Systematic Review of Exposure to Bisphenol A Alternatives and Its Effects on Reproduction and Thyroid Endocrine System in Zebrafish
Abstract
1. Introduction
2. Methods
2.1. Search and Selection of Studies for Inclusion
2.2. Reliability Assessment of Individual Studies
3. Results and Discussion
3.1. Study Selection and Characteristics
3.2. Effects of BPA and Its Alternatives on HPG Axis
| Chemical | Stage (Type) | Exposure Concentration (μg/L) | Exposure Duration | No Observed Adverse Effect Level (μg/L) | Lowest Observed Adverse Effect Level (μg/L) | Toxicity Effect | Ref | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene | Hormone | Protein | Organ | Organism | |||||||
| BPA | Embryo | 0, 0.5, 5, 50, 500 | 96 h | - | 0.5 | ↑esr1, esr2a, vtg1 | - | - | - | - | [27] |
| 0.5 | 5 | ↑cyp19a1b | - | - | - | - | |||||
| 50 | 500 | ↑ar | - | - | - | - | |||||
| BPA | Adult | 0, 1, 10, 100, 1000 | 14 days | - | 1 | ↓cyp19a (♀) | - | ↑VTG (♀) | - | - | [28] |
| BPA | Adult | 0, 100, 300, 600, 800, 1000 | 96 h | >1000 | - | - | - | VTG (♂, ♀) | - | - | [30] |
| 168 h | >1000 | - | - | - | VTG (♂, ♀) | - | - | ||||
| BPA | Adult | 0, 2, 20, 200 | 21 days | - | 2 | - | - | ↑VTG (♀) | - | - | [36] |
| 2 | 20 | - | - | ↑VTG (♂) | - | - | |||||
| 20 | 200 | - | ↓FSH, LH, T, E2 (♂, ♀) | - | - | - | |||||
| BPA | Embryo | 0, 0.02, 0.2, 2, 22, 228, 2282, 6848 (=0, 0.0001, 0.001, 0.01, 0.1, 1, 10, 30 μM) | 5 days | 0.2 | 2 | ↓esr2 | - | - | - | - | [44] |
| 22 | 228 | ↓ar | - | - | - | - | |||||
| 228 | 2282 | ↓esr1 | - | - | - | - | |||||
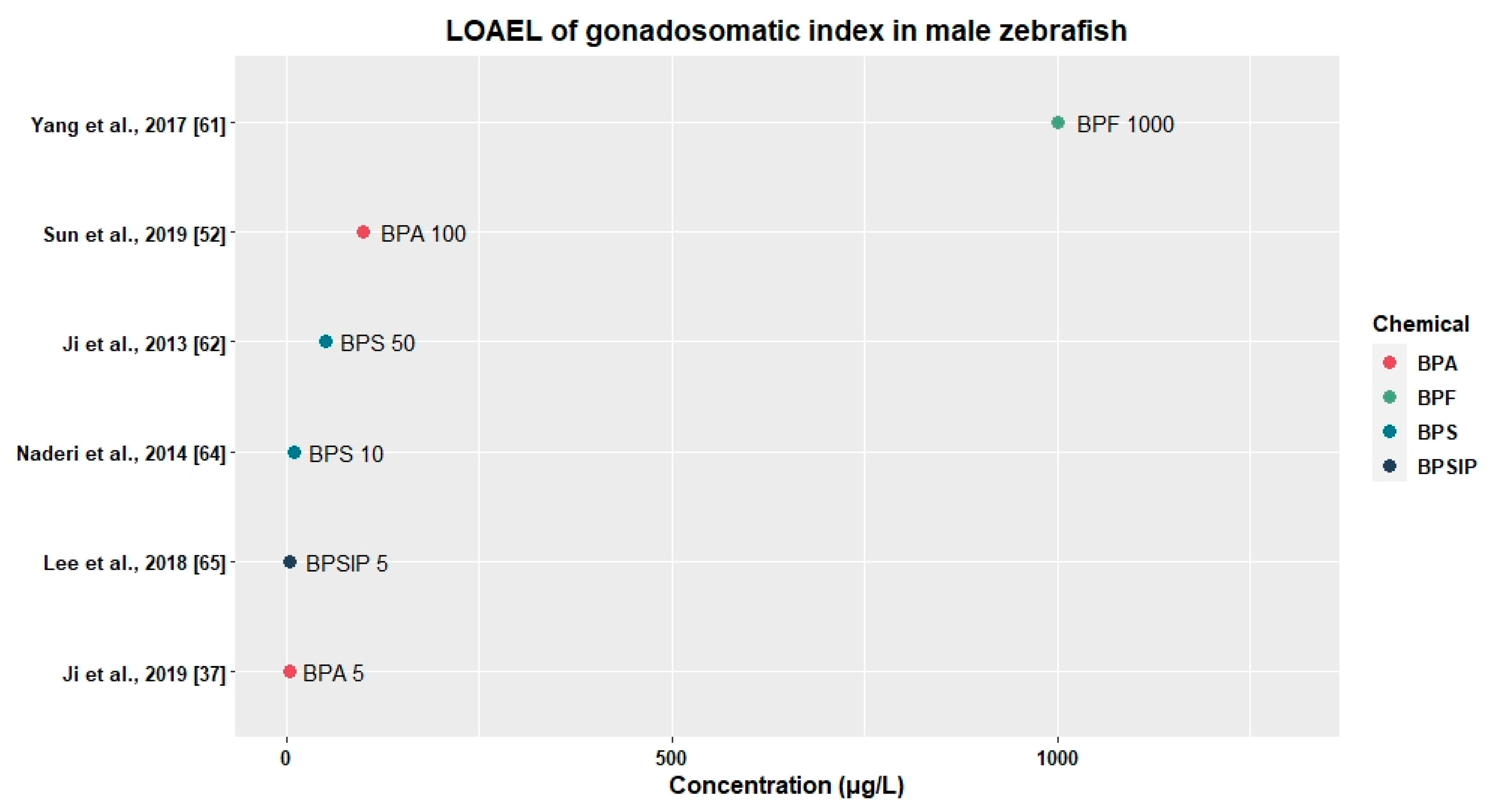
| BPA | Adult | 0, 5 | 21 days | - | 5 | ↑vtg, erα, cyp19a (♂) | ↑E2 (♂), ↓E2 (♀) | - | ↓GSI (♂) | ↓Fecundity | [37] |
| BPA | Adult | 0, 0.01, 0.1, 1, 10, 100 | 96 h | 1 | 10 | - | - | ↑VTG (♂) | - | - | [54] |
| BPA | Adult | 0, 10, 200, 400 | 180 days | - | 10 | - | - | ↑VTG (♂) | - | - | [39] |
| BPA | Embryo | 0, 0.1, 1, 10, 100, 1000 | 120 h | 1 | 10 | ↑gnrh3, lhβ, kiss1r | - | - | - | - | [48] |
| 10 | 100 | ↑erα, kiss1 | - | - | - | - | |||||
| 100 | 1000 | ↑fshβ | - | - | - | - | |||||
| BPA | Adult | 0, 20 | 4 min | - | 20 | ↑F1 vtg2 | ↓E2 (♂),↓T (♀) | ↑F1 VTG2 | - | ↓Fecundity | [32] |
| BPA | Adult | 0, 5, 10, 20 | 21 days | 10 | 20 | ↑star, esr2b, fshr (♀) | - | - | - | - | [50] |
| BPA | Adult (F2) | 0, 20 | 28 days | - | 20 | ↑star, fshr (♀) | - | - | ↓GSI (♀) | ↓Fertility | [49] |
| BPA | Embryo | 0, 100 | 120 h | - | 100 | ↑erα | - | - | - | - | [47] |
| BPA | Juvenile | 0, 100 | 60 days | - | 100 | ↑kiss1, kiss2, gnrh3, erα, cyp19a, cyp19b | - | - | - | - | [46] |
| BPA | Adult | 0, 10, 100, 1000 | 15 days | 10 | 100 | ↓vtg1 (♀) | - | - | - | - | [40] |
| 100 | 1000 | ↑vtg1 (♂) | - | - | - | - | |||||
| BPA | Adult | 0, 100, 2000 | 42 days | - | 100 | ↑vtg1(lai♂) | - | - | ↓GSI (♂) | - | [52] |
| 100 | 2000 | ↑esr1, vtg2 (♂) | - | - | - | - | |||||
| BPA | Embryo | 0, 100, 1000, 5000 | 96 h | - | 100 | ↑vtg1, cyp17a1 | - | - | - | - | [42] |
| 100 | 1000 | ↑esr1, esr2a, esr2b, hsd17b1 | - | - | - | - | |||||
| 1000 | 5000 | ↑cyp19a1 | - | - | - | - | |||||
| BPA | Adult | 0, 25, 50, 100, 250, 500 | 15 days | 100 | 250 | ↑vtg1 (♂) | - | - | - | - | [31] |
| BPA | Adult | 0, 500, 1000, 1500 | 21 days | - | 500 | - | - | ↑VTG (♂) | - | - | [51] |
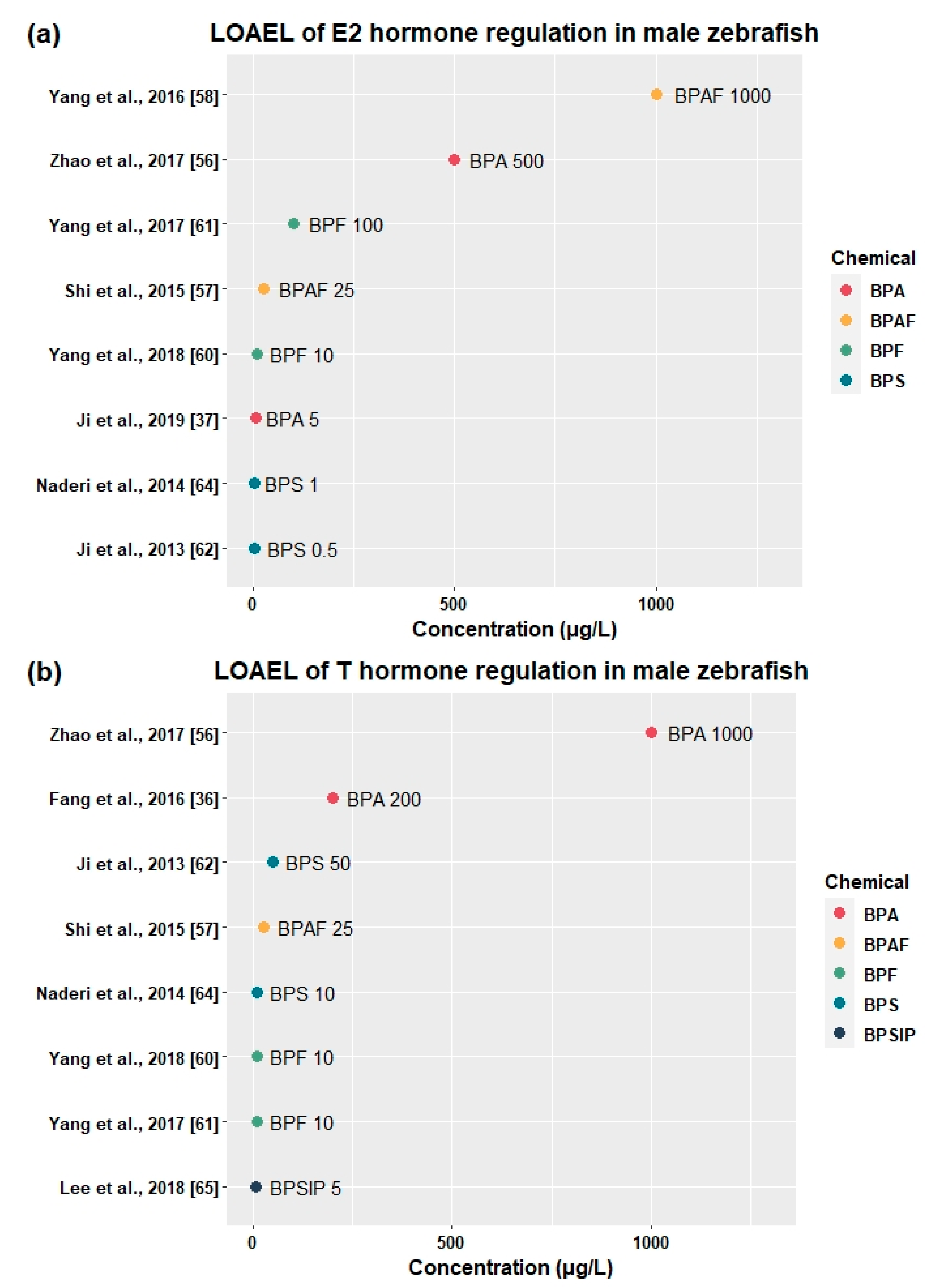
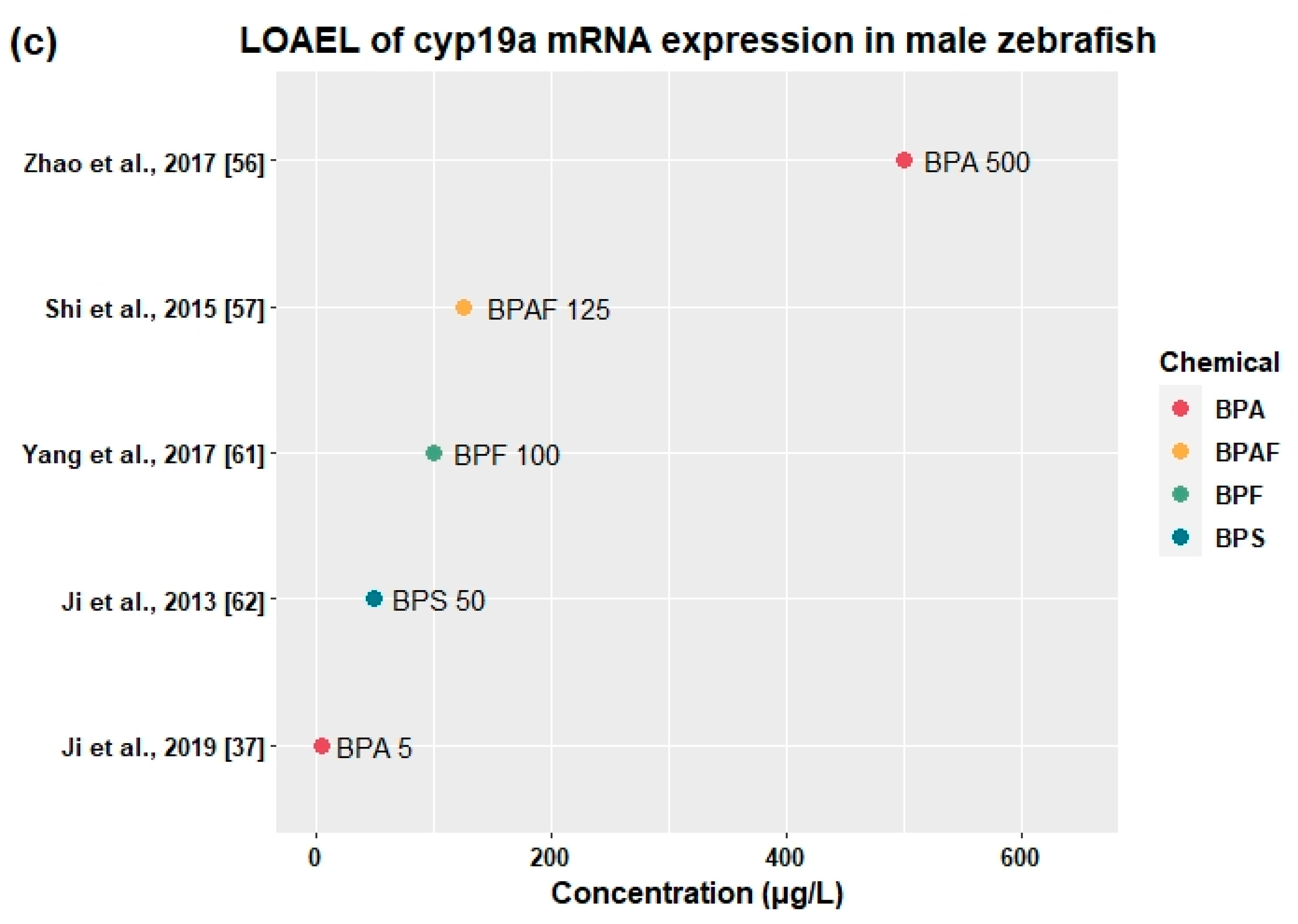
| BPA | Adult | 0, 500, 1000, 1500 | 21 days | - | 500 | ↑vtg1, esr2b, cyp19a1a (♂) | ↑E2 (♂) | ↑VTG (♂) | - | - | [56] |
| 500 | 1000 | ↑esr1 (♂), ↓star, cyp17a1 (♂) | ↓T (♂) | - | - | - | |||||
| BPA | Embryo | 0, 10, 100, 500, 750, 1000, 2500, 5000 | 7 days | 500 | 750 | ↑cyp19b | - | - | - | - | [55] |
| 1000 | 2500 | ↑vtg | - | - | - | - | |||||
| BPA | Adult | 0, 40, 200, 1000 | 21 days | 200 | 1000 | - | - | ↑VTG (♂) | - | - | [53] |
| BPA | Adult | 0, 2000 | 35 days | - | 2000 | ↑vtg (♂) | - | - | - | - | [41] |
| BPA | Embryo | 0, 1141, 2282, 3424 (=0, 5, 10, 15 μM) | 120 h | 1141 | 2282 | ↑vtg1 | - | - | - | - | [43] |
| BPA | Embryo(GFP) | 0, 11, 22, 114, 228, 1141, 2282 (=0, 0.05, 0.1, 0.5, 1, 5, 10 μM) | 2 days | 1141 | 2282 | ↑vtg1, cyp19a1b | - | - | - | - | [45] |
| BPA | Juvenile(albino) | 0, 2282 (=10 μM) | 20 days | - | 2282 | ↓fshβ | - | - | ↓Ovary growth | ↑♀/♂ ratio | [34] |
| BPA | Embryo | 0, 114, 228, 570, 1141, 2282 (=0, 0.5, 1, 2.5, 5, 10 μM) | 7 days | >2282 | - | vtg1 | - | - | - | - | [33] |
| BPA | Embryo | 0, 804, 2010, 4020, 6030 | 96 h | 4020 | 6030 | ↑vtg1 | - | - | - | - | [35] |
| BPA | Adult | 0, 0.1, 2, 20, 200, 400, 1000, 2000 | 11 days | 1000 | 2000 | ↑vtg1 (♂) | - | - | - | - | [38] |
| BPAF | Embryo | 0, 20, 200, 1000 | 96 h | - | 20 | ↑vtg1, esr2b | - | - | - | - | [42] |
| 20 | 200 | ↑esr1, esr2a, cyp19a1, hsd17b1 | - | - | - | - | |||||
| 200 | 1000 | ↑cyp17a1 | - | - | - | - | |||||
| BPAF | Adult | 0, 5, 25, 125 | 120 days | 5 | 25 | ↑vtg1 (♂) | ↑E2 (♂) | - | - | - | [57] |
| ↓T (♂) | |||||||||||
| 25 | 125 | ↑gnrh2, fshβ, lhβ, fshr, star, cyp17, cyp19a, cyp19b (♂) | ↑E2 (♀) | - | - | ↓F1 fertility | |||||
| ↑fshr (♀) | |||||||||||
| ↓star (♀) | |||||||||||
| BPAF | Embryo(GFP) | 0, 16, 33, 168, 336, 1681 ( = 0, 0.05, 0.1, 0.5, 1, 5 μM) | 2 days | 33 | 168 | ↑cyp19a1b | - | - | - | - | [45] |
| 168 | 336 | ↑vtg1 | - | - | - | - | |||||
| BPAF | Adult | 0, 500, 1000, 1500 | 21 days | - | 500 | - | - | ↑VTG (♂) | - | - | [51] |
| BPAF | Adult | 0, 50, 250, 1000 | 28 days | 250 | 1000 | ↑vtg (♂) | ↑E2 (♂) | - | - | - | [58] |
| BPC | Embryo(GFP) | 0, 14, 28, 140, 281, 1405 ( = 0, 0.05, 0.1, 0.5, 1, 5 μM) | 2 days | - | 14 | ↑vtg1 | - | - | - | - | [45] |
| 140 | 281 | ↑cyp19a1b | - | - | - | - | |||||
| BPF | Juvenile | 0, 0.1, 1, 10, 100, 1000 | 60 days | - | 0.1 | - | - | ↑VTG | - | - | [46] |
| 1 | 10 | ↑kiss1r, fshr, vtg, erα, cyp19a | ↑LH, FSH, GnRH | - | - | - | |||||
| 10 | 100 | ↑kiss1, lhr (♀), erβ, cyp19b | - | - | - | - | |||||
| 100 | 1000 | ↑gnrh3, lhr (♂) | - | - | - | - | |||||
| >1000 | - | kiss2, kiss2r, sv2c | - | - | GSI (♀) | - | |||||
| BPF | Adult | 0, 1, 10, 100, 1000 | 21 days | - | 1 | ↑fshβ (♂) | - | - | - | - | [61] |
| 1 | 10 | ↑lhβ, gnrh3, vtg (♂) | ↓T (♂) | - | - | - | |||||
| 10 | 100 | ↑gnrh2, gnrhr1, gnrhr2, cyp19a, fshr, lhr (♂) | ↑E2 (♂) | - | - | - | |||||
| ↑fshr (♀) | |||||||||||
| ↓fshβ, 17βhsd, star (♀) | |||||||||||
| 100 | 1000 | ↑cyp11a (♂, ♀) | ↓T (♀),↑E2 (♀) | - | ↓GSI (♂, ♀) | ↓Fecundity, ↓F1 survival | |||||
| ↓17βhsd, cyp17, star (♂) | |||||||||||
| ↓lhr (♀) | |||||||||||
| BPF | Juvenile | 0, 1, 10, 100, 1000 | 60 days | - | 1 | ↑cyp19a1a, vtg | - | - | - | - | [60] |
| 1 | 10 | ↓amh, foxl2 | ↑E2 (♂, ♀),↓T (♂, ♀) | - | - | - | |||||
| 10 | 100 | ↑dmrt1 | - | - | - | ↑Intersex | |||||
| 100 | 1000 | ↓ff1d | - | - | - | - | |||||
| BPF | Adult(GFP) | 0, 2.28, 22.8, 228, 2282(=0, 0.01, 0.1, 1, 10 μM) | 7 days | 2.28 | 22.8 | - | - | ↑VTG (♂) | - | - | [59] |
| BPF | Embryo | 0, 0.1, 1, 10, 100, 1000 | 120 h | 10 | 100 | ↑erα | - | - | - | - | [47] |
| BPF | Embryo | 0, 200, 2000, 10,000 | 96 h | 200 | 2000 | ↑vtg1 | - | - | - | - | [42] |
| 2000 | 10,000 | ↑esr1, esr2a, esr2b, cyp19a1, cyp17a1, hsd17b1 | - | - | - | - | |||||
| BPS | Adult | 0, 0.5, 5, 50 | 21 days | - | 0.5 | - | ↑E2 (♂) | - | ↓GSI (♀) | ↓Fecundity | [62] |
| 5 | 50 | ↑gnrh3, gnrhr1, gnrhr2, fshβ, lhβ, fshr, lhr, cyp19b, hmgra, hmgrb, cyp11a, 3βhsd, cyp17, 17βhsd, cyp19a (♂) | ↓T (♂) | - | ↓GSI (♂) | - | |||||
| ↑gnrh3, fshβ, hmgra, hmgrb (♀) | ↑E2 (♀) | ||||||||||
| BPS | Adult | 0, 1, 10, 30 | 120 days | - | 1 | ↑esr2a, esr2b (♀) | - | - | - | - | [63] |
| BPS | Adult | 0, 0.1, 1, 10, 100 | 75 days | 0.1 | 1 | - | ↑E2 (♂) | - | - | - | [64] |
| 1 | 10 | - | ↓T (♂),↑E2 (♀) | ↑VTG (♀) | ↓GSI (♂) | ↓Fecundity | |||||
| 10 | 100 | - | ↑VTG (♂) | ↓GSI (♀) | - | ||||||
| BPS | Embryo | 0, 100 | 120 h | 10 | 100 | ↑gnrh3, kiss1, erα | - | - | - | - | [48] |
| BPS | Embryo | 0, 0.1, 1, 10, 100, 1000 | 120 h | 10 | 100 | ↑erα | - | - | - | - | [47] |
| BPS | Juvenile | 0, 100 | 60 days | - | 100 | ↑kiss1, kiss2, kiss1r, gnrh3, erα, erβ, cyp19a, cyp19b | - | - | - | - | [46] |
| BPS | Embryo | 0, 500, 5000, 25,000 | 96 h | >25,000 | - | vtg1, esr1, esr2a, esr2b, cyp19a1, cyp17a1, hsd17b1 | - | - | - | - | [42] |
| BPSIP | Adult | 0, 0.5, 5, 50 | 21 days | 0.5 | 5 | ↓cyp19a, cyp19b, fshr (♂) | ↓T (♂) | - | ↓GSI (♂) | - | [65] |
| 5 | 50 | ↑gnrh2, gnrhr2, gnrhr4, erα (♂) | ↓E2 (♂) | - | - | - | |||||
| ↓fshβ, cyp17, 17βhsd (♂) | |||||||||||
| ↑gnrh2, gnrhr2, gnrhr4, fshβ, cyp19b, erα, er2β, cyp17, cyp19a (♀) | ↑E2, T (♀) | ||||||||||
3.2.1. Effects on Reproduction
3.2.2. Effects on Relative Gonad Weight
3.2.3. Effects on Vitellogenesis
3.2.4. Effects on Sex Hormones and Genes Related to HPG Axis
3.3. Effects of BPA and Its Alternatives on HPT Axis
| Chemical | Stage (Type) | Exposure Concentration (μg/L) | Exposure Duration | No Observed Adverse Effect Level (μg/L) | Lowest Observed Adverse Effect Level (μg/L) | Toxicity Effect | Ref | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Gene | Hormone | Organ | Organism | |||||||
| BPA | Embryo | 0, 2, 22, 228 (=0, 0.01, 0.1, 1 μM) | 24 h | - | 2 | ↑tg, pax8 | - | - | - | [79] |
| 2 | 22 | ↑tsh, pax2a | - | - | - | |||||
| BPA | Adult | 0, 2, 20 | 4 min | 2 | 20 | - | ↓T4(♀) | - | ↓F1 survival | [80] |
| BPA | Embryo | 0, 80, 400, 2000, 10,000 | 120 h | - | 80 | ↑hhex | - | - | - | [83] |
| 80 | 400 | ↑ttr, dio1, ugt1ab | ↑T3 | - | - | |||||
| 400 | 2000 | ↑tg, trα | - | - | ↑Time-to-hatch | |||||
| 2000 | 10,000 | - | - | - | ↓Hatchability | |||||
| >10,000 | - | - | - | - | Length | |||||
| BPA | Embryo | 0, 804, 2010, 4020, 6030 | 96 h | - | 804 | ↑tshβ | - | - | - | [78] |
| BPA | Embryo (GFP) | 0, 2282 (=0, 10 μM) | 168 h | >2282 | - | trα, trβ | - | - | - | [85] |
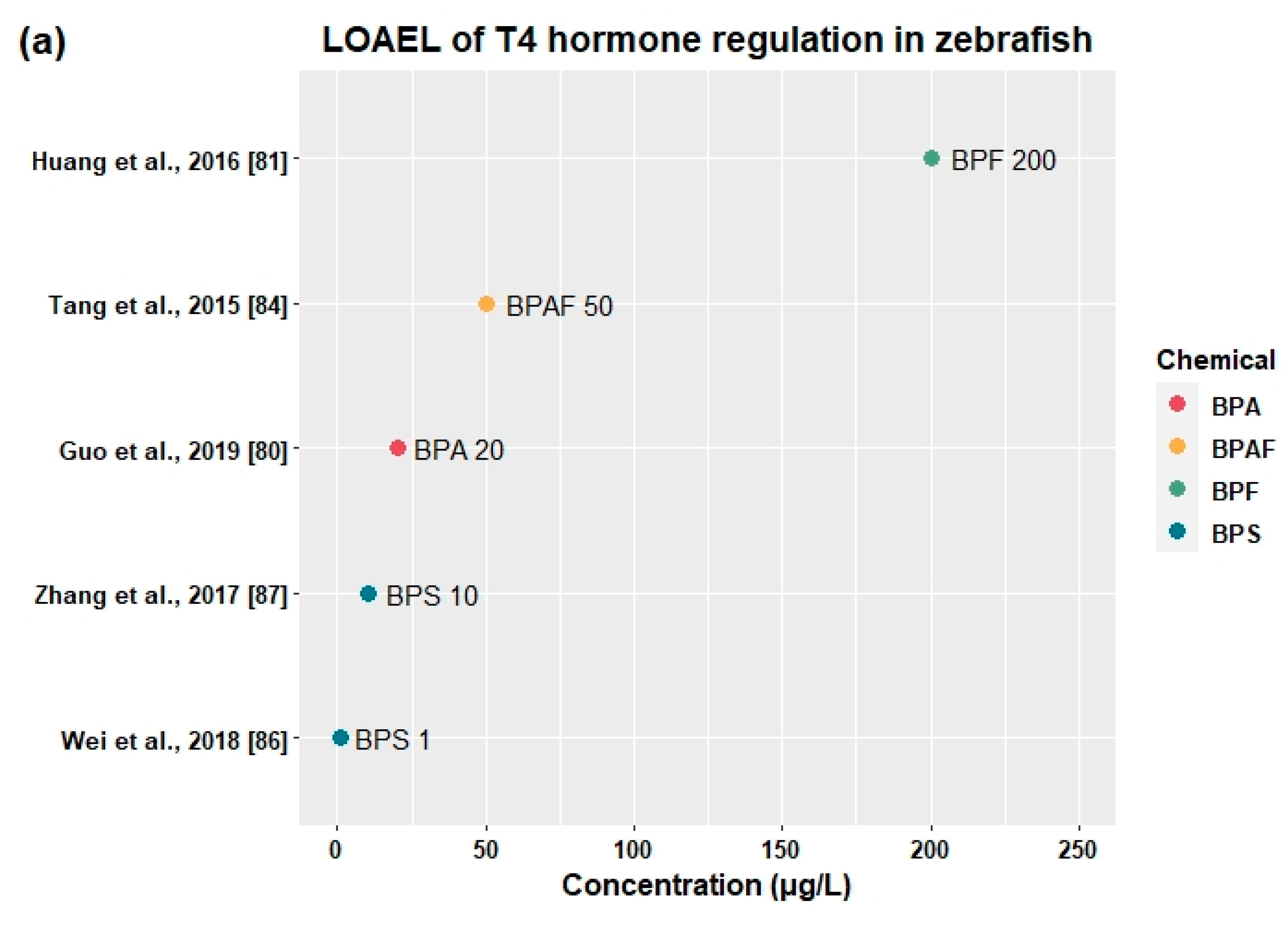
| BPAF | Embryo | 0, 5, 50, 500 | 168 h | - | 5 | ↑ttr | ↓FT3 | - | - | [84] |
| 5 | 50 | ↑tshβ, slc5a5, tg, dio1, dio2 | ↓TT4, FT4, TT3 | - | - | |||||
| ↓trα, trβ | ||||||||||
| BPAF | Adult | 0, 24.7 | 21 days | - | 24.7 | ↑trh, trhr1, tshβ, dio2 (♂) | - | - | - | [82] |
| ↓tpo (♂) | ||||||||||
| BPF | Embryo | 0, 0.2, 2, 20, 200 | 144 h | 0.2 | 2 | ↑crh, tg | - | - | - | [81] |
| 2 | 20 | ↑nis, dio2, ugt1ab | ↑TSH | - | - | |||||
| ↓ttr | ||||||||||
| 20 | 200 | - | ↑T3, ↓T4 | - | - | |||||
| BPF | Embryo | 0, 80, 400, 2000, 10,000 | 120 h | - | 80 | - | - | - | ↑Time-to-hatch | [83] |
| 400 | 2000 | ↑hhex, ugt1ab | ↑T4 | - | - | |||||
| >10,000 | - | - | - | - | Length | |||||
| BPS | Adult | 0, 1, 10, 100 | 120 days | - | 1 | ↑dio2, dio3, ugt1ab (♀), dio2 (♂) | ↑T3(♀), F1 T3 | - | ↓F1 spontaneous movement | [86] |
| ↓crh, tshβ (♂) | ↓T4(♂, ♀), F1 T4 | - | - | |||||||
| 1 | 10 | ↑crh, dio1 (♂, ♀) | - | - | - | |||||
| 10 | 100 | ↑tshβ (♀), dio3 (♂) | - | - | - | |||||
| BPS | Embryo | 0, 1, 3, 10, 30 | 168 h | 1 | 3 | ↓ttr | - | - | - | [87] |
| 3 | 10 | ↑crh, tg, dio1, ugt1ab | ↓T4,↑TSH | - | - | |||||
| BPS | Embryo | 0, 400, 2000, 10,000, 50,000 | 120 h | - | 400 | - | - | - | ↑Time-to-hatch | [83] |
| 400 | 2000 | ↑crh, tshβ, tshr, hhex, tpo, ttr, ugt1ab | - | - | - | |||||
| 10,000 | 50,000 | - | ↑T3 | - | - | |||||
| >50,000 | - | - | - | - | Length | |||||
| BPZ | Embryo | 0, 40, 180, 680, 2900 | 120 h | 180 | 680 | ↑tshβ | - | - | - | [83] |
| 680 | 2900 | - | - | - | ↑Time-to-hatch | |||||
| >2900 | - | - | - | - | Length | |||||
3.3.1. Effects on Development
3.3.2. Effects on Thyroid Hormones and Genes Related to HPT Axis
4. Conclusions
- BPA alternatives have a similar or more significant toxic potential than that of BPA.
- Several BPA alternatives may cause reproductive dysfunction by interfering in the regulatory mechanisms of the HPG axis or inducing vitellogenin in males.
- Males were more sensitive to the adverse effects on sex hormone levels, as well as gene transcriptions, than females.
- Environmentally relevant concentration of BPA alternatives has the potential to inhibit the normal development of embryo/larvae by disrupting thyroid hormone endocrine system.
Author Contributions
Funding
Conflicts of Interest
References
- Kabir, E.R.; Rahman, M.S.; Rahman, I. A review on endocrine disruptors and their possible impacts on human health. Environ. Toxicol. Pharmacol. 2015, 40, 241–258. [Google Scholar] [CrossRef]
- Löhr, H.; Hammerschmidt, M. Zebrafish in Endocrine Systems: Recent Advances and Implications for Human Disease. Annu. Rev. Physiol. 2011, 73, 183–211. [Google Scholar] [CrossRef]
- USEPA. Available online: https://www.epa.gov/endocrine-disruption/what-endocrine-disruption (accessed on 14 December 2020).
- OECD. Available online: http://www.oecd.org/chemicalsafety/testing/OECD%20Work%20on%20Endocrine%20Disrupting%20Chemicals.pdf (accessed on 14 December 2020).
- Lim, M.; Park, J.Y.; Ji, K.; Lee, K. Development and application of a chemical ranking and scoring system for the management of endocrine disrupting chemicals. J. Environ. Health Sci. 2018, 44, 76–89. [Google Scholar]
- Gao, X.; Kang, S.; Xiong, R.; Chen, M. Environment-Friendly Removal Methods for Endocrine Disrupting Chemicals. Sustainability 2020, 12, 7615. [Google Scholar] [CrossRef]
- Casals-Casas, C.; Desvergne, B. Endocrine Disruptors: From Endocrine to Metabolic Disruption. Annu. Rev. Physiol. 2011, 73, 135–162. [Google Scholar] [CrossRef] [PubMed]
- Mathieu-Denoncourt, J.; Wallace, S.J.; de Solla, S.R.; Langlois, V.S. Plasticizer endocrine disruption: Highlighting developmental and reproductive effects in mammals and non-mammalian aquatic species. Gen. Comp. Endocrinol. 2015, 219, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Mnif, W.; Hassine, A.I.H.; Bouaziz, A.; Bartegi, A.; Thomas, O.; Roig, B. Effect of Endocrine Disruptor Pesticides: A Review. Int. J. Environ. Res. Public Health 2011, 8, 2265–2303. [Google Scholar] [CrossRef] [PubMed]
- Iavicoli, I.; Fontana, L.; Bergamaschi, A. The Effects of Metals as Endocrine Disruptors. J. Toxicol. Environ. Health Part B 2009, 12, 206–223. [Google Scholar] [CrossRef]
- Bonefeld-Jørgensen, E.C.; Ghisari, M.; Wielsøe, M.; Bjerregaard-Olesen, C.; Kjeldsen, L.S.; Long, M. Biomonitoring and hormone-disrupting effect biomarkers of persistent organic pollutants in vitro and ex vivo. Basic Clin. Pharmacol. Toxicol. 2014, 115, 118–128. [Google Scholar] [CrossRef]
- Rubin, B.S. Bisphenol A: An endocrine disruptor with widespread exposure and multiple effects. J. Steroid Biochem. Mol. Biol. 2011, 127, 27–34. [Google Scholar] [CrossRef]
- Huang, Y.; Wong, C.; Zheng, J.; Bouwman, H.; Barra, R.; Wahlstrom, B.; Neretin, L.; Wong, M. Bisphenol A (BPA) in chi-na: A review of sources, environmental levels, and potential human health impacts. Environ. Int. 2012, 42, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-H.; Kondo, F.; Katayama, Y. Human exposure to bisphenol A. Toxicology 2006, 226, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.-L.; Corriveau, J.; Popovic, S. Sources of Low Concentrations of Bisphenol A in Canned Beverage Products. J. Food Prot. 2010, 73, 1548–1551. [Google Scholar] [CrossRef]
- Geens, T.; Goeyens, L.; Kannan, K.; Neels, H.; Covaci, A. Levels of bisphenol-A in thermal paper receipts from Belgium and estimation of human exposure. Sci. Total Environ. 2012, 30–33. [Google Scholar] [CrossRef]
- Hengstler, J.G.; Foth, H.; Gebel, T.; Kramer, P.-J.; Lilienblum, W.; Schweinfurth, H.; Völkel, W.; Wollin, K.-M.; Gundert-Remy, U. Critical evaluation of key evidence on the human health hazards of exposure to bisphenol A. Crit. Rev. Toxicol. 2011, 41, 263–291. [Google Scholar] [CrossRef]
- Corrales, J.; Kristofco, L.; Steele, W.; Yates, B.; Breed, C.; Williams, E.; Brooks, B. Global assessment of bisphenol A in the environment: Review and analysis of its occurrence and bioaccumulation. Dose Response 2015, 13, 1–29. [Google Scholar] [CrossRef]
- Van den Berg, L.N.; Chahoud, I.; Heindel, J.J.; Padmanabhan, V.; Paumgartten, F.J.R.; Schoenfelder, G. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ. Health Perspect. 2010, 118, 1055–1070. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Sun, H.; Kannan, K. Blood and Urinary Bisphenol A Concentrations in Children, Adults, and Pregnant Women from China: Partitioning between Blood and Urine and Maternal and Fetal Cord Blood. Environ. Sci. Technol. 2013, 47, 4686–4694. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Alomirah, H.; Cho, H.-S.; Li, Y.-F.; Liao, C.; Minh, T.B.; Mohd, M.A.; Nakata, H.; Ren, N.; Kannan, K. Urinary Bisphenol A Concentrations and Their Implications for Human Exposure in Several Asian Countries. Environ. Sci. Technol. 2011, 45, 7044–7050. [Google Scholar] [CrossRef]
- Wang, W.; Abualnaja, K.; Asimakopoulos, A.; Covaci, A.; Gevao, B.; Johnson-Restrepo, B.; Kumosani, T.; Malarvannan, G.; Minh, T.; Moon, H.; et al. A comparative assessment of human exposure to tetrabromobisphenol A and eight bi-sphenols including bisphenol A via indoor dust ingestion in twelve countries. Environ. Int. 2015, 83, 183–191. [Google Scholar] [CrossRef]
- Yamazaki, E.; Yamashita, N.; Taniyasu, S.; Lam, J.; Lam, P.; Moon, H.; Jeong, Y.; Kannan, P.; Achyuthan, H.; Munuswamy, N.; et al. Bisphenol Aa and other bisphenol analogues including BPS and BPF in surface water samples from Japan, China, Korea and India. Ecotoxicol. Environ. Saf. 2015, 122, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Moermond, C.T.; Kase, R.; Korkaric, M.; Ågerstrand, M. CRED: Criteria for reporting and evaluating ecotoxicity data. Environ. Toxicol. Chem. 2016, 35, 1297–1309. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Ågerstrand, M.; Beronius, A.; Beausoleil, C.; Bergman, Å.; Bero, L.A.; Bornehag, C.-G.; Boyer, C.S.; Cooper, G.S.; Cotgreave, I.; et al. A proposed framework for the systematic review and integrated assessment (SYRINA) of endocrine disrupting chemicals. Environ. Health 2016, 15, 74. [Google Scholar] [CrossRef]
- Klimisch, H.-J.; Andreae, M.; Tillmann, U. A Systematic Approach for Evaluating the Quality of Experimental Toxicological and Ecotoxicological Data. Regul. Toxicol. Pharmacol. 1997, 25, 1–5. [Google Scholar] [CrossRef]
- Xia, L.; Zheng, L.; Zhou, J.L. Transcriptional and morphological effects of tamoxifen on the early development of zebrafish (Danio rerio). J. Appl. Toxicol. 2016, 36, 853–862. [Google Scholar] [CrossRef]
- Molina, A.; Abril, N.; Morales-Prieto, N.; Monterde, J.; Lora, A.; Ayala, N.; Moyano, R. Evaluation of toxicological end-points in female zebrafish after bisphenol A exposure. Food Chem. Toxicol. 2018, 112, 19–25. [Google Scholar] [CrossRef]
- Segner, H. Zebrafish (Danio rerio) as a model organism for investigating endocrine disruption. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2009, 149, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Allner, B.; Hennies, M.; Lerche, C.F.; Schmidt, T.; Schneider, K.; Willner, M.; Stahlschmidt-Allner, P. Kinetic determination of vitellogenin induction in the epidermis of cyprinid and perciform fishes: Evaluation of sensitive enzyme-linked immunosorbent assays. Environ. Toxicol. Chem. 2016, 35, 2916–2930. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, L.; Yu, Q. Determination and reduced life expectancy model and molecular docking analyses of estro-genic potentials of 17beta-estradiol, bisphenol A and nonylphenol on expression of vitellogenin gene (vtg1) in zebrafish. Chemosphere 2019, 221, 727–734. [Google Scholar] [CrossRef]
- Chen, L.; Hu, C.; Guo, Y.; Shi, Q.; Zhou, B. Tio2 nanoparticles and BPA are combined to impair the development of off-spring zebrafish after parental coexposure. Chemosphere 2019, 217, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhang, J.; Pang, S.; Wang, C.; Wang, L.; Sun, Y.; Song, M.; Liang, Y. Evaluating estrogenic and anti-estrogenic effect of endocrine disrupting chemicals (EDCs) by zebrafish (Danio rerio) embryo-based vitellogenin 1 (vtg1) mRNA ex-pression. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2018, 204, 45–50. [Google Scholar] [CrossRef]
- Chen, W.; Lau, S.; Fan, Y.; Wu, R.; Ge, W. Juvenile exposure to bisphenol A promotes ovarian differentiation but sup-presses its growth - potential involvement of pituitary follicle-stimulating hormone. Aquat. Toxicol. 2017, 193, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Chow, W.S.; Chan, W.K.-L.; Chan, K.M. Toxicity assessment and vitellogenin expression in zebrafish (Danio rerio) embryos and larvae acutely exposed to bisphenol A, endosulfan, heptachlor, methoxychlor and tetrabromobisphenol A. J. Appl. Toxicol. 2013, 33, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Shi, Q.; Guo, Y.; Hua, J.; Wang, X.; Zhou, B. Enhanced bioconcentration of bisphenol A in the presence of nano-tio2 can lead to adverse reproductive outcomes in zebrafish. Environ. Sci. Technol. 2016, 50, 1005–1013. [Google Scholar] [CrossRef]
- Ji, K.; Seo, J.; Kho, Y.; Choi, K. Co-exposure to ketoconazole alters effects of bisphenol A in Danio rerio and H295R cells. Chemosphere 2019, 237, 124414. [Google Scholar] [CrossRef] [PubMed]
- Kausch, U.; Alberti, M.; Haindl, S.; Budczies, J.; Hock, B. Biomarkers for exposure to estrogenic compounds: Gene ex-pression analysis in zebrafish (Danio rerio). Environ. Toxicol. 2008, 23, 15–24. [Google Scholar] [CrossRef]
- Keiter, S.; Baumann, L.; Färber, H.; Holbech, H.; Skutlarek, D.; Engwall, M.; Braunbeck, T. Long-term effects of a binary mixture of perfluorooctane sulfonate (PFOS) and bisphenol A (BPA) in zebrafish (Danio rerio). Aquat. Toxicol. 2012, 116–129. [Google Scholar] [CrossRef]
- Laing, L.; Viana, J.; Dempster, E.L.; Trznadel, M.; Trunkfield, L.A.; Webster, T.M.U.; Van Aerle, R.; Paull, G.C.; Wilson, R.J.; Mill, J.; et al. Bisphenol A causes reproductive toxicity, decreases dnmt1 transcription, and reduces global DNA methylation in breeding zebrafish (Danio rerio). Epigenetics 2016, 11, 526–538. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, Y.; Li, H.; Qiao, F.; Wu, J.; Du, Z.-Y.; Zhang, M. Influence of Endogenous and Exogenous Estrogenic Endocrine on Intestinal Microbiota in Zebrafish. PLOS ONE 2016, 11, e0163895. [Google Scholar] [CrossRef]
- Mu, X.; Huang, Y.; Li, X.; Lei, Y.; Teng, M.; Li, X.; Wang, C.; Li, Y. Developmental Effects and Estrogenicity of Bisphenol A Alternatives in a Zebrafish Embryo Model. Environ. Sci. Technol. 2018, 52, 3222–3231. [Google Scholar] [CrossRef]
- Muncke, J.; Junghans, M.; Eggen, R.I.L. Testing estrogenicity of known and novel (xeno-)estrogens in the MolDarT using developing zebrafish (Danio rerio). Environ. Toxicol. 2007, 22, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Olsvik, P.; Whatmore, P.; Penglase, S.; Skjaerven, K.; Angles d’Auriac, M.; Ellingsen, S. Associations between behavioral effects of bisphenol A and DNA methylation in zebrafish embryos. Front. Genet. 2019, 10, 184. [Google Scholar] [CrossRef]
- Pinto, C.; Hao, R.; Grimaldi, M.; Thrikawala, S.; Boulahtouf, A.; Aït-Aïssa, S.; Brion, F.; Gustafsson, J.-Å.; Balaguer, P.; Bondesson, M. Differential activity of BPA, BPAF and BPC on zebrafish estrogen receptors in vitro and in vivo. Toxicol. Appl. Pharmacol. 2019, 380, 114709. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Fang, M.; Liu, J.; Fu, C.; Zheng, C.; Chen, B.; Wang, K. In vivo actions of bisphenol F on the reproductive neu-roendocrine system after long-term exposure in zebrafish. Sci. Total Environ. 2019, 665, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Shao, H.; Lei, P.; Zheng, C.; Qiu, C.; Haiyang, S.; Zheng, Y. Immunotoxicity of bisphenol S and F are similar to that of bisphenol A during zebrafish early development. Chemosphere 2018, 194, 1–8. [Google Scholar] [CrossRef]
- Qiu, W.; Zhao, Y.; Yang, M.; Farajzadeh, M.; Pan, C.; Wayne, N. Actions of bisphenol A and bisphenol S on the reproduc-tive neuroendocrine system during early development in zebrafish. Endocrinology 2016, 157, 636–647. [Google Scholar] [CrossRef] [PubMed]
- Santangeli, S.; Consales, C.; Pacchierotti, F.; Habibi, H.; Carnevali, O. Transgenerational effects of BPA on female re-production. Sci. Total Environ. 2019, 685, 1294–1305. [Google Scholar] [CrossRef] [PubMed]
- Santangeli, S.; Maradonna, F.; Gioacchini, G.; Cobellis, G.; Piccinetti, C.; Dalla Valle, L.; Carnevali, O. BPA-induced de-regulation of epigenetic patterns: Effects on female zebrafish reproduction. Sci. Rep. 2016, 6, 21982. [Google Scholar] [CrossRef]
- Song, M.; Liang, D.; Liang, Y.; Chen, M.; Wang, F.; Wang, H.; Jiang, G. Assessing developmental toxicity and estrogenic activity of halogenated bisphenol A on zebrafish (Danio rerio). Chemosphere 2014, 112, 275–281. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, Y.; Lu, D.; Wang, W.; Limbu, S.; Chen, L.; Zhang, M.; Du, Z. Concentration-dependent effects of 17beta-estradiol and bisphenol A on lipid deposition, inflammation and antioxidant response in male zebrafish (Danio rerio). Chemosphere 2019, 237, 124422. [Google Scholar] [CrossRef]
- Van den Belt, K.; Verheyen, R.; Witters, H. Comparison of vitellogenin responses in zebrafish and rainbow trout fol-lowing exposure to environmental estrogens. Ecotoxicol. Environ. Saf. 2003, 56, 271–281. [Google Scholar] [CrossRef]
- Villeneuve, D.L.; Garcia-Reyero, N.; Escalon, B.L.; Jensen, K.M.; Cavallin, J.E.; Makynen, E.A.; Durhan, E.J.; Kahl, M.D.; Thomas, L.M.; Perkins, E.J.; et al. Ecotoxicogenomics to Support Ecological Risk Assessment: A Case Study with Bisphenol A in Fish. Environ. Sci. Technol. 2011, 46, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shi, X.; Du, Y.; Zhou, B. Effects of xenoestrogens on the expression of vitellogenin (vtg) and cytochrome p450 aromatase (cyp19a and b) genes in zebrafish (Danio rerio) larvae. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2011, 46, 960–967. [Google Scholar] [CrossRef]
- Zhao, F.; Wei, P.; Wang, J.; Yu, M.; Zhang, X.; Tian, H.; Wang, W.; Ru, S. Estrogenic effects associated with bisphenol A exposure in male zebrafish (Danio rerio) is associated with changes of endogenous 17beta-estradiol and gene specific DNA methylation levels. Gen. Comp. Endocrinol. 2017, 252, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Jiao, Z.; Zheng, S.; Li, M.; Zhang, J.; Feng, Y.; Yin, J.; Shao, B. Long-term effects of bisphenol AF (BPAF) on hor-monal balance and genes of hypothalamus-pituitary-gonad axis and liver of zebrafish (Danio rerio), and the impact on offspring. Chemosphere 2015, 128, 252–257. [Google Scholar] [CrossRef]
- Yang, X.; Liu, Y.; Li, J.; Chen, M.; Peng, D.; Liang, Y.; Song, M.; Zhang, J.; Jiang, G. Exposure to Bisphenol AF disrupts sex hormone levels and vitellogenin expression in zebrafish. Environ. Toxicol. 2016, 31, 285–294. [Google Scholar] [CrossRef]
- Le Fol, V.; Ait-Aissa, S.; Sonavane, M.; Porcher, J.; Balaguer, P.; Cravedi, J.; Zalko, D.; Brion, F. In vitro and in vivo es-trogenic activity of BPA, BPF and BPS in zebrafish-specific assays. Ecotoxicol. Environ. Saf. 2017, 142, 150–156. [Google Scholar] [CrossRef]
- Yang, Q.; Yang, X.; Liu, J.; Chen, Y.; Shen, S. Effects of exposure to BPF on development and sexual differentiation during early life stages of zebrafish (Danio rerio). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2018, 210, 44–56. [Google Scholar] [CrossRef]
- Yang, Q.; Yang, X.; Liu, J.; Ren, W.; Chen, Y.; Shen, S. Effects of BPF on steroid hormone homeostasis and gene expres-sion in the hypothalamic-pituitary-gonadal axis of zebrafish. Environ. Sci. Pollut. Res. Int. 2017, 24, 21311–21322. [Google Scholar] [CrossRef]
- Ji, K.; Hong, S.; Kho, Y.; Choi, K. Effects of Bisphenol S Exposure on Endocrine Functions and Reproduction of Zebrafish. Environ. Sci. Technol. 2013, 47, 8793–8800. [Google Scholar] [CrossRef] [PubMed]
- Naderi, M.; Salahinejad, A.; Attaran, A.; Chivers, D.; Niyogi, S. Chronic exposure to environmentally relevant concen-trations of bisphenol S differentially affects cognitive behaviors in adult female zebrafish. Environ. Pollut. 2020, 261, 114060. [Google Scholar] [CrossRef]
- Naderi, M.; Wong, M.Y.; Gholami, F. Developmental exposure of zebrafish (Danio rerio) to bisphenol-S impairs subsequent reproduction potential and hormonal balance in adults. Aquat. Toxicol. 2014, 148, 195–203. [Google Scholar] [CrossRef]
- Lee, J.; Park, N.-Y.; Kho, Y.; Ji, K. Effects of 4-Hydroxyphenyl 4-Isoprooxyphenylsulfone (BPSIP) Exposure on Reproduction and Endocrine System of Zebrafish. Environ. Sci. Technol. 2018, 52, 1506–1513. [Google Scholar] [CrossRef]
- Ma, Y.; Han, J.; Guo, Y.; Lam, P.K.; Wu, R.S.; Giesy, J.P.; Zhang, X.; Zhou, B. Disruption of endocrine function in in vitro H295R cell-based and in in vivo assay in zebrafish by 2,4-dichlorophenol. Aquat. Toxicol. 2012, 106-107, 173–181. [Google Scholar] [CrossRef]
- Liu, C.; Deng, J.; Yu, L.; Ramesh, M.; Zhou, B. Endocrine disruption and reproductive impairment in zebrafish by expo-sure to 8:2 fluorotelomer alcohol. Aquat. Toxicol. 2010, 96, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, T.; Ankley, G.; Segner, H.; Tyler, C. Screening and testing for endocrine disruption in fish-biomarkers as “signposts,” not “traffic lights,” in risk assessment. Environ. Health Perspect. 2006, 114, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Belt, K.V.D.; Verheyen, R.; Witters, H. Reproductive Effects of Ethynylestradiol and 4t-Octylphenol on the Zebrafish (Danio rerio). Arch. Environ. Contam. Toxicol. 2001, 41, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.-X.; Xu, Y.; Hui, Y. Reproductive effects of prenatal exposure to nonylphenol on zebrafish (Danio rerio). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2006, 142, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Peteffi, G.P.; Fleck, J.D.; Kael, I.M.; Rosa, D.C.; Antunes, M.V.; Linden, R. Ecotoxicological risk assessment due to the presence of bisphenol A and caffeine in surface waters in the Sinos River Basin - Rio Grande do Sul-Brazil. Braz. J. Biol. 2019, 79, 712. [Google Scholar] [CrossRef]
- Kallivretaki, E.; Eggen, R.; Neuhauss, S.; Alberti, M.; Kausch, U.; Segner, H. Aromatase in zebrafish: A potential target for endocrine disrupting chemicals. Mar. Environ. Res. 2006, 62, S187–S190. [Google Scholar] [CrossRef]
- Trant, J.M.; Gavasso, S.; Ackers, J.; Chung, B.-C.; Place, A.R. Developmental expression of cytochrome P450 aromatase genes (CYP19a and CYP19b) in zebrafish fry (Danio rerio). J. Exp. Zool. 2001, 290, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Schally, A.; Arimura, A.; Kastin, A.; Matsuo, H.; Baba, Y.; Redding, T.; Nair, R.; Debeljuk, L.; White, W. Gonadotro-pin-releasing hormone: One polypeptide regulates secretion of luteinizing and follicle-stimulating hormones. Science 1971, 173, 1036–1038. [Google Scholar] [CrossRef] [PubMed]
- Tello, J.A.; Wu, S.; Rivier, J.E.; Sherwood, N.M. Four functional GnRH receptors in zebrafish: Analysis of structure, signaling, synteny and phylogeny. Integr. Comp. Biol. 2008, 48, 570–587. [Google Scholar] [CrossRef]
- Ji, K.; Choi, K. Endocrine disruption potentials of bisphenol A alternatives - are bisphenol A alternatives safe from en-docrine disruption? Korean J. Environ. Health Sci. 2013, 39, 1–18. [Google Scholar] [CrossRef][Green Version]
- Kitamura, S.; Suzuki, T.; Sanoh, S.; Kohta, R.; Jinno, N.; Sugihara, K.; Yoshihara, S.; Fujimoto, N.; Watanabe, H.; Ohta, S. Comparative Study of the Endocrine-Disrupting Activity of Bisphenol A and 19 Related Compounds. Toxicol. Sci. 2005, 84, 249–259. [Google Scholar] [CrossRef]
- Chan, W.; Chan, K. Disruption of the hypothalamic-pituitary-thyroid axis in zebrafish embryo-larvae following wa-terborne exposure to BDE-47, TBBPA and BPA. Aquat. Toxicol. 2012, 108, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Gentilcore, D.; Porreca, I.; Rizzo, F.; Ganbaatar, E.; Carchia, E.; Mallardo, M.; De Felice, M.; Ambrosino, C. Bisphenol A interferes with thyroid specific gene expression. Toxicology 2013, 304, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Chen, L.; Wu, J.; Hua, J.; Yang, L.; Wang, Q.; Zhang, W.; Lee, J.; Zhou, B. Parental co-exposure to bisphenol A and nano-Tio2 causes thyroid endocrine disruption and developmental neurotoxicity in zebrafish offspring. Sci. Total Environ. 2019, 650, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.-M.; Tian, X.-F.; Fang, X.-D.; Ji, F.-J. Waterborne exposure to bisphenol F causes thyroid endocrine disruption in zebrafish larvae. Chemosphere 2016, 147, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Kwon, B.; Kho, Y.; Kim, P.-G.; Ji, K. Thyroid endocrine disruption in male zebrafish following exposure to binary mixture of bisphenol AF and sulfamethoxazole. Environ. Toxicol. Pharmacol. 2016, 48, 168–174. [Google Scholar] [CrossRef]
- Lee, S.; Kim, C.; Shin, H.; Kho, Y.L.; Choi, K. Comparison of thyroid hormone disruption potentials by bisphenols A, S, F, and Z in embryo-larval zebrafish. Chemosphere 2019, 221, 115–123. [Google Scholar] [CrossRef]
- Tang, T.; Yang, Y.; Chen, Y.; Tang, W.; Wang, F.; Diao, X. Thyroid Disruption in Zebrafish Larvae by Short-Term Exposure to Bisphenol AF. Int. J. Environ. Res. Public Health 2015, 12, 13069–13084. [Google Scholar] [CrossRef] [PubMed]
- Terrien, X.; Fini, J.; Demeneix, B.; Schramm, K.; Prunet, P. Generation of fluorescent zebrafish to study endocrine disrup-tion and potential crosstalk between thyroid hormone and corticosteroids. Aquat. Toxicol. 2011, 105, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Zhao, F.; Zhang, X.; Liu, W.; Jiang, G.; Wang, H.; Ru, S. Transgenerational thyroid endocrine disruption induced by bisphenol S affects the early development of zebrafish offspring. Environ. Pollut. 2018, 243, 800–808. [Google Scholar] [CrossRef]
- Zhang, D.-H.; Zhou, E.-X.; Yang, Z.-L. Waterborne exposure to BPS causes thyroid endocrine disruption in zebrafish larvae. PLoS ONE 2017, 12, e0176927. [Google Scholar] [CrossRef]
- Ong, K.J.; Zhao, X.; Thistle, M.E.; MacCormack, T.J.; Clark, R.J.; Ma, G.; Martinez-Rubi, Y.; Simard, B.; Loo, J.S.C.; Veinot, J.G.; et al. Mechanistic insights into the effect of nanoparticles on zebrafish hatch. Nanotoxicology 2014, 8, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Orozco, A.; Valverde-R, C. Thyroid Hormone Deiodination in Fish. Thyroid 2005, 15, 799–813. [Google Scholar] [CrossRef]
- Zaccaroni, A.; Gamberoni, M.; Mandrioli, L.; Sirri, R.; Mordenti, O.; Scaravelli, D.; Sarli, G.; Parmeggiani, A. Thyroid hormones as a potential early biomarker of exposure to 4-nonylphenol in adult male shubunkins (Carassius auratus). Sci. Total Environ. 2009, 407, 3301–3306. [Google Scholar] [CrossRef]
- Houbrechts, A.; Delarue, J.; Gabriels, I.; Sourbron, J.; Darras, V. Permanent deiodinase type 2 deficiency strongly per-turbs zebrafish development, growth, and fertility. Endocrinology 2016, 157, 3668–3681. [Google Scholar] [CrossRef]
- Castañeda Cortés, D.; Langlois, V.; Fernandino, J. Crossover of the hypothalamic pituitary–adrenal/interrenal–thyroid, and–gonadal axes in testicular development. Front. Endocrinol. 2014, 5, 139. [Google Scholar] [CrossRef]
- Iyigündoğdu, I.; Üstündağ, A.; Duydu, Y. Toxicological Evaluation of Bisphenol A and Its Analogues. Turk. J. Pharm. Sci. 2020, 17, 457–462. [Google Scholar] [CrossRef] [PubMed]



| Classification | Bisphenol | Bisphenol A (BPA) | Bisphenol AF (BPAF) | Bisphenol C (BPC) |
| Structure a |  | 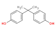 |  |  |
| CAS number | X, Y: regulating factor for estrogenic andanti-and rogenic effects | 80-05-7 | 1478-61-1 | 14868-03-2 |
| Molecular formula | C15H16O2 | C15H10F6O2 | C14H10Cl2O2 | |
| Molecular weight (g/mol) | 228.29 | 336.24 | 281.14 | |
| Classification | Bisphenol F (BPF) | Bisphenol S (BPS) | Bisphenol SIP (BPSIP) | Bisphenol Z (BPZ) |
| Structure a | 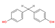 | 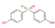 | 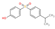 |  |
| CAS number | 620-92-8 | 80-09-1 | 95235-30-6 | 843-55-0 |
| Molecular formula | C13H12O2 | C12H10O4S1 | C15H16O4S1 | C18H20O2 |
| Molecular weight (g/mol) | 200.24 | 250.27 | 292.35 | 268.36 |
| Element | Explanation | Inclusion Criteria | Exclusion Criteria |
|---|---|---|---|
| (P) Population | What are the characteristics of the receptors? |
|
|
| (E) Exposures | What are the types of chemicals and the timing of exposure? |
|
|
| (C) Comparator | Which exposure groups will be compared to each other? |
|
|
| (O) Outcome | Which outcomes will be included or covered? |
|
|
| Publication parameters | - |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Moon, K.W.; Ji, K. Systematic Review of Exposure to Bisphenol A Alternatives and Its Effects on Reproduction and Thyroid Endocrine System in Zebrafish. Appl. Sci. 2021, 11, 1837. https://doi.org/10.3390/app11041837
Lee J, Moon KW, Ji K. Systematic Review of Exposure to Bisphenol A Alternatives and Its Effects on Reproduction and Thyroid Endocrine System in Zebrafish. Applied Sciences. 2021; 11(4):1837. https://doi.org/10.3390/app11041837
Chicago/Turabian StyleLee, Jiyun, Kyong Whan Moon, and Kyunghee Ji. 2021. "Systematic Review of Exposure to Bisphenol A Alternatives and Its Effects on Reproduction and Thyroid Endocrine System in Zebrafish" Applied Sciences 11, no. 4: 1837. https://doi.org/10.3390/app11041837
APA StyleLee, J., Moon, K. W., & Ji, K. (2021). Systematic Review of Exposure to Bisphenol A Alternatives and Its Effects on Reproduction and Thyroid Endocrine System in Zebrafish. Applied Sciences, 11(4), 1837. https://doi.org/10.3390/app11041837







