Use of Potabilized Water Sludge in the Production of Low-Energy Blended Calcium Sulfoaluminate Cements
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Test Methods and Characterization Techniques
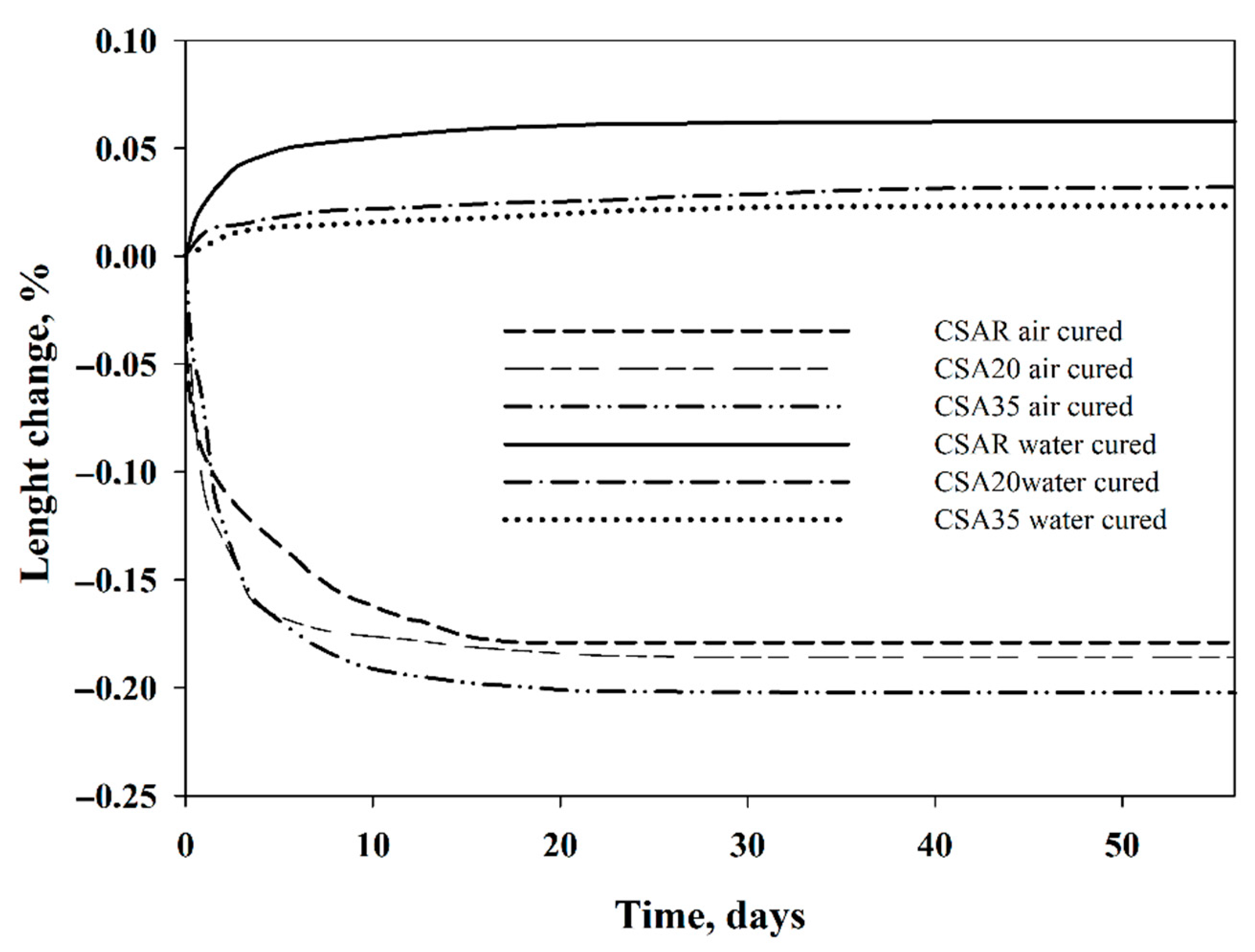
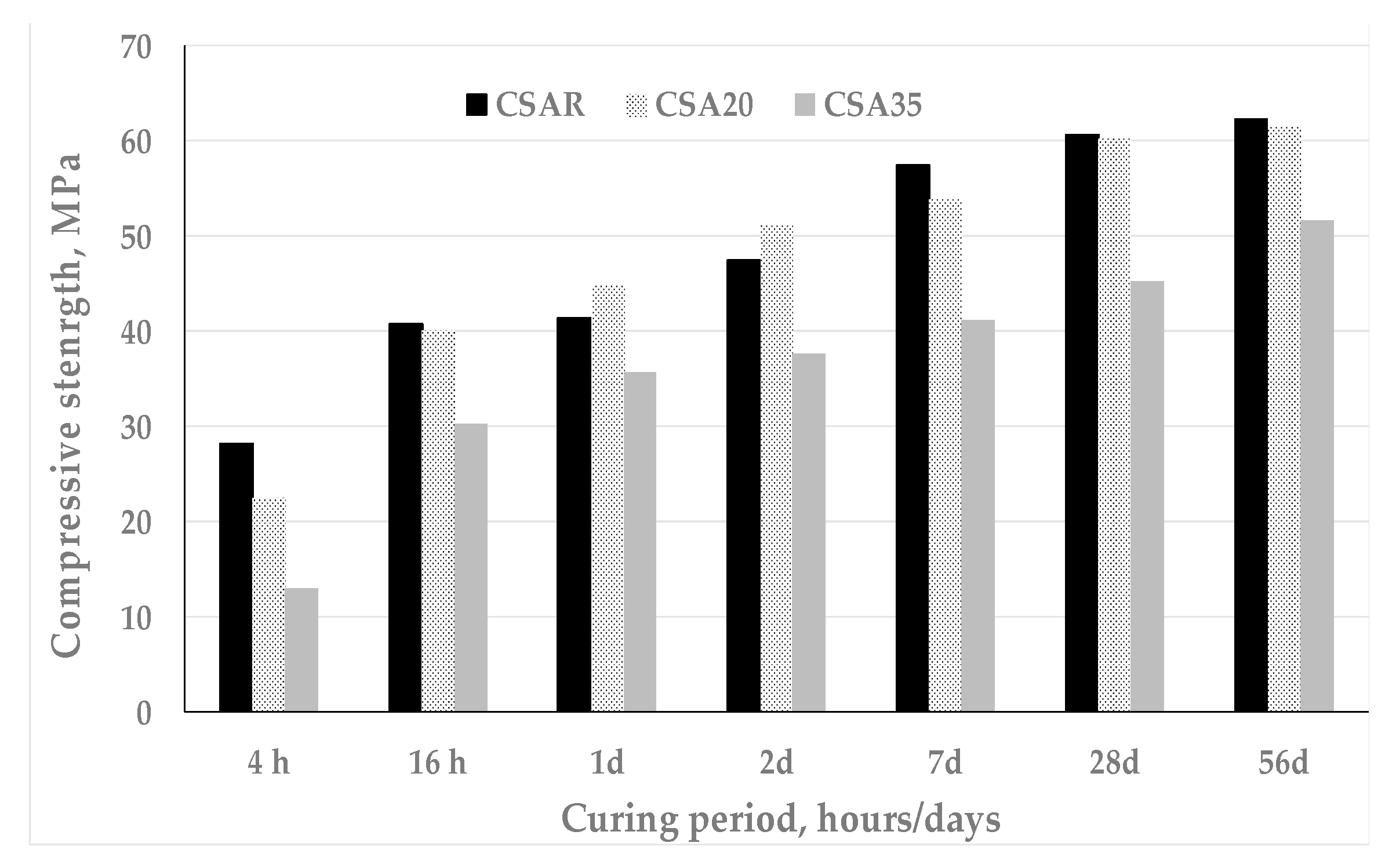
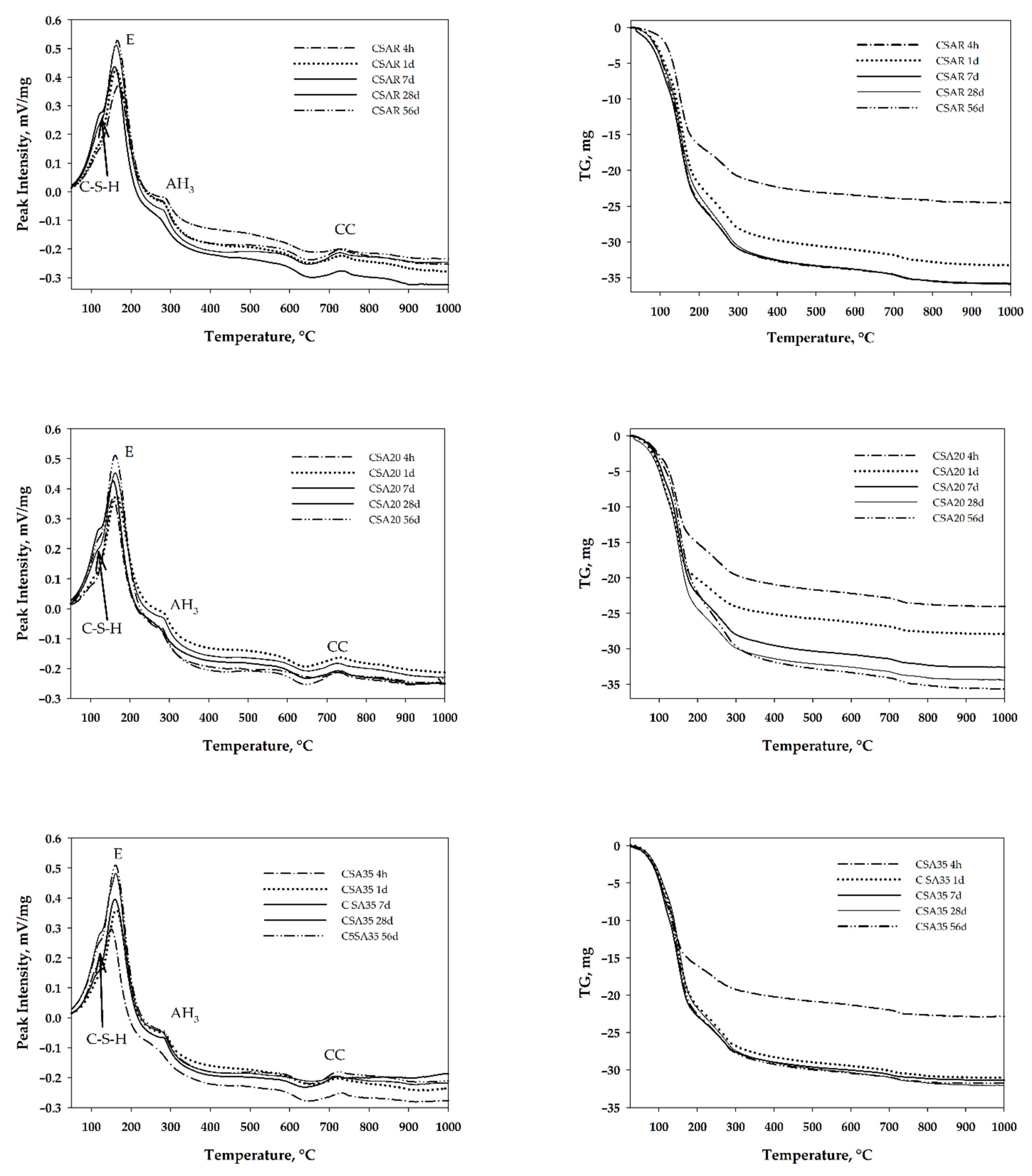
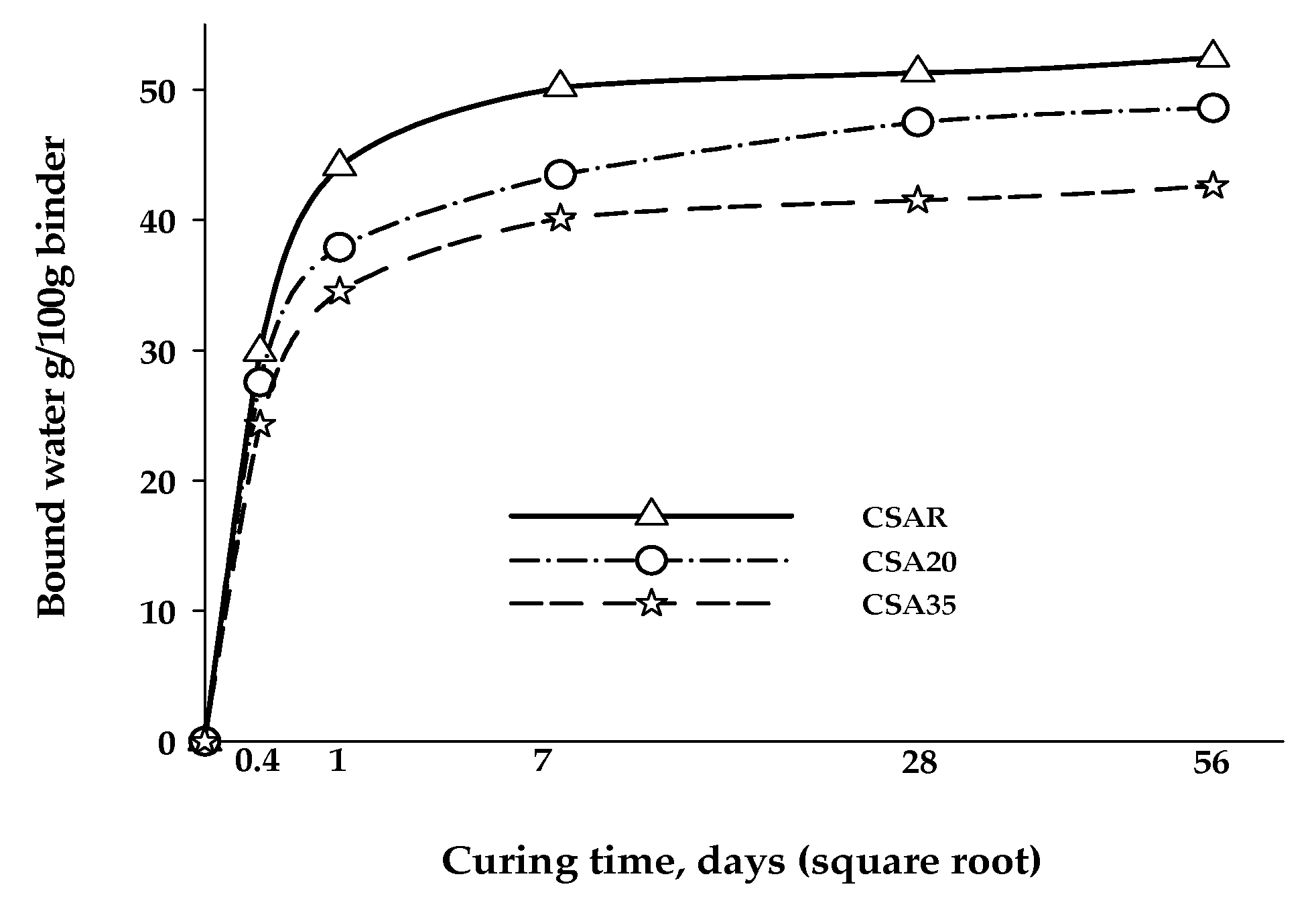
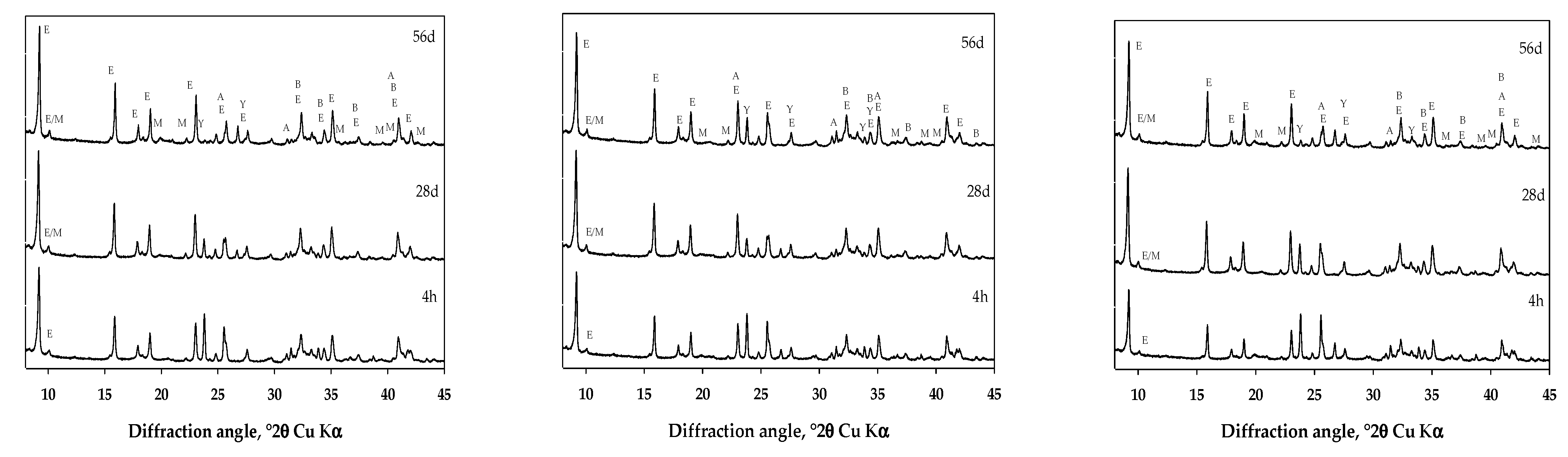
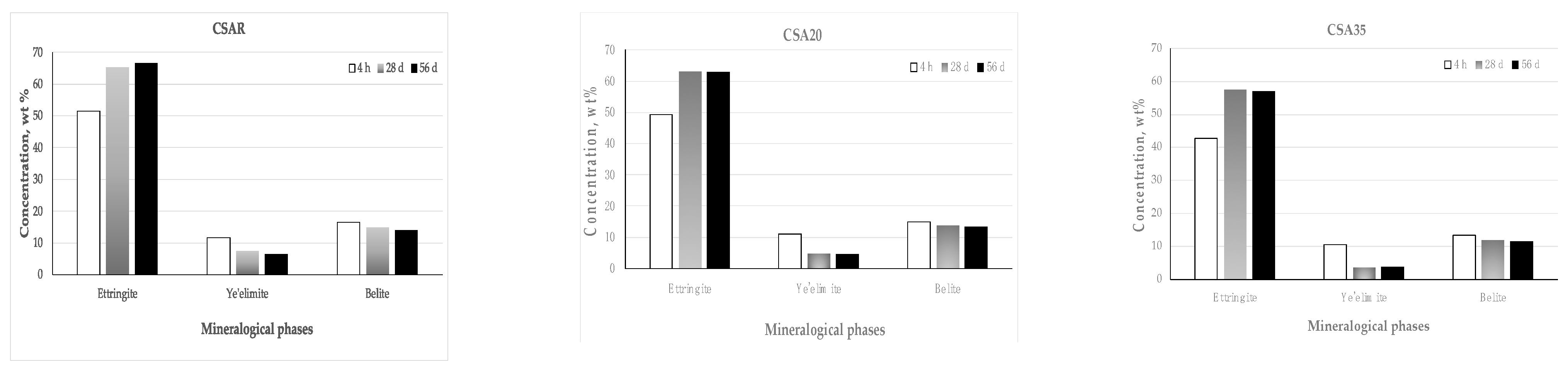
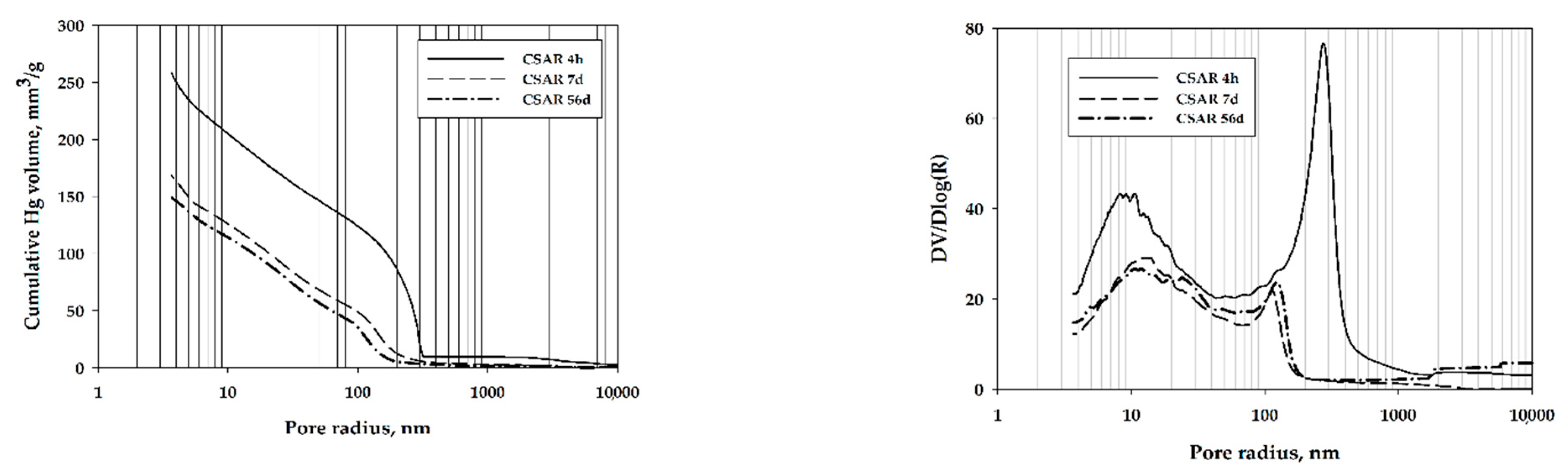
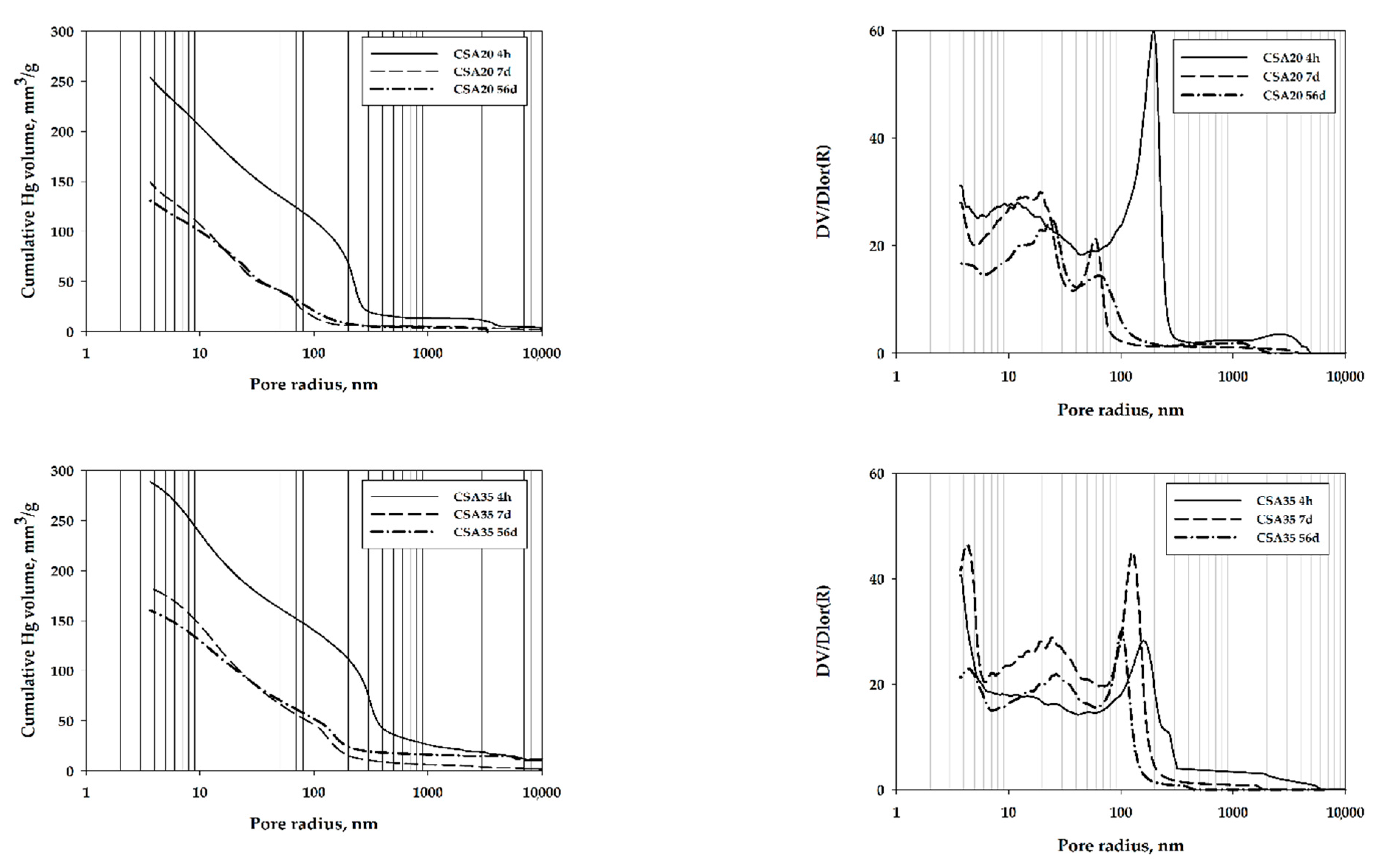
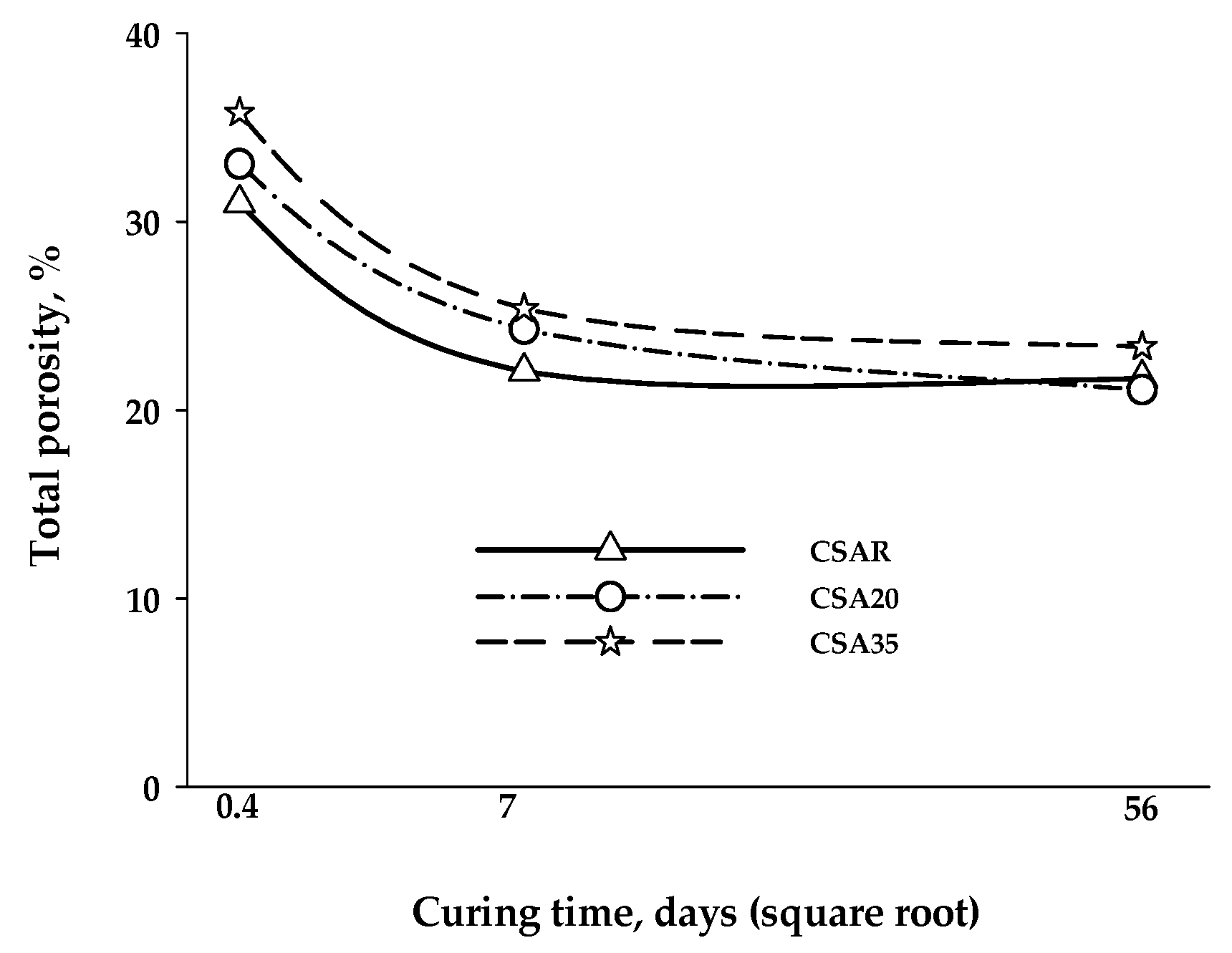
3. Results and Discussion
4. Environmental Implications of the Manufacture of CSA-Based Cements: Kiln Thermal Requirement and CO2 Emissions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Telesca, A.; Matschei, T.; Marroccoli, M. Study of eco-friendly belite-calcium sulfoaluminate cements obtained from special wastes. Appl. Sci. 2020, 10, 8650. [Google Scholar] [CrossRef]
- IEA. 2020. Available online: https://www.iea.org/reports/global-energy-review-2020/global-energy-and-co2-emissions-in-2020 (accessed on 27 January 2021).
- Barcelo, L.; Kline, J.; Walenta, G.; Gartner, E. Cement and carbon emissions. Mater. Struct. 2014, 47, 1055–1065. [Google Scholar] [CrossRef]
- Schneider, M. The cement industry on the way to a low-carbon future. Cem. Concr. Res. 2019, 124, 105792. [Google Scholar] [CrossRef]
- Shi, C.; Qu, B.; Provis, J.L. Recent progress in low-carbon binders. Cem. Concr. Res. 2019, 122, 227–250. [Google Scholar] [CrossRef]
- Gartner, E.; Sui, T. Alternative cement clinkers. Cem. Concr. Res. 2018, 114, 27–39. [Google Scholar] [CrossRef]
- Tregambi, C.; Solimene, R.; Montagnaro, F.; Salatino, P.; Marroccoli, M.; Ibris, N.; Telesca, A. Solar-driven production of lime for ordinary Portland cement formulation. Sol. Energy 2018, 173, 759–768. [Google Scholar] [CrossRef]
- Gartner, E.; Hirao, H. A review of alternative approaches to the reduction of CO2 emissions associated with the manufacture of the binder phase in concrete. Cem. Concr. Res. 2015, 78, 126–142. [Google Scholar] [CrossRef]
- Juenger, M.C.G.; Winnefeld, F.; Provis, J.L.; Ideker, J.H. Advances in alternative cementitious binders. Cem. Concr. Res. 2011, 41, 1232–1243. [Google Scholar] [CrossRef]
- Telesca, A.; Marroccoli, M.; Tomasulo, M.; Valenti, G.L.; Dieter, H.; Montagnaro, F. Calcium looping spent sorbent as a limestone replacement in the manufacture of Portland and calcium sulfoaluminate cements. Environ. Sci. Technol. 2015, 49, 6865–6871. [Google Scholar] [CrossRef] [PubMed]
- Marroccoli, M.; Montagnaro, F.; Pace, M.L.; Telesca, A.; Valenti, G.L. Utilization of coal combustion ashes for the synthesis of ordinary and special cements. Combust. Sci. Technol. 2010, 182, 588–599. [Google Scholar] [CrossRef]
- Cardinale, T.; D’Amato, M.; Sulla, R.; Cardinale, N. Mechanical and Physical Characterization of Papercrete as New Eco-Friendly Construction Material. Appl. Sci. 2021, 11, 1011. [Google Scholar] [CrossRef]
- Diaz-Loya, I.; Juenger, M.; Seraj, S.; Minkara, R. Extending supplementary cementitious material resources: Reclaimed and remediated fly ash and natural pozzolans. Cem. Concr. Compos. 2019, 101, 44–51. [Google Scholar] [CrossRef]
- Juenger, M.C.G.; Snellings, R.; Bernal, S.A. Supplementary cementitious materials: New sources, characterization, and performance insights. Cem. Concr. Res. 2019, 122, 257–273. [Google Scholar] [CrossRef]
- Snellings, R. Assessing, understanding and unlocking supplementary cementitious materials. RILEM Tech. Lett. 2016, 1, 50–55. [Google Scholar] [CrossRef]
- Juenger, M.C.G.; Siddique, R. Recent advances in understanding the role of supplementary cementitious materials in concrete. Cem. Concr. Res. 2015, 78, 71–80. [Google Scholar] [CrossRef]
- De Weerdt, K.; Ben Haha, M.; Le Saout, G.; Kjellsen, K.O.; Justnes, H.; Lothenbach, B. Hydration mechanisms of ternary Portland cements containing limestone powder and fly ash. Cem. Concr. Res. 2011, 41, 279–291. [Google Scholar] [CrossRef]
- Mobili, A.; Telesca, A.; Marroccoli, M.; Tittarelli, F. Calcium sulfoaluminate and alkali-activated fly ash cements as alternative to Portland cement: Study on chemical, physical-mechanical, and durability properties of mortars with the same strength class. Constr. Build. Mater. 2020, 246, 118436. [Google Scholar] [CrossRef]
- Dung, N.T.; Unluer, C. Development of MgO concrete with enhanced hydration and carbonation mechanisms. Cem. Concr. Res. 2018, 103, 160–169. [Google Scholar] [CrossRef]
- Gomez, C.M.; de Oliveira, A.D.S. Chemical phases and microstructural analysis of pastes based on magnesia cement. Constr. Build. Mater. 2018, 188, 615–620. [Google Scholar] [CrossRef]
- Luukkonen, T.; Abdollahnejad, Z.; Yliniemi, J.; Kinnunen, P.; Illikainen, M. One-part alkali-activated materials: A review. Cem. Concr. Res. 2018, 103, 21–34. [Google Scholar] [CrossRef]
- Mobili, A.; Belli, A.; Giosué, C.; Telesca, A.; Marroccoli, M.; Tittarelli, F. Calcium sulfoaluminate, geopolimeric, and cementitious mortars for structural applications. Environments 2017, 4, 64. [Google Scholar] [CrossRef]
- Myers, R.J.; Lothenbach, B.; Bernal, S.A.; Provis, J.L. Thermodynamic modelling of alkali-activated slag cements. J. Appl. Geochem. 2015, 61, 233–247. [Google Scholar] [CrossRef]
- Walling, S.A.; Provis, J.L. Magnesia-based cements: A journey of 150 years, and cements for the future? Chem. Rev. 2016, 116, 4170–4204. [Google Scholar] [CrossRef]
- Provis, J.L.; van Deventer, J.S.J. Alkali-Activated Materials: State-of-the-Art Report; RILEM TC 224-AAM, 2014; Provis, J., van Deventer, J., Eds.; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Marroccoli, M.; Montagnaro, F.; Telesca, A.; Valenti, G.L. Environmental implications of the manufacture of calcium sulfoaluminate-based cements. In Proceedings of the 2nd International Conference on Sustainable Construction Materials and Technologies, Ancona, Italy, 28–30 June 2010; Zachar, J., Claisse, P., Naik, T.R., Ganjiam, E., Eds.; UWM Center for By-Products Utilization: Milwaukee, WI, USA, 2010. [Google Scholar]
- Marroccoli, M.; Nobili, M.; Telesca, A.; Valenti, G.L. Early hydration of calcium sulfoaluminate-based cements for structural applications. In Proceedings of the International Conference on Sustainable Construction Materials and Technology, Coventry, UK, 11–13 June 2007; pp. 389–395. [Google Scholar]
- Wang, L.; Zhan, S.; Tang, X.; Xu, Q.; Qian, K. Pore Solution Chemistry of Calcium Sulfoaluminate Cement and Its Effects on Steel Passivation. Appl. Sci. 2019, 9, 1092. [Google Scholar] [CrossRef]
- Telesca, A.; Marroccoli, M.; Pace, M.L.; Tomasulo, M.; Valenti, G.L.; Monteiro, P.J.M. A hydration study of various calcium sulfoaluminate cements. Cem. Concr. Comp. 2014, 53, 224–232. [Google Scholar] [CrossRef]
- Chen, I.A.; Hargis, C.W.; Juenger, M.C.G. Understanding expansion in calcium sulfoaluminate-belite cements. Cem. Concr. Res. 2012, 42, 51–60. [Google Scholar] [CrossRef]
- Winnefeld, F.; Lothenbach, B. Hydration of calcium sulfoaluminate cements—Experimental findings and thermodynamic modelling. Cem. Concr. Res. 2010, 40, 1239–1247. [Google Scholar] [CrossRef]
- Glasser, F.P.; Zhang, L. High-performance cement matrices based on calcium sulphoaluminate-belite compositions. Cem. Concr. Res. 2001, 31, 1881–1886. [Google Scholar] [CrossRef]
- Telesca, A.; Marroccoli, M.; Winnefeld, F. Synthesis and characterization of calcium sulfoaluminate cements produced by different chemical gypsums. Adv. Cem. Res. 2019, 31, 113–123. [Google Scholar] [CrossRef]
- Xu, L.; Wu, K.; Li, N.; Zhou, X.; Wang, P. Utilization of flue gas desulfurization gypsum for producing calcium sulfoaluminate cement. J. Clean. Prod. 2017, 161, 803–811. [Google Scholar] [CrossRef]
- Telesca, A.; Marroccoli, M.; Tomasulo, M.; Valenti, G.L.; Dieter, H.; Montagnaro, F. Low-CO2 Cements from Fluidized Bed Process Wastes and Other Industrial By-Products. Comb. Sci. Tech. 2016, 188, 492–503. [Google Scholar] [CrossRef]
- Shen, Y.; Qian, J.; Chai, J.; Fan, Y. Calcium sulphoaluminate cements made with phosphogypsum: Production issues and material properties. Cem. Concr. Res. 2014, 48, 67–74. [Google Scholar] [CrossRef]
- Wu, K.; Shi, H.; Guo, X. Utilization of municipal solid waste incineration fly ash for sulfoaluminate cement clinker production. Waste Manag. 2011, 31, 2001–2008. [Google Scholar] [CrossRef]
- Marroccoli, M.; Pace, M.L.; Telesca, A.; Valenti, G.L. Synthesis of calcium sulfoaluminate cements from Al2O3-rich by-products from aluminium manufacture. In Proceedings of the 2nd International Conference on Sustainable Construction Materials and Technologies, Ancona, Italy, 28–30 June 2010; Zachar, J., Claisse, P., Naik, T.R., Ganjiam, E., Eds.; UWM Center for By-Products Utilization: Milwaukee, WI, USA, 2010. [Google Scholar]
- Marroccoli, M.; Montagnaro, F.; Pace, M.L.; Telesca, A.; Valenti, G.L. Use of fluidized bed combustion ash and other industrial wastes as raw materials for the manufacture of calcium sulphoaluminate cements. In Proceedings of the 20th International Conference on Fluidized Bed Combustion, Xian, China, 18–21 May 2009; Yue, G., Zhang, H., Zhao, C., Luo, Z., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 389–395. [Google Scholar]
- Adolfsson, D.; Menad, N.; Viggh, E.; Bjorkman, B. Steelmaking slags as raw material for sulphoaluminate belite cement. Adv. Cem. Res. 2007, 19, 147–156. [Google Scholar] [CrossRef]
- Gao, D.; Zhang, Z.; Meng, Y.; Tang, J.; Yang, L. Effect of Flue Gas Desulfurization Gypsum on the Properties of Calcium Sulfoaluminate Cement Blended with Ground Granulated Blast Furnace Slag. Materials 2021, 14, 382. [Google Scholar] [CrossRef]
- Kim, T.; Ki_Young, Y.S.; Kang, C.; Lee, T.-K. Development of Eco-Friendly Cement Using a Calcium Sulfoaluminate Expansive Agent Blended with Slag and Silica Fume. Appl. Sci. 2021, 11, 394. [Google Scholar] [CrossRef]
- Yoon, H.N.; Seo, J.; Kim, S.; Lee, H.K.; Park, S. Hydration of calcium sulfoaluminate cement blended with blast-furnace slag. Const. Build. Mat. 2021, 268, 121214. [Google Scholar] [CrossRef]
- Niu, M.; Zhang, J.; Li, G.; Song, Z.; Wang, X. Mechanical properties of polyvinyl alcohol fiber reinforced sulfoaluminate cement mortar containing high-volume of fly ash. J. Build. Eng. 2021, 35, 101988. [Google Scholar] [CrossRef]
- Coppola, L.; Coffetti, D.; Crotti, E.; Dell’Aversano, R.; Gazzaniga, G.; Pastore, T. Influence of Lithium Carbonate and Sodium Carbonate on Physical and Elastic Properties and on Carbonation Resistance of Calcium Sulphoaluminate-Based Mortars. Appl. Sci. 2020, 10, 176. [Google Scholar] [CrossRef]
- Ding, H.; Zhang, S. Quicklime and Calcium Sulfoaluminate Cement Used as Mineral Accelerators to Improve the Properties of Cemented Paste Backfill with a High Volume of Fly Ash. Materials 2020, 13, 4018. [Google Scholar] [CrossRef]
- Ke, G.; Zhang, J.; Xie, S.; Pei, T. Rheological behavior of calcium sulfoaluminate cement paste with supplementary cementitious materials. Constr. Build. Mater. 2020, 243, 118234. [Google Scholar] [CrossRef]
- Liao, Y.; Jiang, G.; Wang, K.; Qunaynah, S.A.; Yuan, W. Effect of steel slag on the hydration and strength development of calcium sulfoaluminate cement. Constr. Build. Mater. 2020, 265, 12030. [Google Scholar] [CrossRef]
- Bertola, F.; Gastaldi, D.; Canonico, F.; Paul, G. CSA and slag: Towards CSA composite binders. Adv. Cem. Res. 2019, 31, 147–158. [Google Scholar] [CrossRef]
- Bullerjahn, F.; Zajac, M.; Skocek, J.; Ben Haha, M. The role of boron during the early hydration of belite ye’elimite ferrite cements. Constr. Build. Mat. 2019, 215, 252–263. [Google Scholar] [CrossRef]
- Gao, D.; Meng, Y.; Yang, L.; Tang, J.; Lv, M. Effect of ground granulated blast furnace slag on the properties of calcium sulfoaluminate cement. Constr. Build. Mater. 2019, 227, 116665. [Google Scholar] [CrossRef]
- Jun, Y.; Hong Kim, J.; Kim, T. Hydration of Calcium Sulfoaluminate-Based Binder Incorporating Red Mud and Silica Fume. Appl. Sci. 2019, 9, 2270. [Google Scholar] [CrossRef]
- Martin, L.H.J.; Winnefeld, F.; Tschopp, E.; Müller, C.J.; Lothenbach, B. Influence of fly ash on the hydration of calcium sulfoaluminate cement. Cem. Concr. Res. 2017, 95, 152–163. [Google Scholar] [CrossRef]
- Martin, L.H.J.; Winnefeld, F.; Müller, C.J.; Lothenbach, B. Contribution of limestone to the hydration of calcium sulfoaluminate cement. Cem. Con. Comp. 2015, 62, 204–211. [Google Scholar] [CrossRef]
- Hargis, G.W.; Telesca, A.; Monteiro, P.J.M. Calcium sulfoaluminate (ye’elimite) hydration in the presence of gypsum, calcite and vaterite. Cem. Concr. Res. 2014, 65, 15–20. [Google Scholar] [CrossRef]
- Ioannou, S.; Reig, L.; Paine, K.; Quillin, K. Properties of a ternary calcium sulfoaluminate-calcium sulfate-fly ash cement. Cem. Concr. Res. 2014, 56, 75–83. [Google Scholar] [CrossRef]
- García-Maté, M.; De la Torre, A.G.; León-Reina, L.; Aranda, M.A.; Santacruz, I. Hydration studies of calcium sulfoaluminate cements blended with fly ash. Cem. Concr. Compos. 2013, 54, 12–20. [Google Scholar] [CrossRef]
- Martin, L.H.J.; Winnefeld, F.; Müller, C.J.; Lothenbach, B. Influence of limestone on the hydration of calcium sulfoaluminate cement. In Proceedings of the First International Conference on Sulphoaluminate Cement: Materials and Engineering Technology, Wuhan, China, 23–24 October 2013; Xu, Y., Zhang, Q., Eds.; WUT Press: Wuhan, China, 2013; pp. 229–245. [Google Scholar]
- Chen, I.A.; Juenger, M.C.J. Incorporation of coal combustion residuals into calcium sulfoaluminate- belite cement clinkers. Cem. Concr. Res. 2012, 34, 893–902. [Google Scholar] [CrossRef]
- Pelletier-Chaignat, L.; Winnefeld, F.; Lothenbach, B.; Müller, C.J. Beneficial use of limestone filler with calcium sulfoaluminate cement. Constr. Build. Mater. 2012, 26, 619–627. [Google Scholar] [CrossRef]
- Ferone, C.; Capasso, I.; Bonati, A.; Roviello, G.; Montagnaro, F.; Santoro, L.; Turco, R.; Cioffi, R. Sustainable management of water potabilization sludge by means of geopolymers production. J. Clean. Prod. 2019, 229, 1–9. [Google Scholar] [CrossRef]
- Ahmad, T.; Ahmad, K.; Alam, M. Sustainable management of water treatment sludge through 3’R’ concept. J. Clean. Prod. 2016, 124, 1–13. [Google Scholar] [CrossRef]
- Mohammed, S. Processing effect and reactivity assessment of artificial pozzolans obtained from clays and clay wastes: A review. Constr. Build. Mater. 2017, 140, 10–19. [Google Scholar] [CrossRef]
- Telesca, A. (University of Basilicata, Potenza, Italy); Marroccoli, M. (University of Basilicata, Potenza, Italy). Personal communication. 2019. [Google Scholar]
- Taylor, H.F.W. Cement Chemistry, 2nd ed.; Academic Press: London, UK, 1997; p. 480. [Google Scholar] [CrossRef]
- Naqi, A.; Jang, J.G. Recent Progress in Green Cement Technology Utilizing Low-Carbon Emission Fuels and Raw Materials: A Review. Sustainability 2019, 11, 537. [Google Scholar] [CrossRef]
- Coppola, L.; Coffetti, D.; Crotti, E. An holistic approach to a sustainable future in concrete construction. IOP Conf. Ser. Mater. Sci. Eng. 2018, 442, 012024. [Google Scholar] [CrossRef]
- Madlool, N.A.; Saidur, R.; Rahim, N.A.; Kamalisarvestani, M. An overview of energy savings measures for cement industries, Renew. Sustain. Energ. Rev. 2013, 19, 18–29. [Google Scholar] [CrossRef]
- Van den Heede, P.; De Belie, N. Environmental impact and life cycle assessment (LCA) of traditional and ‘green’ concretes: Literature review and theoretical calculations. Cem. Concr. Res. 2012, 34, 431–442. [Google Scholar] [CrossRef]
- Imbabi, M.S.; Carrigan, C.; McKenna, S. Trends and developments in green cement and concrete technology. Int. J. Sustain. Built Env. 2012, 1, 194–216. [Google Scholar] [CrossRef]
- Damtoft, J.S.; Lukasik, J.; Herfort, D.; Sorrentino, D.; Gartner, E.M. Sustainable development and climate change initiatives. Cem. Concr. Res. 2008, 38, 115–127. [Google Scholar] [CrossRef]
- Worrell, E.; Martin, N.; Price, L. Potentials for energy efficiency improvement in the US cement industry. Energy 2000, 25, 1189–1214. [Google Scholar] [CrossRef]
- Chatterjee, A.; Sui, T. Alternative fuels—Effects on clinker process and properties. Cem. Concr. Res. 2019, 103, 105777. [Google Scholar] [CrossRef]
- Kajaste, R.; Hurme, M. Cement industry greenhouse gas emissions e management options and abatement cost. J. Clean. Prod. 2016, 112, 4041–4052. [Google Scholar] [CrossRef]
- Coppola, L.; Coffetti, D.; Crotti, E.; Gazzaniga, G.; Pastore, T. An Empathetic Added Sustainability Index (EASI) for cementitious based construction materials. J. Clean. Prod. 2019, 220, 475–482. [Google Scholar] [CrossRef]
- Ludwig, H.M.; Zhang, W. Research review of cement clinker chemistry. Cem. Concr. Res. 2015, 78, 24–37. [Google Scholar] [CrossRef]
- Schneider, M. Process technology for efficient and sustainable cement production. Cem. Concr. Res. 2015, 78, 14–23. [Google Scholar] [CrossRef]
- Technology Roadmap: Low-Carbon Transition in the Cement Industry; International Energy Agency. 2018. Available online: https://www.wbcsd.org/Sector-Projects/Cement-Sustainability-Initiative/Resources/Technology-Roadmap-Low-Carbon-Transition-in-the-Cement-Industry (accessed on 27 January 2021).
- Jaskulski, R.; Jóźwiak-Niedźwiedzka, D.; Yakymechko, Y. Calcined Clay as Supplementary Cementitious Material. Materials 2020, 13, 4734. [Google Scholar] [CrossRef]
- Dãaz, Y.C.; Berriel, S.S.; Heierli, U.; Favier, A.R.; Machado, I.R.S.; Scrivener, K.L.; Hernã¡ndez, J.F.M.; Habert, G. Limestone calcined clay cement as a low-carbon solution to meet expanding cement demand in emerging economies. Dev. Eng. 2017, 2, 82–91. [Google Scholar] [CrossRef]
| Chemical Composition | Mineralogical Phase Composition | |||||
|---|---|---|---|---|---|---|
| CSA Cement | WPSs | TTWPSs | CSA Cement | ICDD Ref. Number | ||
| CaO | 44.58 | 4.95 | 6.26 | Ye’elimite | 30-0256 | 43.0 |
| SiO2 | 8.95 | 37.71 | 47.66 | β-belite | 33-0302 | 21.7 |
| Al2O3 | 22.42 | 27.52 | 34.78 | Celite | 38-1429 | 3.8 |
| Fe2O3 | 1.86 | 2.37 | 3.00 | Anhydrite | 37-1496 | 19.1 |
| TiO2 | 1.10 | 0.35 | 0.44 | Calcite | 05-0586 | 1.1 |
| K2O | 0.30 | 1.01 | 1.28 | Brownmillerite | 30-0256 | 4.5 |
| MnO | 0.08 | 0.33 | 0.42 | Gehlenite | 73-2041 | 1.6 |
| Na2O | 0.08 | 0.35 | 0.44 | Others | - | 5.2 |
| MgO | 0.94 | 1.18 | 1.49 | |||
| Cl | 0.07 | 0.01 | 0.01 | |||
| SO3 | 16.85 | 0.53 | 0.67 | |||
| P2O5 | 0.05 | 1.18 | 1.49 | |||
| l.o.i * | 2.16 | 22.12 | 1.56 | |||
| Total | 99.44 | 99.61 | 99.50 | Total | 100.0 | |
| Kiln Thermal Requirement | CO2 Emissions | |
|---|---|---|
| OPC CEM I | 3280 | 0.82 |
| CSAR | 2295 | 0.51 |
| CSA20 | 2278 | 0.45 |
| CSA35 | 2265 | 0.40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Telesca, A.; Ibris, N.; Marroccoli, M. Use of Potabilized Water Sludge in the Production of Low-Energy Blended Calcium Sulfoaluminate Cements. Appl. Sci. 2021, 11, 1679. https://doi.org/10.3390/app11041679
Telesca A, Ibris N, Marroccoli M. Use of Potabilized Water Sludge in the Production of Low-Energy Blended Calcium Sulfoaluminate Cements. Applied Sciences. 2021; 11(4):1679. https://doi.org/10.3390/app11041679
Chicago/Turabian StyleTelesca, Antonio, Neluta Ibris, and Milena Marroccoli. 2021. "Use of Potabilized Water Sludge in the Production of Low-Energy Blended Calcium Sulfoaluminate Cements" Applied Sciences 11, no. 4: 1679. https://doi.org/10.3390/app11041679
APA StyleTelesca, A., Ibris, N., & Marroccoli, M. (2021). Use of Potabilized Water Sludge in the Production of Low-Energy Blended Calcium Sulfoaluminate Cements. Applied Sciences, 11(4), 1679. https://doi.org/10.3390/app11041679







