3.1.1. X-ray Powder Diffraction
The phase composition of cement clinkers KMO1 and KMO2, sintered at different temperatures, was determined by Rietveld refinement (
Table 5). In both types of clinker, the overall content of the main clinker phases was closest to the targeted values following sintering at 1250 °C (
Table 5). The most abundant phase was C
2S, which was mainly presented in beta form. During cooling, less than 1 wt.% of the beta C
2S was transformed into gamma modification in both KMO1 and KMO2 (
Table 5). The amount of belite did not significantly vary between clinkers sintered at 1200, 1250, and 1300 °C (
Table 5). The second most abundant phase was C
4A
3Ś, which presented in orthorhombic and cubic form. The orthorhombic modification dominated in both clinkers at all sintering temperatures (
Table 5). The highest amount of C
4A
3Ś developed at 1250 °C in both KMO1 and KMO2 clinkers (
Table 5).
With regards to the C
4AF phase, the targeted amount was reached at all three sintering temperatures in KMO1, while the amount of C
4AF detected in KMO2 was much lower than the value predicted, only reaching around 2 wt.% (
Table 5). The amount of ferrite remained constant between 1250 and 1300 °C, and then increased slightly between 1200 and 1250 °C (
Table 5). However, perovskite was present in both clinkers, with less than 1 wt.% detected in KMO1, compared to more than 5 wt.% in KM02 (
Table 5). Ferrite is the artificial equivalent of natural mineral brownmillerite, having a structure similar to perovskite, which allows the substitution of various cations [
31]. However, Ti was mainly incorporated into the raw clinker mixtures as a result of the presence of bauxite (
Table 1), meaning the higher amount of perovskite in KMO2 can be explained by the larger amount of bauxite used in the KMO2 mixture (
Table 3).
Among the minor phases, mayenite and periclase were detected in both clinkers. The amount of mayenite was higher in the KMO2 sample due to an increased content of the calcium sulfoaluminate phase. In both KMO1 and KMO2, the highest amount of mayenite was detected in samples sintered at 1200 °C, when the C
4A
3Ś was forming, while the lowest amount of C
4A
3Ś occurred at 1250 °C, which corresponds to the optimal sintering temperature at which the targeted values were reached. The periclase (free magnesia (f-MgO)) content was similar for KMO1 and KMO2, with values remaining constant across all sintering temperatures (
Table 5). The amount of periclase, determined by Rietveld refinement, was slightly enhanced in the two clinkers. For BCSA clinkers synthesized by including either bottom ash or fly ash, values are reported to be between 0.4 and 2.3 wt.% [
16,
32,
33]. The main source of Mg was steel slag (
Table 1); however, the overall amount of MgO introduced in both raw clinker mixtures was around 2 wt.%. The values are below the limit, if compared to the OPC clinker requirements, where the content of MgO should not exceed 5 wt.% [
34] in order to avoid the formation of dead-burned MgO with a very slow hydration rate, as the late expansion of MgO would jeopardize the cement soundness [
35]. At all sintering temperatures, some aphthitalite (K
3Na(SO
4)
2), langbeinite (K
2Ca
2(SO
4)
3), and arcanite (K
2SO
4) were also detected in KMO1 (
Table 5), as a consequence of the higher content of fly ash, which contains alkalis.
3.1.2. SEM/EDS
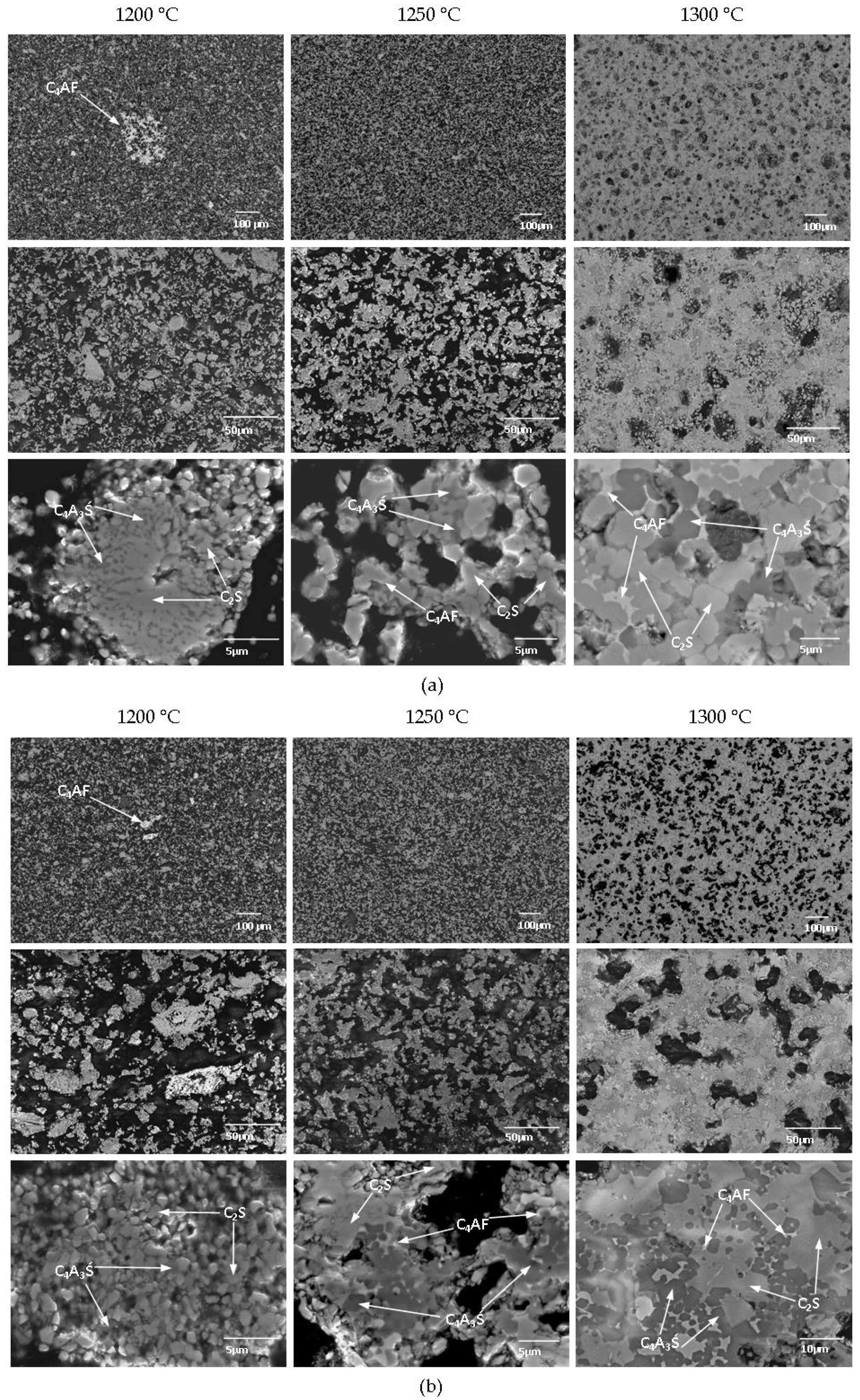
Clinkers showed a clear decrease in porosity as the sintering temperature increased (
Figure 2). In both mixtures, a temperature of 1200 °C was sufficient for developing the main reactive phases. However, X-ray Powder Diffraction (XRD) analysis revealed that the targeted amounts were not achieved at 1200 °C. Subhedral to euhedral C
2S and C
4A
3Ś grains were already clearly developed at 1200 °C in both KMO1 and KMO2 clinkers. However, at 1200 °C, the clinkers showed an inhomogeneous structure, as C
4AF was not present in the interstitial areas, but formed in individual nests (
Figure 2). In both mixtures, sintering at 1250 °C resulted in a homogeneous structure, formed by subhedral to euhedral C
2S and C
4A
3Ś grains and C
4AF in the interstitial area (
Figure 2). Raising the sintering temperature to 1300 °C led to structure densification, and increased the number of euhedral C
2S and C
4A
3Ś grains (
Figure 2).
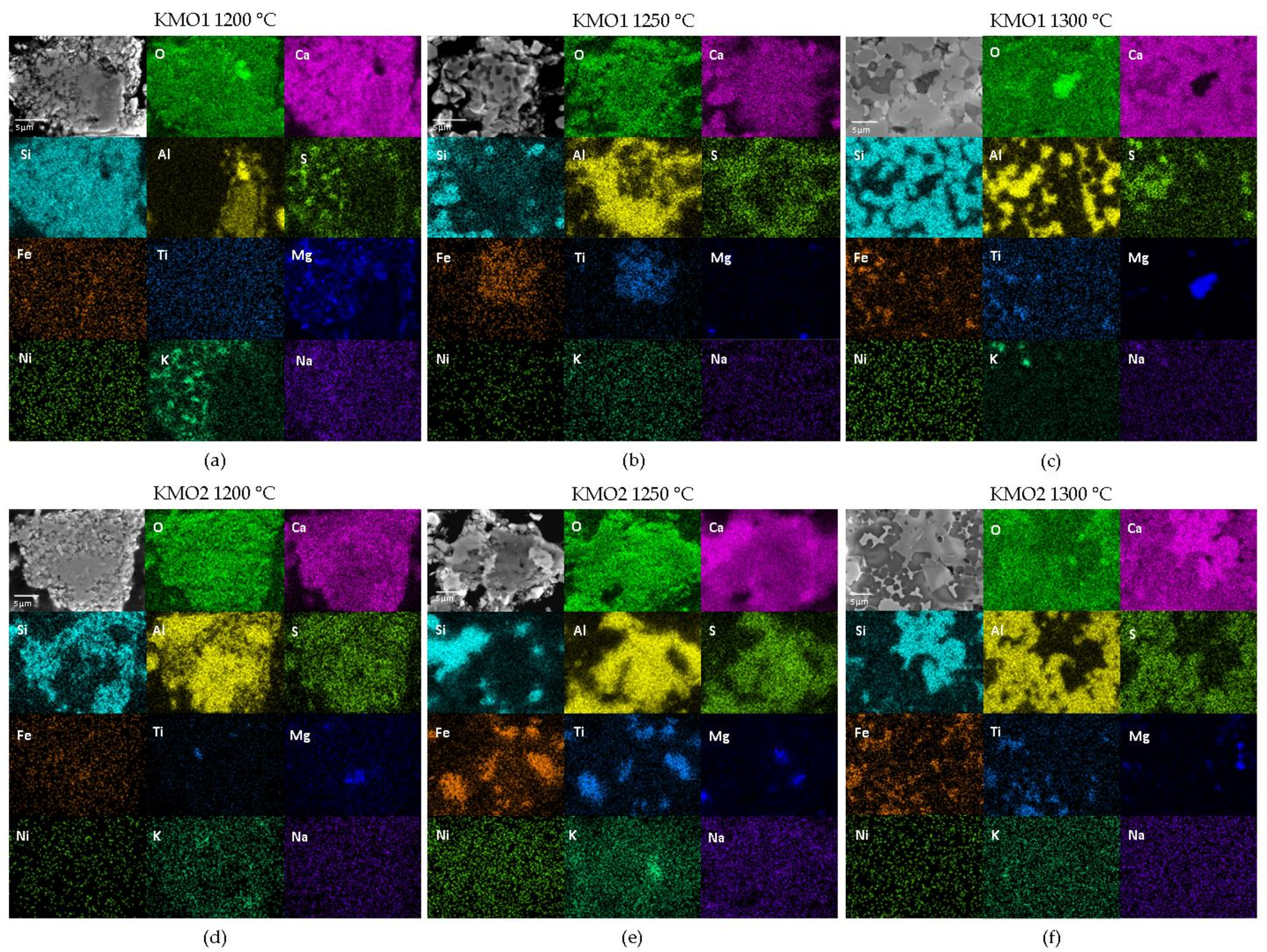
Figure 3 shows elemental EDS maps for the KMO1 and KMO2 clinkers sintered at 1200, 1250, and 1300 °C.
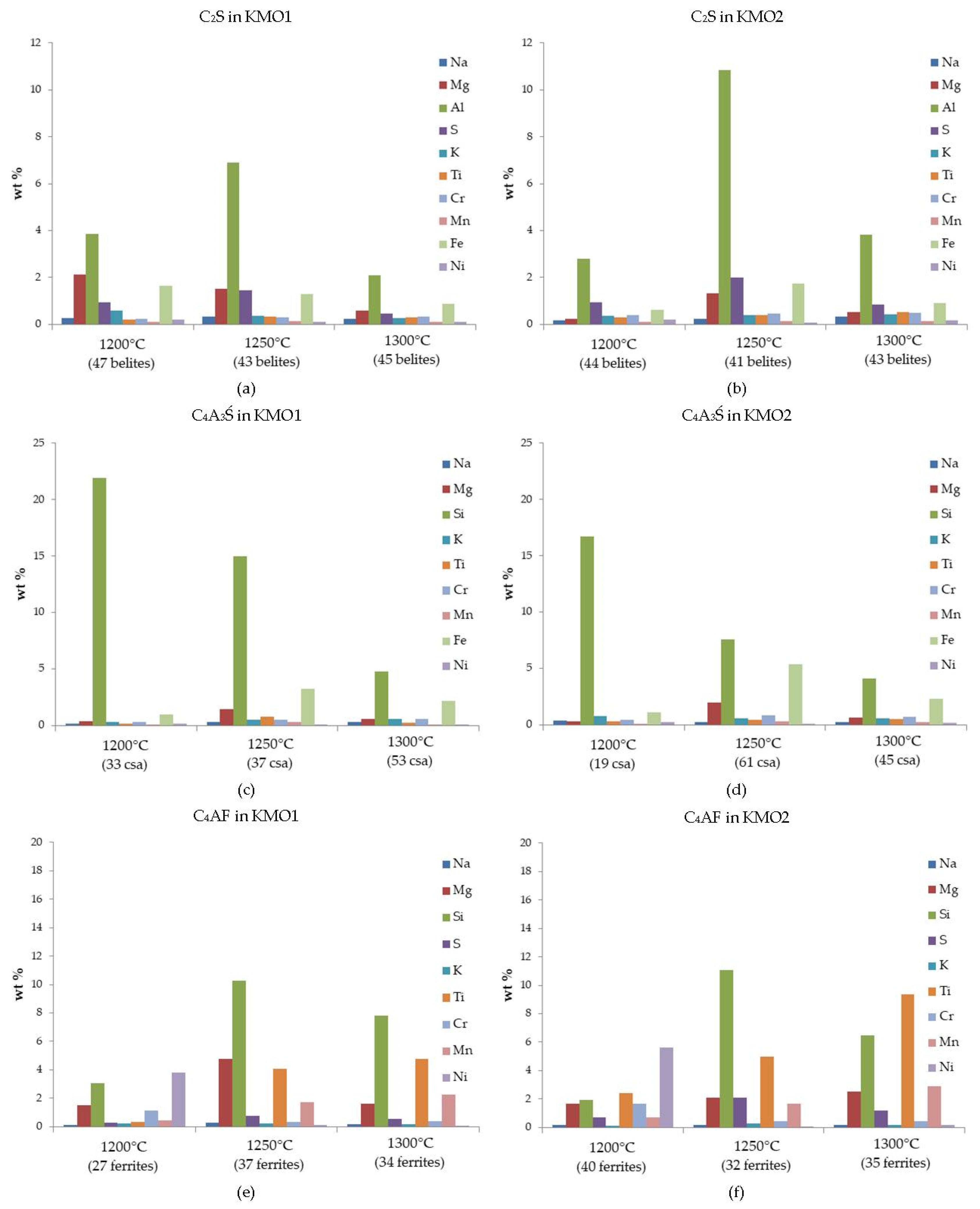
Furthermore, the amounts of minor individual elements measured (EDS point measurements and SEM statistics), incorporated in the main clinker phases, are presented as sequences, in order of the maximum to minimum quantity detected. Belite enables various element substitutions [
12,
23,
36,
37,
38]. It exhibited Al, Mg, Fe, S, K, Cr, Ti, Na, Ni, and Mn in the KMO1 clinker and Al, S, Fe, Mg, Cr, Ti, K, Na, Ni, and Mn in the KMO2 clinker. The higher amount of S detected in KMO2 corresponds to a higher gypsum content (
Table 3). On the other hand, the higher Mg content of the KMO1 belites, as detected by EDS analysis, cannot be explained by a higher input from the raw materials (
Table 1 and
Table 3). However, the highest amount of Mg in KMO1 belites was detected at 1200 °C, where the sintering was not complete, as evident from the EDS maps (
Figure 4a). In both clinker mixtures, the highest amount of Al was incorporated into belite at 1250 °C (
Figure 4a,b). On the other hand, the amount of Mg incorporated varied between the clinker mixtures. In KMO1, the amount of Mg in the belite phase clearly decreased with an increase in the sintering temperature (
Figure 4a), while in KMO2 belites, the maximum amount of Mg was detected at 1250 °C (
Figure 4b). In both clinkers, belites included the maximum amount of S at 1250 °C. The amount of Fe in the KMO1 belites decreased with a rise in the sintering temperature (
Figure 4a), while in KMO2, the maximum amount of Fe incorporated in belite occurred at 1250 °C (
Figure 4b). The maximum amount of K incorporated into belites occurred at 1200 °C in KMO1, while in KMO2, the K content of belites did not change as a result of the sintering temperature.
In both clinkers, calcium sulfoaluminate included minor elements in the same sequence, with Si, Fe, and Mg, followed by K, Cr, Ti, Na, Mn, and Ni. The quantity of Si decreased with rising temperatures in both KMO1 and KMO2, while the maximum amount of Fe and Mg occurred at 1250 °C (
Figure 4c,d). The high Si content can be attributed to the fact that calcium sulfoaluminate is an end member of sodalite solid solution, in which Si is replaced by Al [
39,
40]. Consequently, increasing the sintering temperature resulted in decreased amounts of Si (
Figure 4c,d).
In KMO1, ferrite included Si, Ti, Mg, Mn, Ni, Cr, S, K, and Na, which slightly differed to the sequence of Si, Ti, Mg, Ni, Mn, S, Cr, K, and Na incorporated in KMO2. In both KMO1 and KMO2, the maximum amount of Si was incorporated into the ferrite phase at 1250 °C (
Figure 4e,f). However, it has already been reported that Si and S commonly replace Fe or Al in a ferrite structure [
41]. In both clinkers, the amount of Ti incorporated into the ferrite phase increased when raising the sintering temperature (
Figure 4e,f). In KMO1, the maximum amount of Mg was incorporated into ferrites at 1250 °C, while in KMO2, the amount of Mg was constant across all sintering temperatures. In both clinkers, the maximum amounts of Ni and Cr were incorporated into ferrite at 1200 °C (
Figure 4e,f).
Amongst the minor clinker phases, EDS mapping identified Mg-rich areas of periclase, which were seen in both clinker mixtures across all three sintering temperatures (
Figure 3). Although steel slag contains a large amount of MgO (
Table 1), the overall amount of Mg in clinkers was not substantially increased as clinker raw mixtures only incorporated around 8 or 11 wt.% of steel slag (
Table 3). Moreover, the amount of periclase, determined by Rietveld refinement, was not very considerable (
Table 5). The Ti-enriched areas correspond to perovskite, which was also identified in all clinkers (
Figure 3a–d), but it was present to a greater degree in the KMO2 mixture (
Figure 3d–f), as also supported by the XRD results (
Table 5). Ca- and Al-enriched interstitial areas were observed in all clinkers, which can be attributed to the mayenite phase (
Figure 3). KMO1 clinker mixtures also showed some K- and S-enriched areas of K-sulphate minor phases (
Figure 3a–c).