Structural, Electronic, and Optical Properties of Group 6 Doped Anatase TiO2: A Theoretical Approach
Abstract
1. Introduction
2. Materials and Methods
3. Results
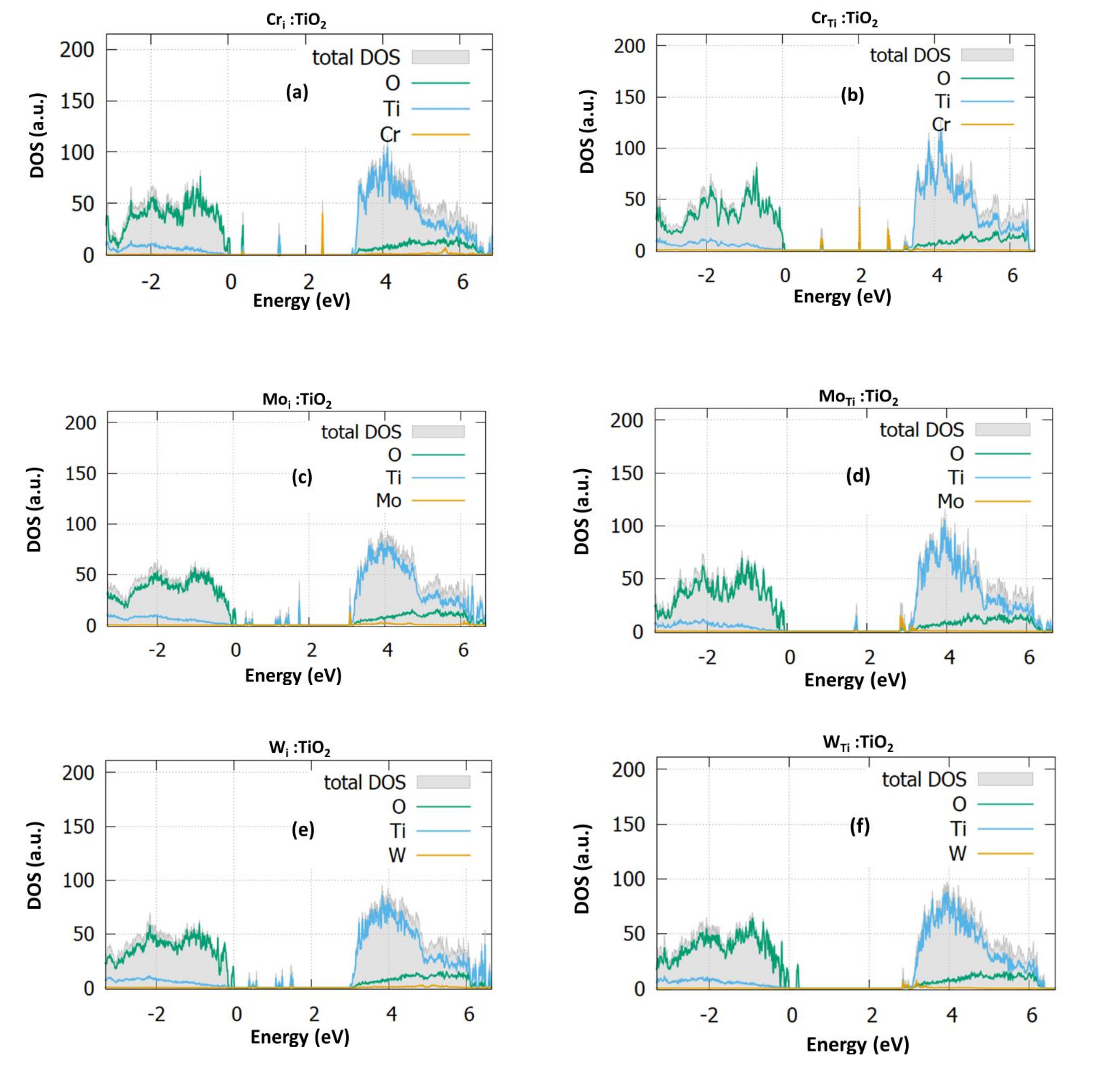
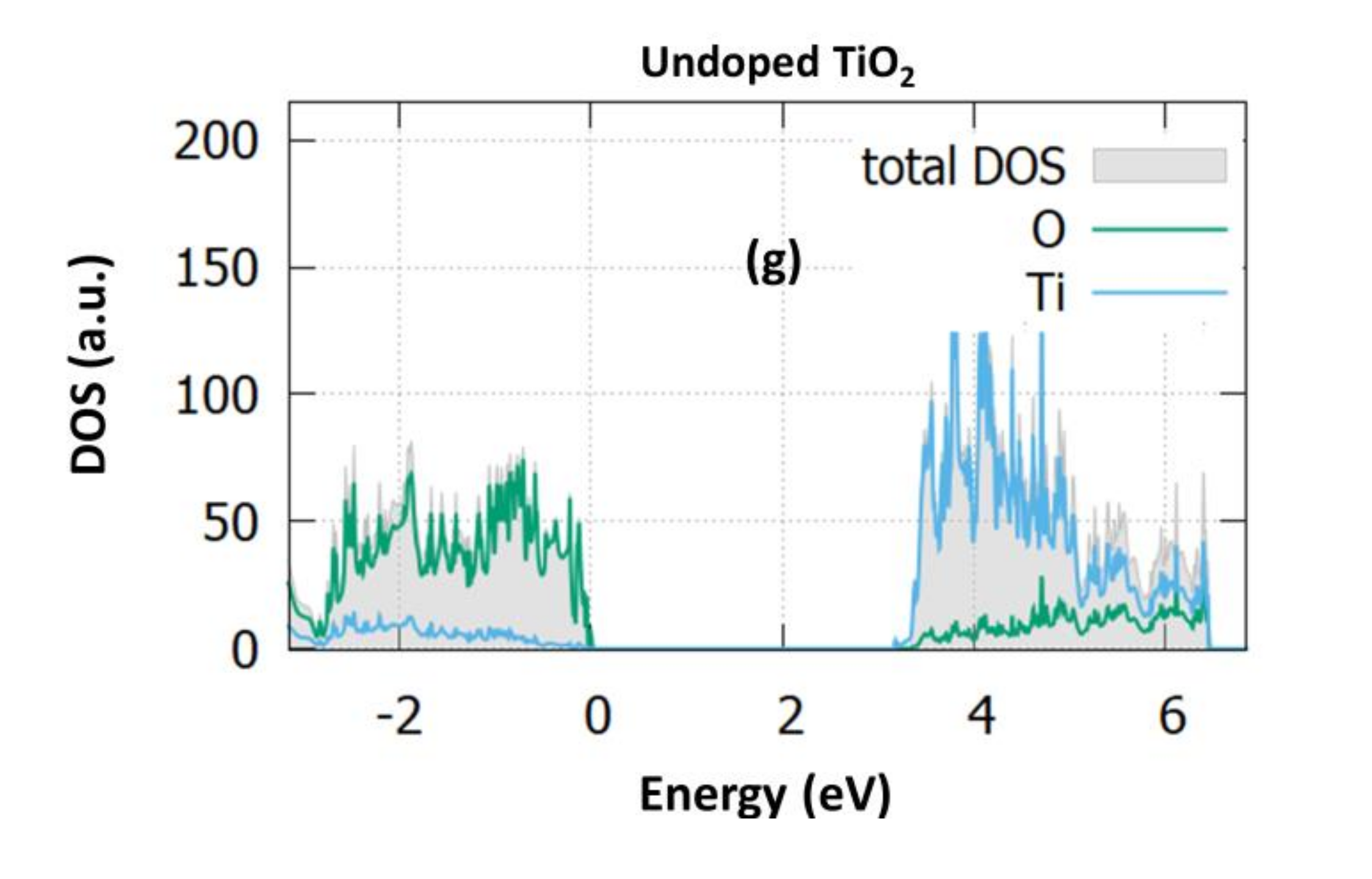
3.1. Structural and Electronic Properties
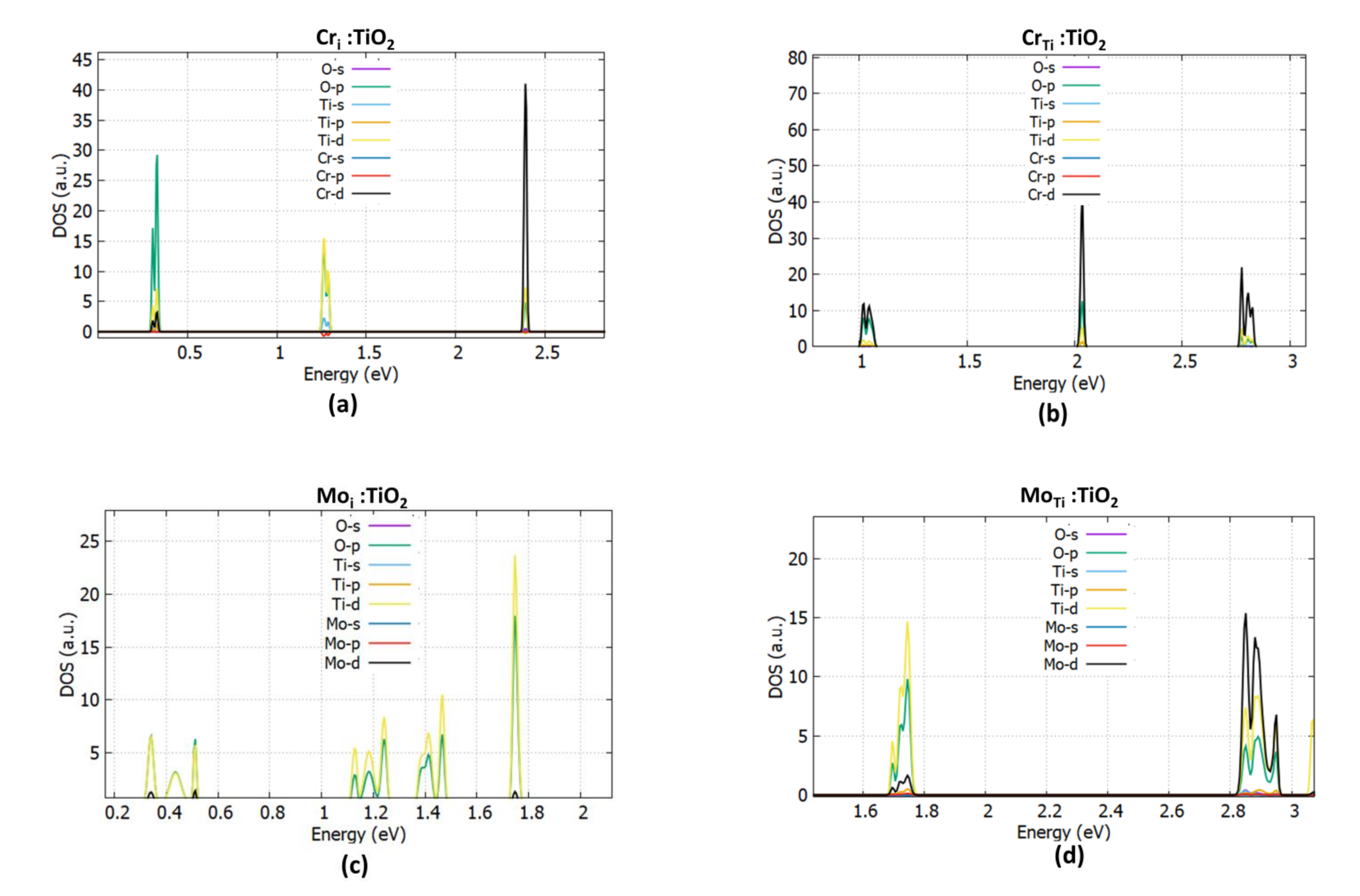
3.1.1. Cr Doping
3.1.2. Mo Doping
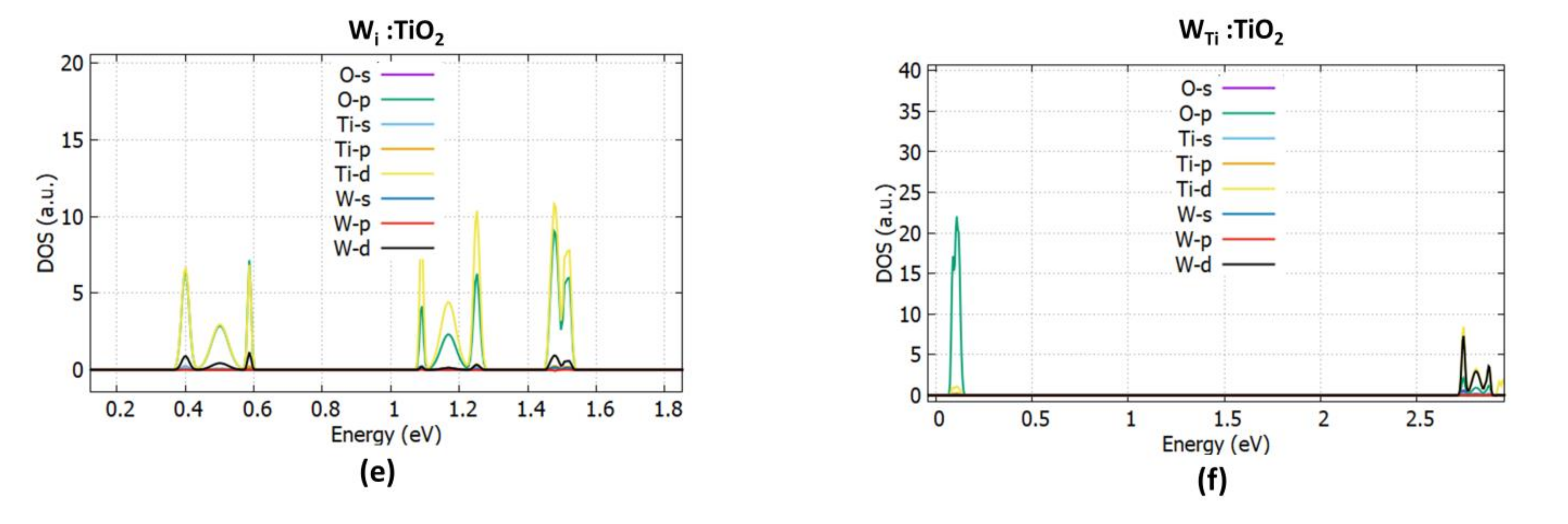
3.1.3. W Doping
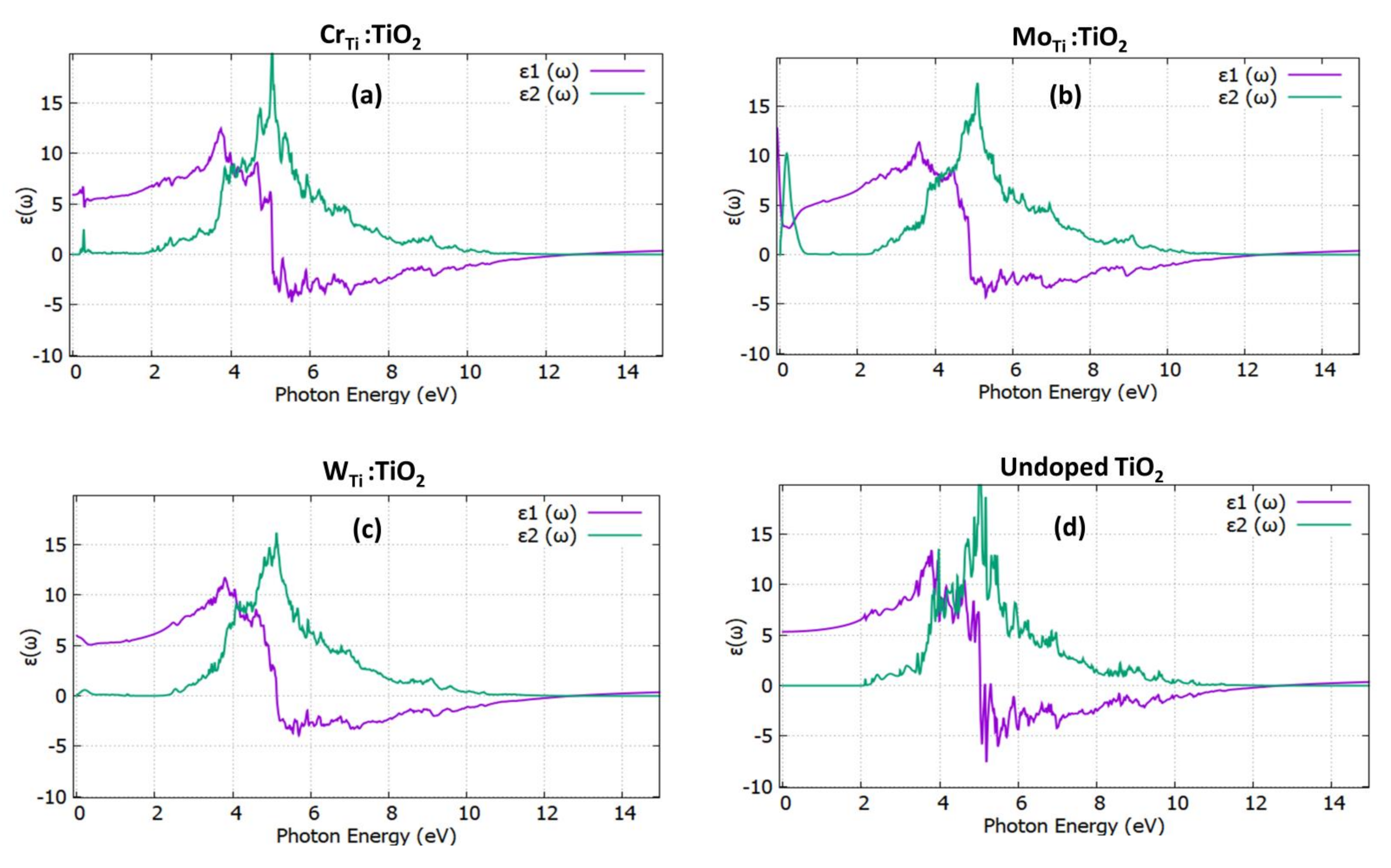
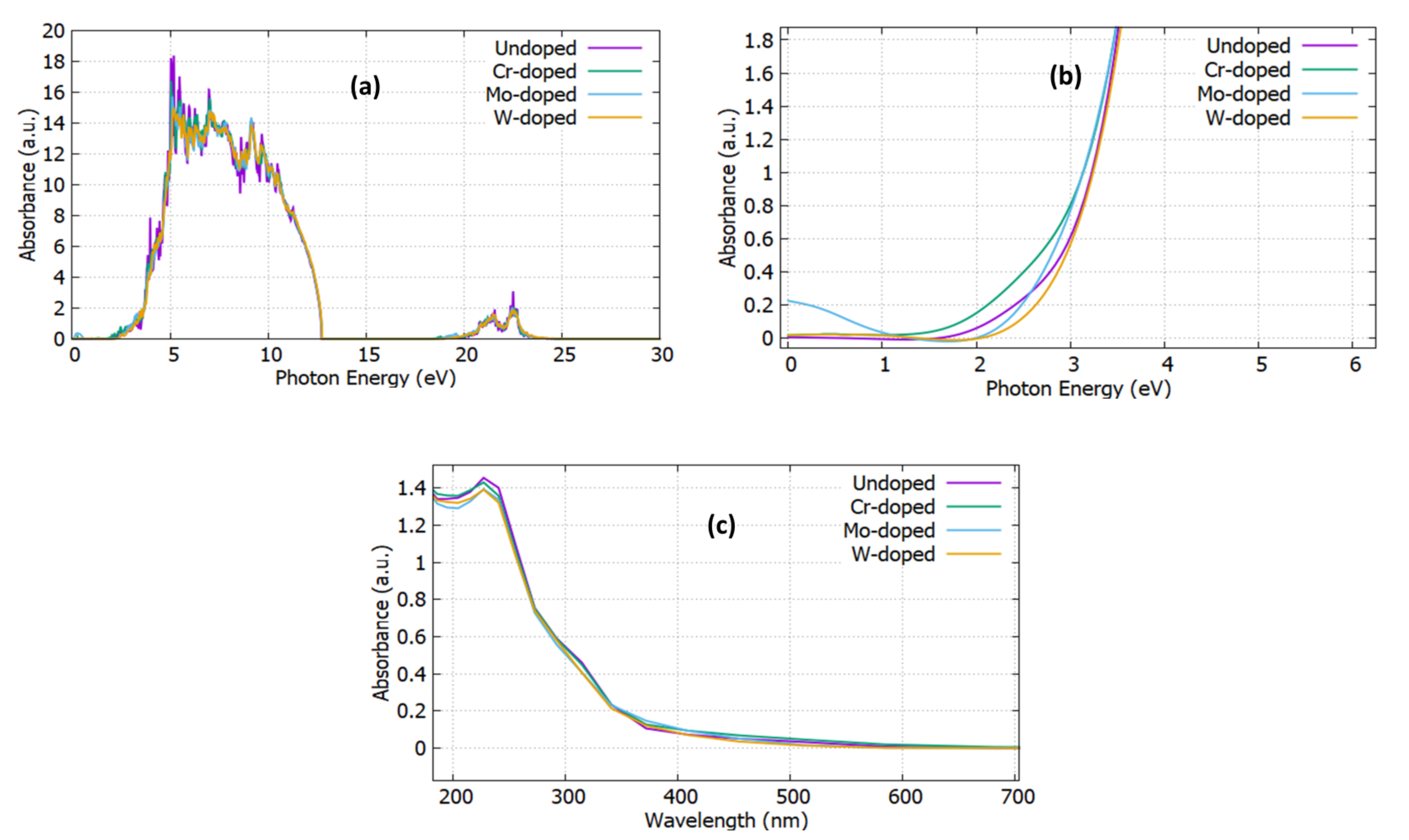
3.2. Optical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37. [Google Scholar] [CrossRef]
- Gratzel, M. Photoelectrochemical cells. Nature 2001, 414, 338. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269. [Google Scholar] [CrossRef]
- Khan, S.U.M.; Al-Shahry, M.; Ingler, W.B. Efficient photochemical water splitting by a chemically modified n-TiO2. Science 2002, 297, 2243. [Google Scholar] [CrossRef] [PubMed]
- Russo, S.P.; Grey, I.E.; Wilson, N.C. Nitrogen/hydrogen codoping of anatase: A DFT study. J. Phys. Chem. C 2008, 112, 7653. [Google Scholar] [CrossRef]
- Yang, H.G.; Sun, C.H.; Qiao, S.Z.; Zou, J.; Liu, G.; Smith, S.C.; Cheng, H.M.; Lu, G.Q. Anatase TiO2 single crystals with a large percentage of reactive facets. Nature 2008, 453, 638. [Google Scholar] [CrossRef]
- Gai, Y.; Li, J.; Li, S.-S.; Xia, J.-B.; Wei, S.-H. Design of narrow-gap TiO2: A passivated codoping approach for enhanced photoelectrochemical activity. Phys. Rev. Lett. 2009, 102, 036402. [Google Scholar] [CrossRef]
- Vasilopoulou, M.; Georgiadou, D.G.; Soultati, A.; Boukos, N.; Gardelis, S.; Palilis, L.C.; Fakis, M.; Skoulatakis, G.; Kennou, S.; Botzakaki, M.; et al. Atomic-layer-deposited aluminum and zirconium oxides for surface passivation of TiO2 in high-efficiency organic photovoltaics. Adv. Energy Mater. 2014, 4, 1400214. [Google Scholar] [CrossRef]
- Sivula, K.; van de Krol, R. Semiconducting materials for photoelectrochemical energy conversion. Nat. Mater. Rev. 2016, 1, 15010. [Google Scholar] [CrossRef]
- Filippatos, P.P.; Kelaidis, N.; Vasilopoulou, M.; Davazoglou, D.; Chroneos, A. Defect Processes in Halogen Doped SnO2. Appl. Sci. 2021, 11, 551. [Google Scholar] [CrossRef]
- Qiu, Y.; Chen, W.; Yang, S. Double-layered photoanodes from variable-size anatase TiO2 nanospindles: A candidate for high-efficiency dye-sensitized solar cells. Angew. Chem. Int. Ed. 2010, 49, 3675–3679. [Google Scholar] [CrossRef]
- Ullattil, S.G.; Thelappurath, A.V.; Tadka, S.N.; Kavil, J.; Vijayan, B.K.; Periyat, P. A sol-solvothermal processed ‘Black TiO2’as photoanode material in dye sensitized solar cells. Sol. Energy 2017, 155, 490–495. [Google Scholar] [CrossRef]
- Govindaraj, R.; Santhosh, N.; Pandian, M.S.; Ramasamy, P.; Sumita, M. Fabrication of stable dye-sensitized solar cell with hydrothermally synthesized titanium dioxide nanorods as a photoanode material. J. Mater. Sci. Mater. Electron. 2018, 29, 3736–3743. [Google Scholar] [CrossRef]
- Sauvage, F.; Di Fonzo, F.; Li Bassi, A.; Casari, C.S.; Russo, V.; Divitini, G.; Graetzel, M. Hierarchical TiO2 photoanode for dye-sensitized solar cells. Nano Lett. 2010, 10, 2562–2567. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Neale, N.R.; Miedaner, A.; Frank, A.J. Enhanced charge-collection efficiencies and light scattering in dye-sensitized solar cells using oriented TiO2 nanotubes arrays. Nano Lett. 2007, 7, 69–74. [Google Scholar] [CrossRef]
- Mor, G.K.; Shankar, K.; Paulose, M.; Varghese, O.K.; Grimes, C.A. Use of highly-ordered TiO2 nanotube arrays in dye-sensitized solar cells. Nano Lett. 2006, 6, 215–218. [Google Scholar] [CrossRef]
- Wang, Z.; Fang, J.; Mi, Y.; Zhu, X.; Ren, H.; Liu, X.; Yan, Y. Enhanced performance of perovskite solar cells by ultraviolet-ozone treatment of mesoporous TiO2. Appl. Surf. Sci. 2018, 436, 596–602. [Google Scholar] [CrossRef]
- Zhang, D.; Xie, F.; Lin, P.; Choy, W.C. Al-TiO2 composite-modified single-layer graphene as an efficient transparent cathode for organic solar cells. ACS Nano 2013, 7, 1740–1747. [Google Scholar] [CrossRef]
- Seo, H.O.; Park, S.Y.; Shim, W.H.; Kim, K.D.; Lee, K.H.; Jo, M.Y.; Kim, J.H.; Lee, E.; Kim, D.W.; Kim, Y.D.; et al. Ultrathin TiO2 films on ZnO electron-collecting layers of inverted organic solar cell. J. Phys. Chem. C 2011, 115, 21517–21520. [Google Scholar] [CrossRef]
- Zhang, D.; Choy, W.C.; Xie, F.; Sha, W.E.; Li, X.; Ding, B.; Zhang, K.; Huang, F.; Cao, Y. Plasmonic electrically functionalized TiO2 for high-performance organic solar cells. Adv. Funct. Mater. 2013, 23, 4255–4261. [Google Scholar] [CrossRef]
- Lira-Cantu, M.; Chafiq, A.; Faissat, J.; Gonzalez-Valls, I.; Yu, Y. Oxide/polymer interfaces for hybrid and organic solar cells: Anatase vs. Rutile TiO2. Sol. Energy Mat. Sol. Cells 2011, 95, 1362–1374. [Google Scholar] [CrossRef]
- Lin, Z.; Jiang, C.; Zhu, C.; Zhang, J. Development of inverted organic solar cells with TiO2 interface layer by using low-temperature atomic layer deposition. ACS Appl. Mater. Interfaces 2013, 5, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Green, M.A.; Ho-Baillie, A.; Snaith, H.J. The emergence of perovskite solar cells. Nat. Photonics 2014, 8, 506–514. [Google Scholar] [CrossRef]
- Kim, H.S.; Park, N.G. Parameters affecting I–V hysteresis of CH3NH3PbI3 perovskite solar cells: Effects of perovskite crystal size and mesoporous TiO2 layer. J. Phys. Chem Lett. 2014, 5, 2927–2934. [Google Scholar] [CrossRef]
- Pathak, S.K.; Abate, A.; Ruckdeschel, P.; Roose, B.; Gödel, K.C.; Vaynzof, Y.; Santhala, A.; Watanabe, S.I.; Hollman, D.J.; Noel, N.; et al. Performance and stability enhancement of dye-sensitized and perovskite solar cells by Al doping of TiO2. Adv. Funct. Mater. 2014, 24, 6046–6055. [Google Scholar] [CrossRef]
- Bessekhouad, Y.; Robert, D.; Weber, J.V. Bi2S3/TiO2 and CdS/TiO2 heterojunctions as an available configuration for photocatalytic degradation of organic pollutant. J. Photochem. Photobiol. A 2004, 163, 569. [Google Scholar] [CrossRef]
- Vasilopoulou, M.; Kelaidis, N.; Polydorou, E.; Soultati, A.; Davazoglou, D.; Argitis, P.; Papadimitropoulos, G.; Tsikritzis, D.; Kennou, S.; Auras, F.; et al. Hydrogen and nitrogen codoping of anatase TiO2 for efficiency enhancement in organic solar cells. Sci. Rep. 2017, 7, 17839. [Google Scholar] [CrossRef]
- Wang, J.; Tafen, D.N.; Lewis, J.P.; Hong, Z.; Manivannan, A.; Zhi, M.; Li, M.; Wu, N. Origin of photocatalytic activity of nitrogen-doped TiO2 nanobelts. J. Am. Chem. Soc. 2009, 131, 12290–12297. [Google Scholar] [CrossRef]
- Czoska, A.M.; Livraghi, S.; Chiesa, M.; Giamello, E.; Agnoli, S.; Granozzi, G.; Finazzi, E.; Valentin, C.D.; Pacchioni, G. The nature of defects in fluorine-doped TiO2. J. Phys. Chem. C 2008, 112, 8951–8956. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, C.; Liu, X.; Gu, F.; Du, H.L.; Shi, L. Zn-doped TiO2 nanoparticles with high photocatalytic activity synthesized by hydrogen–oxygen diffusion flame. Appl. Catal. B Environ. 2008, 79, 208–215. [Google Scholar] [CrossRef]
- Opra, D.P.; Gnedenkov, S.V.; Sokolov, A.A.; Podgorbunsky, A.B.; Ustinov, A.Y.; Mayorov, V.Y.; Kuryavyi, V.G.; Sinebryukhov, S.L. Vanadium-doped TiO2-B/Anatase Mesoporous Nanotubes with Improved Rate and Cycle Performance for Rechargeable Lithium and Sodium Batteries. J. Mater. Sci. Technol. 2020, 54, 181–189. [Google Scholar] [CrossRef]
- Sharma, S.D.; Singh, D.; Saini, K.K.; Kant, C.; Sharma, V.; Jain, S.C.; Sharma, C.P. Sol–gel-derived super-hydrophilic nickel doped TiO2 film as active photo-catalyst. Appl. Catal. A Gen. 2006, 314, 40–46. [Google Scholar] [CrossRef]
- Kordatos, A.; Kelaidis, N. Chroneos, A. Defect pair formation in fluorine and nitrogen codoped TiO2. J. Appl. Phys. 2018, 123, 161510. [Google Scholar] [CrossRef]
- Filippatos, P.P.; Kelaidis, N.; Vasilopoulou, M.; Davazoglou, D.; Lathiotakis, N.N.; Chroneos, A. Defect processes in F and Cl doped anatase TiO2. Sci. Rep. 2019, 9, 19970. [Google Scholar] [CrossRef]
- Babu, V.J.; Nair, A.S.; Peining, Z.; Ramakrishna, S. Synthesis and characterization of rice grains like Nitrogen-doped TiO2 nanostructures by electrospinning–photocatalysis. Mater. Lett. 2011, 65, 3064–3068. [Google Scholar] [CrossRef]
- Khan, S.; Cho, H.; Kim, D.; Han, S.S.; Lee, K.H.; Cho, S.H.; Song, T.; Choi, H. Defect engineering toward strong photocatalysis of Nb-doped anatase TiO2: Computational predictions and experimental verifications. Appl. Catal. B 2017, 206, 520–530. [Google Scholar] [CrossRef]
- Payne, M.C.; Teter, M.P.; Allan, D.C.; Arias, T.A.; Joannopoulos, J.D. Iterative minimization techniques for ab initio total-energy calculations: Molecular dynamics and conjugate gradients. Rev. Mod. Phys. 1992, 64, 1045. [Google Scholar] [CrossRef]
- Segall, M.D.; Lindan, P.J.D.; Probert, M.J.; Pickard, C.J.; Hasnip, P.J.; Clark, S.J.; Payne, M.C. First-principles simulation: Ideas, illustrations and the CASTEP code. J. Phys. Condens. Matter 2002, 14, 2717. [Google Scholar] [CrossRef]
- Perdew, J.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- Vanderbilt, D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism. Phys. Rev. B 1990, 41, 7892. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188. [Google Scholar] [CrossRef]
- Kiarii, E.M.; Govender, K.K.; Ndungu, P.G.; Govender, P.P. The generation of charge carriers in semi conductors–A theoretical study. Chem. Phys. Lett. 2017, 678, 167. [Google Scholar] [CrossRef]
- Liu, C.; Song, Y.; Yu, X.; Liu, J.; Deng, J. Electronic Structure and Optical Absorption Spectra of C–Cr Co-Doped Anatase TiO2 Based on First Principles. Phys. Status Solidi B 2018, 255, 1700616. [Google Scholar] [CrossRef]
- Zhang, J.; Deng, M.; Ren, F.; Wu, Y.; Wang, Y. Effects of Mo/W codoping on the visible-light photocatalytic activity of monoclinic BiVO4 within the GGA+ U framework. RSC Adv. 2016, 6, 1229. [Google Scholar] [CrossRef]
- Di Valentin, C.; Pacchioni, G. Trends in non-metal doping of anatase TiO2: B, C, N and F. Catal. Today 2013, 206, 12–18. [Google Scholar] [CrossRef]
- Samsudin, E.M.; Hamid, S.B.A. Effect of band gap engineering in anionic-doped TiO2 photocatalyst. Appl. Surf. Sci. 2017, 391, 326–336. [Google Scholar] [CrossRef]
- Nagpal, P.; Klimov, V.I. Role of mid-gap states in charge transport and photoconductivity in semiconductor nanocrystal films. Nat. Commun. 2011, 2, 486. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Dai, Y.; Huang, B. Density Functional Characterization of the Electronic Structure and Visible-Light Absorption of Cr-Doped Anatase TiO2. ChemPhysChem 2009, 10, 2327. [Google Scholar] [CrossRef] [PubMed]
- Gönüllü, Y.; Haidry, A.A.; Saruhan, B. Nanotubular Cr-doped TiO2 for use as high-temperature NO2 gas sensor. Sens. Actuators B Chem. 2015, 217, 78. [Google Scholar] [CrossRef]
- Luttrell, T.; Halpegamage, S.; Tao, J.; Kramer, A.; Sutter, E.; Batzill, M. Why is anatase a better photocatalyst than rutile?-Model studies on epitaxial TiO2 films. Sci. Rep. 2014, 4, 4043. [Google Scholar] [CrossRef] [PubMed]
- Kelaidis, N.; Kordatos, A.; Christopoulos, S.-R.G.; Chroneos, A. A roadmap of strain in doped anatase TiO2. Sci. Rep. 2018, 8, 12790. [Google Scholar] [CrossRef]
- Takaoka, G.H.; Nose, T.; Kawashita, M. Photocatalytic properties of Cr-doped TiO2 films prepared by oxygen cluster ion beam assisted deposition. Vacuum 2008, 83, 679. [Google Scholar] [CrossRef]
- Yu, X.; Hou, T.; Sun, X.; Li, Y. The influence of defects on Mo-doped TiO2 by first-principles studies. ChemPhysChem 2012, 13, 1514. [Google Scholar] [CrossRef]
- Yu, C.J.; Yu, J.; Ho, W.; Jiang, Z.; Zhang, L. Effects of F-doping on the photocatalytic activity and microstructures of nanocrystalline TiO2 powders. Chem. Mater. 2002, 14, 3808. [Google Scholar] [CrossRef]
- Kubacka, A.; Colón, G.; Fernández-García, M. Cationic (V, Mo, Nb, W) doping of TiO2–anatase: A real alternative for visible light-driven photocatalysts. Catal. Today 2009, 143, 286. [Google Scholar] [CrossRef]
- Kubacka, A.; Colon, G.; Garcia, M. N-and/or W-(co) doped TiO2-anatase catalysts: Effect of the calcination treatment on photoactivity. Appl. Catal. B Environ. 2010, 9, 238. [Google Scholar] [CrossRef]
- Samat, M.H.; Ali, A.M.M.; Taib, M.F.M.; Hassan, O.H.; Yahya, M.Z.A. Hubbard U calculations on optical properties of 3d transition metal oxide TiO2. Res. Phys. 2016, 6, 891. [Google Scholar]
- Abdel-Aziz, M.M.; Yahia, I.S.; Wahab, L.A.; Fadel, M.; Afifi, M.A. Determination and analysis of dispersive optical constant of TiO2 and Ti2O3 thin films. Appl. Surf. Sci. 2006, 252, 8163. [Google Scholar] [CrossRef]
- Yu, X.; Li, C.; Ling, Y.; Tang, T.A.; Wu, Q.; Kong, J. First principles calculations of electronic and optical properties of Mo-doped rutile TiO2. J. Alloys Compd. 2010, 507, 33. [Google Scholar] [CrossRef]
- Khan, M.; Xu, J.; Chen, N.; Cao, W. Electronic and optical properties of pure and Mo doped anatase TiO2 using GGA and GGA+ U calculations. Phys. B 2012, 407, 3610. [Google Scholar] [CrossRef]
- Mergel, D. Modeling thin TiO2 films of various densities as an effective optical medium. Thin Solid Films 2001, 397, 216. [Google Scholar] [CrossRef]
- Hajjaji, A.; Labidi, A.; Gaidi, M.; Ben Rabha, M.; Smirani, R.; Bejaoui, A.; Bessais, B.; El Khakani, M.A. Structural, optical and sensing properties of Cr-doped TiO2 thin films. J. Sens. Lett. 2011, 9, 1697. [Google Scholar] [CrossRef]
- Lin, S.S. Properties of heavily W-doped TiO2 films deposited on Al2O3-deposited glass by simultaneous rf and dc magnetron sputtering. Ceram. Int. 2014, 40, 217. [Google Scholar] [CrossRef]
- Opra, D.P.; Gnedenkov, S.V.; Sinebryukhov, S.L. Recent efforts in design of TiO2(B) anodes for high-rate lithium-ion batteries: A review. J. Power Sources 2019, 442, 227225. [Google Scholar] [CrossRef]

| a (Å) | c (Å) | Volume (Å3) | |
|---|---|---|---|
| TiO2 | 3.80 | 9.73 | 140.80 |
| Cri:TiO2 | 3.80 | 9.80 | 143.75 |
| CrTi:TiO2 | 3.81 | 9.69 | 140.29 |
| Moi:TiO2 | 3.83 | 9.70 | 142.29 |
| MoTi:TiO2 | 3.81 | 9.72 | 141.10 |
| Wi:TiO2 | 3.83 | 9.70 | 142.30 |
| WTi:TiO2 | 3.82 | 9.69 | 141.40 |
| Band Gap (eV) | |
|---|---|
| TiO2 | 3.12 |
| Cri:TiO2 | 3.04 |
| CrTi:TiO2 | 3.09 |
| Moi:TiO2 | 2.87 |
| MoTi:TiO2 | 2.77 |
| Wi:TiO2 | 2.89 |
| WTi:TiO2 | 2.88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filippatos, P.-P.; Kelaidis, N.; Vasilopoulou, M.; Davazoglou, D.; Chroneos, A. Structural, Electronic, and Optical Properties of Group 6 Doped Anatase TiO2: A Theoretical Approach. Appl. Sci. 2021, 11, 1657. https://doi.org/10.3390/app11041657
Filippatos P-P, Kelaidis N, Vasilopoulou M, Davazoglou D, Chroneos A. Structural, Electronic, and Optical Properties of Group 6 Doped Anatase TiO2: A Theoretical Approach. Applied Sciences. 2021; 11(4):1657. https://doi.org/10.3390/app11041657
Chicago/Turabian StyleFilippatos, Petros-Panagis, Nikolaos Kelaidis, Maria Vasilopoulou, Dimitris Davazoglou, and Alexander Chroneos. 2021. "Structural, Electronic, and Optical Properties of Group 6 Doped Anatase TiO2: A Theoretical Approach" Applied Sciences 11, no. 4: 1657. https://doi.org/10.3390/app11041657
APA StyleFilippatos, P.-P., Kelaidis, N., Vasilopoulou, M., Davazoglou, D., & Chroneos, A. (2021). Structural, Electronic, and Optical Properties of Group 6 Doped Anatase TiO2: A Theoretical Approach. Applied Sciences, 11(4), 1657. https://doi.org/10.3390/app11041657








