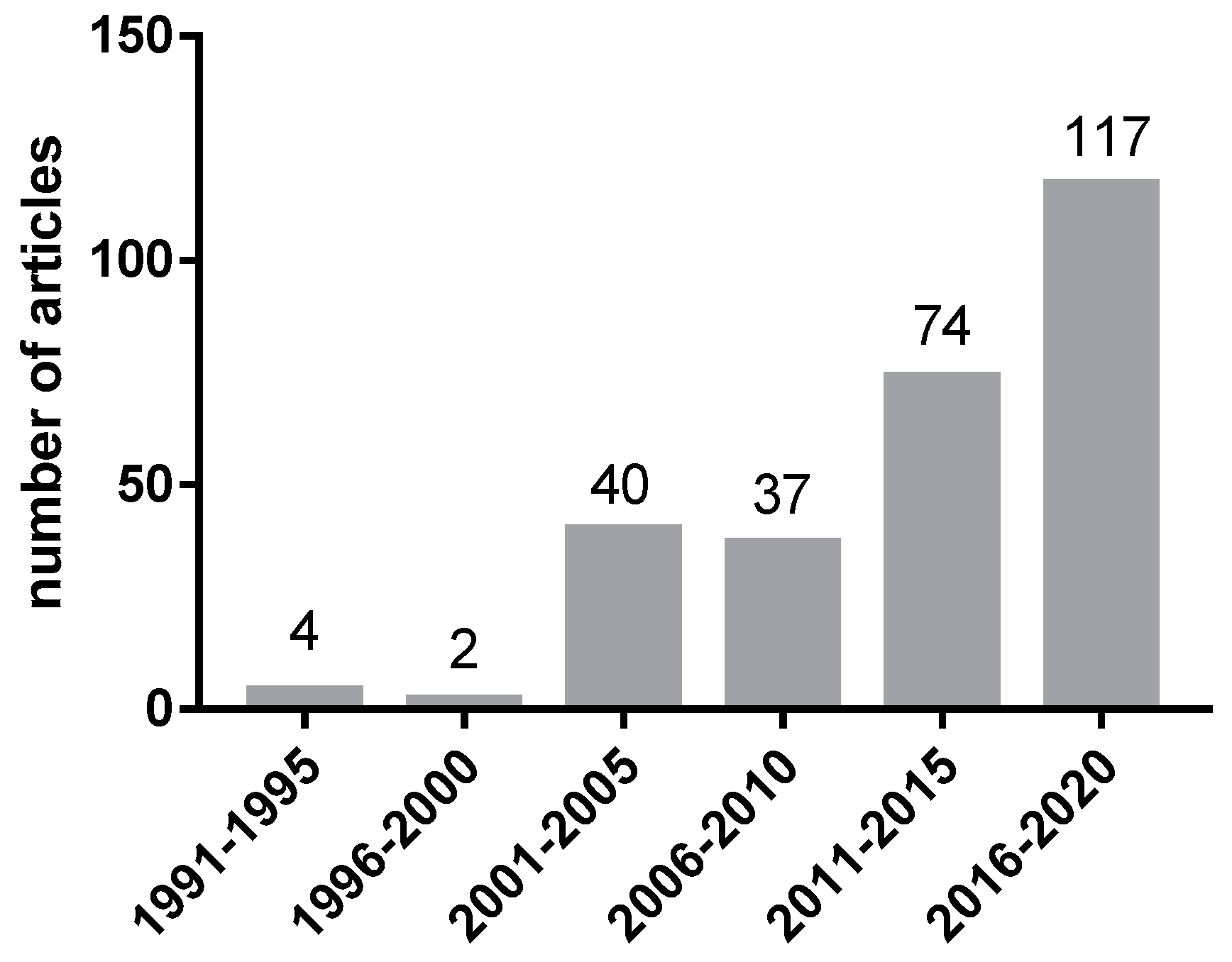
| Method | Condition | Efficiency 1 | Reference |
|---|---|---|---|
| Microwave-assisted | pH 2, 10 min extraction time, 700 W microwave power | ↑ 20% | [32] |
| Ultrasonic-assisted | 40 kHz frequency, 10 min extraction time, 70% amplitude | ↑ 20% from freeze-dried samples, → from frozen or over-dried samples | [33] |
| Ultrasonic-assisted | 45 kHz frequency, 15 min extraction time, 90 °C | → | [34] |
| Ultrasonic-assisted | Ultrasonic bath, 40 min extraction time | → | [35] |
| Enzyme-assisted | Pectinolytic enzyme preparations, 30 min treatment, 60 °C | → or ↑ | [36] |
| Enzyme-assisted | Pectinolytic enzyme preparations or protease, 10 min treatment, 100 °C | ↓ or → | [37] |
| Pulsed electrical field | 1318 V/cm and 315 pulses | ↑ 6% | [38] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, L.; Park, Y.; Lee, S.; Kim, D.-O. Extraction, Identification, and Health Benefits of Anthocyanins in Blackcurrants (Ribes nigrum L.). Appl. Sci. 2021, 11, 1863. https://doi.org/10.3390/app11041863
Cao L, Park Y, Lee S, Kim D-O. Extraction, Identification, and Health Benefits of Anthocyanins in Blackcurrants (Ribes nigrum L.). Applied Sciences. 2021; 11(4):1863. https://doi.org/10.3390/app11041863
Chicago/Turabian StyleCao, Lei, Yena Park, Sanggil Lee, and Dae-Ok Kim. 2021. "Extraction, Identification, and Health Benefits of Anthocyanins in Blackcurrants (Ribes nigrum L.)" Applied Sciences 11, no. 4: 1863. https://doi.org/10.3390/app11041863
APA StyleCao, L., Park, Y., Lee, S., & Kim, D.-O. (2021). Extraction, Identification, and Health Benefits of Anthocyanins in Blackcurrants (Ribes nigrum L.). Applied Sciences, 11(4), 1863. https://doi.org/10.3390/app11041863







