Abstract
Background: Cynara cardunculus L. var. altilis (DC) is a plant generally associated as an ingredient in the Mediterranean diet. The polyphenols present in this plant provide pharmacological and nutritional properties. C. cardunculus L. has been used throughout animal studies, which demonstrated an anti-inflammatory effect. Inflammatory bowel disease (IBD) is a chronic inflammatory disorder of the gastrointestinal tract. Since there is not a known cure, the research of new possible pharmacological approaches is essential. This study aims to evaluate the effect of an aqueous extract of C. cardunculus L. dry leaves in a 2,4,6-Trinitrobenzenesulfonic acid (TNBS)-induced colitis model. Methods: CD-1 mice with TNBS-induced colitis received an intraperitoneal (IP) administration of C. cardunculus L. once per day for 4 days. Results: The C. cardunculus L. demonstrated a beneficial effect in this experimental model of IBD with anti-inflammatory action through the reduction of tumor necrosis factor (TNF)-α levels. It also demonstrated a beneficial influence on the extra-intestinal manifestations related to IBD, with the absence of significant side effects of its use. Conclusions: The extract of C. cardunculus L. dry leaves can become an interesting tool for new possible pharmacological approaches in the management of IBD.
1. Introduction
Cynara cardunculus L. var. altilis (DC), commonly named cardoon, is a complex species that belongs to the Asteraceae family and is native to the Mediterranean basin [1,2,3,4,5]. This plant combines organoleptic properties with a rich source of nutraceutical compounds [5]. C. cardunculus L. is a good source of components beneficial to health, such as polyphenols, which play a relevant role in its pharmacological properties [6,7]. The leaves are recognized in traditional medicine for their therapeutic potential as a diuretic, cardiotonic, antidiabetic, antihemorrhoidal, and antimicrobial agent, among others [1,8,9,10]. Additionally, it has been used throughout animal studies, demonstrating its pharmacological properties, namely its anti-inflammatory effect. C. cardunculus L. has demonstrated its anti-inflammatory effect through the inhibition of NF-κB activation and, consequently, reducing the expression of ciclooxigenase-2 and matrix metalloproteinase-2 proteins [11,12]. Specifically, cardoon leaves are constituted by several active compounds such as caffeoylquinic acids, flavonoids, cynarin, chlorogenic acid, luteolin, and inulin [7,13,14]. Eventually, C. cardunculus L. extract (CLE), represented by the presence of these compounds, may be used to ameliorate the symptoms associated with inflammatory bowel disease (IBD).
IBD is categorized as a chronic relapsing idiopathic inflammation response disorder of the gastrointestinal tract [15,16,17]. IBD is represented by two chronic phenotypes, namely, Crohn’s disease (CD) and ulcerative colitis (UC) [15,18,19]. Neither UC nor CD is fatal; however, both are debilitating conditions [19]. The evolution of IBD follows the advancement of society, and it is estimated that nearly 3.9 million females and 3 million males are living with this disease [20,21]. This disease is predominantly observed in developed countries; however, its global incidence and prevalence are increasing, which appears to accompany the growing adoption of a westernized lifestyle in several countries. Additionally, IBD appears to have different frequencies depending on age, ethnicity, and global localization [19,22,23]. IBD promotes inflammation of the gut and gastrointestinal symptoms such as blood in feces, vomiting, abdominal pain, rectal bleeding, and tenesmus. There are other extra-intestinal manifestations, such as fever, anemia, fatigue, weight loss, arthritis, ankylosing spondylitis, sclerosing cholangitis, uveitis, iritis, pyoderma gangrenosum, and erythema nodosum [16,19,24,25,26].
Currently, IBD is not curable, and the conventional pharmacological approach to treat patients with this disease is to induce and maintain clinical remission [19,27]. Pharmacological therapy for IBD includes anti-inflammatory drugs, such as 5-aminosalicylates (5-ASA), corticosteroids, immunosuppressants (e.g., azathioprine, mercaptopurine, methotrexate, and cyclosporine), biologic agents, antibiotics (e.g., metronidazole and ciprofloxacin), agents to inhibit tumor necrosis factor (TNF)-α, leucocyte adhesion, and migration (natalizumab) [15,24,28]. These therapies are effective; however, they are inappropriate for long-term use due to their side effects and complications related to a higher incidence of malignancies or infectious diseases [16,24]. Additionally, it has been investigated that natural products might have a positive influence on the IBD setting, such as berberine and Wedelia chinensis through the suppression of Th1/Th17 cell proliferation; probiotis by regulating gut microbiota; green tea polyphenols by suppressing mast cells and macrophage activity; catechine and curcumin through inhibition of TNF-α expression, among others that are still under investigation [29,30]. Taking into account all the information above, it is important to investigate new possible pharmacological tools for the management of IBD. This study aims to evaluate the effect of aqueous extracts of C. cardunculus L. dry leaves in the 2,4,6-Trinitrobenzenesulfonic acid (TNBS)-induced colitis model.
2. Materials and Methods
2.1. Reagents
2,4,6-Trinitrobenzene sulphonic acid (TNBS 5%) was acquired from Sigma-Aldrich Chemical. Xylazine (Rompun® 2%) was acquired from Bayer®, and Ketamine (Imalgene® 1000) was acquired from Merial. The CLE was provided by Instituto Politécnico de Viseu, by Escola Superior Agrária de Viseu. An ADVIA® kit was acquired from Siemens Healthcare Diagnostics. For TNF-α measurements, an ELISA assay kit was obtained from Hycult Biotechnology.
2.2. Animals
CD-1 mice (6–10 weeks old, male) were used for this study. They were provided standard polypropylene cages, with access to standard food and water for the animals. The temperature (18–23 °C), humidity (40–60%), and lighting conditions (controlled 12-h light/dark) were monitored during this time. Animal care followed the internationally accepted principles for laboratory animal use and care available in Directive 2010/63/EU. The present experiment was accepted by the Institutional Animal Ethics Committee (approval code: 35/2014).
2.3. Experimental Induction of Colitis
The TNBS-induced colitis model was obtained with the instillation of TNBS by a single intracolonic dose as described by Mateus et al. (2017) [24]. The animals fasted for 24 h to reduce stool from the colon as much as possible, facilitating the insertion of the cannula through the anus. The induction was performed on anesthetized mice. Anesthetization was obtained with an administration of ketamine 100 mg/kg + xylazine 10mg/kg through an intraperitoneal (IP) injection. Colitis was induced chemically through an intracolonic administration of 100 µL of TNBS 2.5% (in ethanol 50%), carefully inserting the cannula until 4 cm into the colon. Then, mice were maintained in a Trendelenburg position for 1 min to avoid reflux.
On day 4, the mice were anesthetized as previously described. In order to collect the blood samples, a cardiac puncture was performed. After that, the mice were sacrificed by cervical dislocation. Colon tissue was separated and removed, liberated from surrounding tissues, and finally, washed with phosphate-buffered saline (PBS).
2.4. Groups Distribution
Groups were categorized based on the influence of CLE in the experimental model of colitis induced with TNBS. Thus, experimental groups were: TNBS group (5 mice)—disease control group, represented by mice with TNBS-induced colitis; TNBS + CLE100 group (10 mice)—experimental group, represented by mice with TNBS-induced colitis treated daily with 100mg/kg of CLE by IP injection; TNBS + CLE500 group (10 mice)—experimental group, represented by mice with TNBS-induced colitis treated daily with 500mg/kg of CLE by IP injection; CLE group (5 mice)—drug control group, represented by healthy mice treated daily with 500mg/kg of CLE by IP injection; ethanol group (5 mice)—TNBS’s vehicle control group, represented by healthy mice with ethanol 50% administered rectally; Sham group (5 mice)—control group, represented by healthy mice with NaCl 0.9% administered rectally.
2.5. CLE Preparation
For CLE preparation, the hot aqueous extraction was used that consisted of: weighing 1500 mg of crushed C. cardunculus L. dry leaves; boiling 50 mL of water; placing the powder in the boiling water and magnetically stirring for 30 min; centrifuging the aqueous extract for 15 min at 3500× g rpm; freezing the supernatant.
2.6. Monitoring of Clinical Signs of Illness
Daily observation of all mice and monitorization of body weight, morbidity, stool consistency, presence of blood, and anus appearance was performed.
2.7. Biochemical Markers
Centrifugation (2000 rpm for 15 min) was performed to separate the serum from the blood samples, and its analysis was possible using an automated clinical chemistry analyzer (ADVIA®1200). Alkaline phosphatase (ALP), alanine aminotransferase (ALT), urea, and creatinine were the biochemical markers analyzed. A quantitative method by immunoturbidimetry (Kroma Systems) was used to evaluate fecal hemoglobin.
2.8. Measurement of Cytokines
TNF-α, an inflammation marker, was quantified and expressed as pg/mL. Colon tissue from each animal was weighed, and then the samples were subjected to homogenization in phosphate-buffered saline (Ultra-turrax T25, 13.500 rev/min, twice for 30 s). Posteriorly, the samples of colonic tissue were subjected to centrifugation (15.000× g rpm for 15 min at 4 °C). The supernatant was conserved in aliquots at −20 °C until use. Spectrophotometric measurement of TNF-α levels was performed at 450 nm (ELISA kit Quantikine, Hycult Biotechnology).
2.9. Statistics
The results were represented as mean ± SEM of N observations (N = number of animals analyzed). SPSS software was used to analyze the data. It was applied to a one-way ANOVA to analyze the results, determining statistical significance between experimental and control groups. For multiple comparisons, Tukey’s post hoc test was used. To be statistically significant, a p-value < 0.05 was considered.
3. Results
3.1. Clinical Signs of Illness
The TNBS group showed changes in the anus appearance, such as the presence of edema, and changes in stool consistency, represented by softer stools and diarrhea. On this hand, the disease control group showed changes in intestinal motility. In the TNBS + CLE groups, the intestinal motility presented only a slight improvement. The CLE500, ethanol, and sham groups did not reveal alterations.
Variations of the body weight were monitored during the 4-day experimental period (Figure 1). The TNBS group showed a reduction in body weight throughout the experimental period (−3.3 ± 1.1%). In the TNBS + CLE groups, no significant differences were observed in relation to the TNBS group, as well as the CLE500 group (−0.07 ± 0.8%). The TNBS + CLE100 group presented a variation of −0.07 ± 0.8% of its initial weight, and the TNBS + CLE500 group presented a variation of −0.6 ± 0.9%. The ethanol and sham groups showed a variation of 14.8 ± 3.8% (p < 0.01 in comparison to the TNBS group) and 24.3 ± 6.1% (p < 0.0001 in comparison to the TNBS group) of its initial weight, respectively.
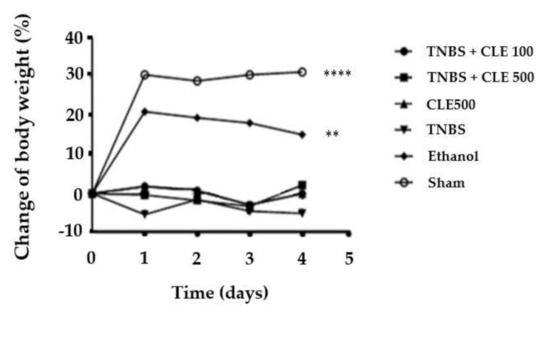
Figure 1.
CLE influence on body weight changes in IBD context. Legend: Two-way ANOVA for multiple comparisons; ** p < 0.01; **** p < 0.0001 in comparison to the TNBS group. Abbreviations: CLE, C. cardunculus L. extract; IBD, inflammatory bowel disease; TNBS, 2,4,6-trinitrobenzene sulfonic acid.
3.2. Biochemical Markers
The mice from the TNBS group showed a significant reduction in colon length (8.4 ± 0.1 cm) in comparison to mice in the sham group (11.2 ± 0.2 cm), which is considered a length of healthy tissue (p < 0.0001). The TNBS + CLE500 group (9.5 ± 0.2 cm) demonstrated a significant increase in colon length in comparison to the TNBS group (p < 0.1). However, the TNBS + CLE100 group revealed similar results with the TNBS group. The CLE500, ethanol, and sham groups demonstrated similar results among them, such as 10.5 ± 0.4 cm, 11.1 ± 0.3 cm, and 11.2 ± 0.2 cm, respectively (Figure 2).
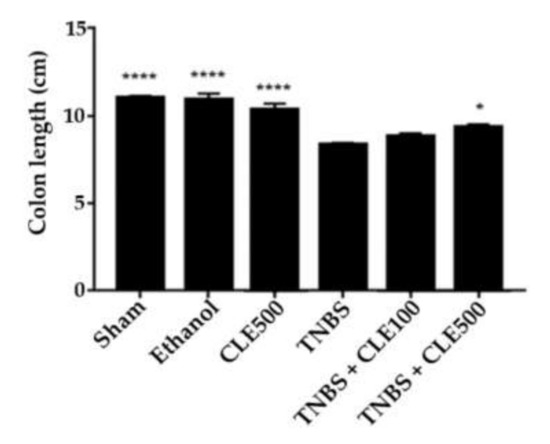
Figure 2.
CLE influence on colon length in IBD context. Legend: One-way ANOVA and Tuckey’s post hoc test; * p < 0.1; **** p < 0.0001; in comparison to the TNBS group. Abbreviations: CLE, C. cardunculus L. extract; TNBS, 2,4,6-trinitrobenzene sulfonic acid.
Fecal hemoglobin was quantified and then compared between all groups (Figure 3). Mice in the TNBS group presented a more severe presence of blood in feces compared to the mice in the sham group (12.0 ± 0.7 and 0.7 ± 0.1 μmol/g feces, respectively, p < 0.0001), as well as the CLE500 group (1.3 ± 0.1 μmol/g feces) and the ethanol group (1.1 ± 0.1 μmol/g feces) (p <0.0001). The TNBS + CLE100 group showed 6.1 ± 0.1 μmol/g feces and the TNBS + CLE500 group presented 4.3 ± 0.3 μmol/g feces (p < 0.0001, in comparison to the TNBS group). Therefore, treatment with CLE showed a positive influence on the severity of focal hemorrhaging. Since the fecal hemoglobin decreased when the dose of CLE was increased, it can be assumed that it has a dose-dependent effect (p < 0.01).
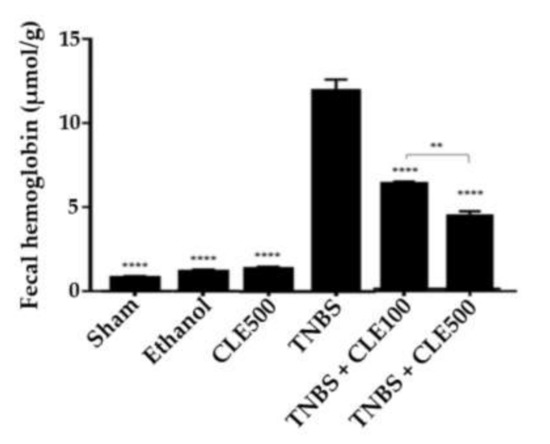
Figure 3.
CLE influence on fecal hemoglobin levels in IBD context. Legend: One-way ANOVA and Tuckey’s post hoc test; ** p < 0.01, **** p < 0.0001 in comparison to the TNBS group or between groups. Abbreviations: CLE, C. cardunculus L. extract; TNBS, 2,4,6-trinitrobenzene sulfonic acid.
Concerning ALP levels, the TNBS group demonstrated a significantly higher concentration in comparison to the sham group (67.8 ± 2.6 and 19.2 ± 1.8 U/L, respectively, p < 0.0001). The TNBS + CLE100 group revealed concentrations of 39.4 ± 1.4 U/L, and the TNBS + CLE500 group presented 39.8 ± 1.4 U/L. In this sense, CLE treatment showed a positive influence in this parameter by decreasing the ALP concentration with statistical significance at both doses (p < 0.0001, in comparison to the TNBS group); however, this effect was not considered dose-dependent. Additionally, ALP concentration in the CLE500 and ethanol groups was significantly lower in comparison to the TNBS group (39.8 ± 0.9 U/L and 38.0 ± 0.8 U/L, p < 0.001, respectively) (Figure 4).
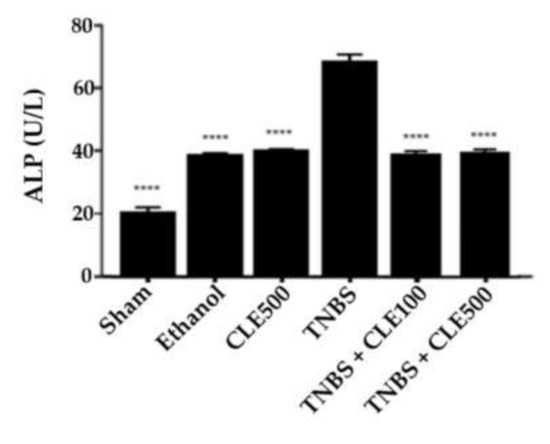
Figure 4.
CLE influence on total ALP levels in IBD context. Legend: One-way ANOVA and Tuckey’s post hoc test; **** p < 0.0001, in comparison to the TNBS group. Abbreviations: ALP, alkaline phosphatase; CLE, C. cardunculus L. extract; TNBS, 2,4,6-trinitrobenzene sulfonic acid.
3.3. Measurement of Cytokines
Through the measurement and comparison of TNF-α levels among all groups, it was demonstrated that the TNBS group showed a significantly higher concentration of this cytokine compared to the sham group (244.6 ± 8.0 vs. 11.2 ± 0.2 pg/mL, p < 0.0001). The TNBS + CLE100 group presented 160.5 ± 8.4 pg/mL of TNF-α (p < 0.01), whereas the TNBS + CLE500 group exhibited 147.3 ± 5.0 pg/mL of TNF-α (p < 0.0001), in comparison to the TNBS group. In control groups, the CLE500 group (42.8 ± 3.7 pg/mL) presented TNF-α levels slightly higher than the sham group; however, the ethanol group (14.9 ± 2.0 pg/mL) presented a TNF-α concentration similar to the sham group (Figure 5). Therefore, mice treated with CLE demonstrated a considerable reduction in TNF-α levels; however, this effect was not considered dose-dependent.
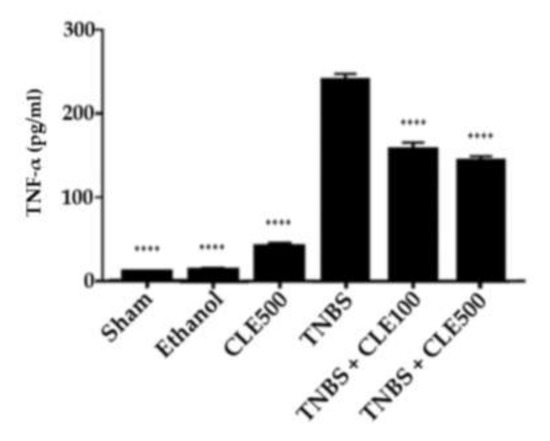
Figure 5.
CLE influence on TNF-α levels in IBD context. Legend: One-way ANOVA and Tuckey’s post hoc test; **** p < 0.0001, in comparison to the TNBS group. Abbreviations: CLE, C. cardunculus L. extract; TNBS, 2,4,6-trinitrobenzene sulfonic acid; TNF-α, tumor necrosis factor-α.
3.4. Renal and Hepatic Functions
In order to evaluate renal function, the concentrations of urea and creatinine were considered (Figure 6 and Figure 7). The TNBS group (66.6 ± 1.4 mg/dL) revealed a significant difference in urea concentration compared to the sham group (44.7 ± 3.8 mg/dL, p < 0.01). The TNBS + CLE100 (43.0 ± 1.8 mg/dL), TNBS + CLE500 (46.5 ± 1.6 mg/dL), and ethanol (40.7 ± 0.7 mg/dL) groups did not exhibit different urea levels in comparison with the sham group. However, CLE treatment groups demonstrated a significant reduction in urea levels compared to the TNBS group (p < 0.0001). Concerning creatinine levels, the TNBS group presented results considerably higher in comparison to the sham group (0.28 ± 0.01 vs. 0.2 ± 0.02 mg/dL, p < 0.0001). CLE treatment (TNBS + CLE100 group with 0.21 ± 0.01 and TNBS + CLE500 group with 0.20 ± 0.01 mg/dL) promoted a decrease in creatinine levels compared to the disease control group (p < 0.001). Creatinine concentrations obtained with the CLE treatment were similar to the resting control groups.
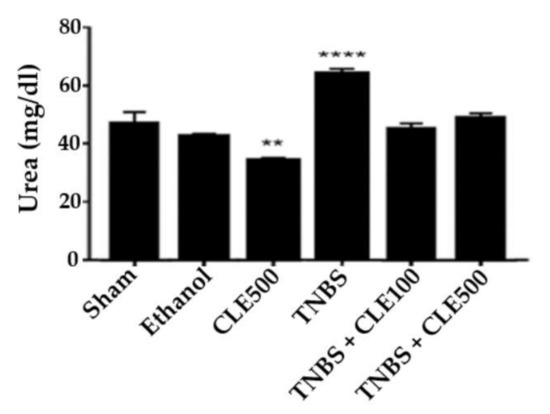
Figure 6.
CLE influence on urea levels in IBD context. Legend: One-way ANOVA and Tuckey’s post hoc test; ** p < 0.01, **** p < 0.0001, in comparison to the sham group. Abbreviations: CLE, C. cardunculus L. extract; TNBS, 2,4,6-trinitrobenzene sulfonic acid.
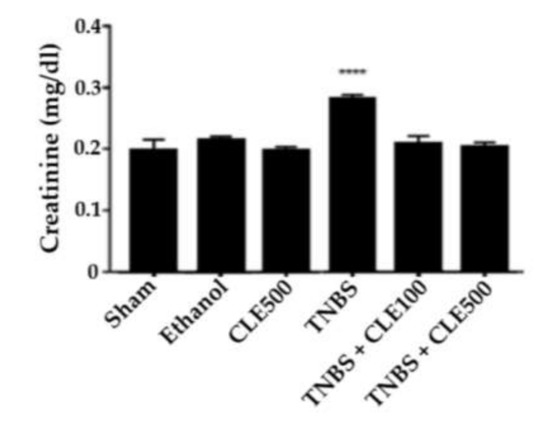
Figure 7.
CLE influence on creatinine levels in IBD context. Legend: One-way ANOVA and Tuckey’s post hoc test; **** p < 0.0001, in comparison to with the sham group. Abbreviations: CLE, C. cardunculus L. extract; TNBS, 2,4,6-trinitrobenzene sulfonic acid.
Regarding hepatic function, the TNBS group showed a significantly higher concentration of ALT compared to the sham group (32.0 ± 1.6 vs. 17.5 ± 0.8 U/L, p < 0.0001). The mice treated with CLE 100 mg/kg and 500 mg/kg daily kept high ALT levels with 29.2 ± 1.6 and 31.7 ± 1.5 U/L, respectively (p < 0.001 and p < 0.0001, compared to the sham group). Treatment with both doses of CLE did not promote a reduction in ALT levels compared with the TNBS group. The CLE500 (34.8 ± 1.4 UI/L) and ethanol (32.7 ± 2.1 UI/L) groups also presented considerably higher concentrations of ALT compared to the sham group (p < 0.0001) (Figure 8).
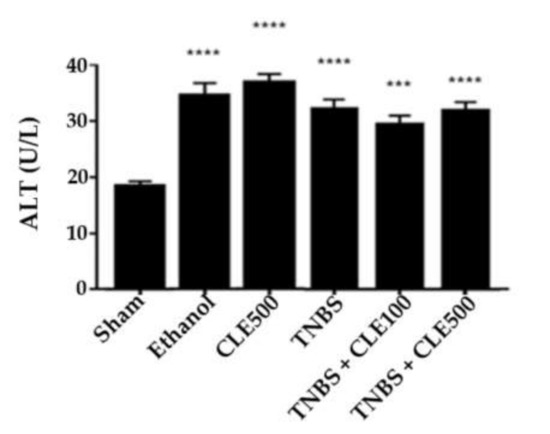
Figure 8.
CLE influence on ALT levels in IBD context. Legend: One-way ANOVA and Tuckey’s post hoc test; ***p < 0.001, ****p < 0.0001, in comparison to the sham group. Abbreviations: ALT, alanine aminotransferase; CLE, C. cardunculus L. extract; TNBS, 2,4,6-trinitrobenzene sulfonic acid.
4. Discussion
The plant C. cardunculus L. is generally used in food, and it is also known to serve medicinal purposes throughout the Mediterranean basin. It presents several health benefits derived mostly from its high concentration of antioxidant components such as inulin and polyphenols such as caffeic acid derivates, luteolin, and cymaroside [1,9,31,32,33]. This plant has been shown in several studies to have the capability of being hepatoprotective, antioxidative, anticarcinogenic, antibacterial, anti-HIV, bile-expelling, urinative activities, an inhibitor of cholesterol biosynthesis, and low-density lipoprotein (LDL) oxidation [7,9,31,34,35].
During the experimental study, colitic mice revealed an alteration of intestinal motility that suggests a correct induction of experimental colitis. However, the CLE treatment was able to attenuate diarrhea and the moderate edema present in the anus in comparison to mice that were not treated. Regarding changes in body weight, CLE treatment did not promote recovery of weight loss. These results were not expected since C. cardunculus L. presents phenolic compounds in its constitution, and there are several studies that report the benefits of these compounds in weight loss [36]. In this case, the colon lesions may be so severe that the administered doses of C. cardunculus L. were not able to recover the body weight reduction seen.
Colon length, which is a parameter for tissue integrity evaluation, showed differences between groups, verifying a shortening of the colon in colitic mice, which is in agreement with other studies [24]. After CLE treatment, a statistically significant increase in colon length due to the phenolic content of this plant was verified, which several studies had reported as protection of the polyphenols against shortening of the colon [36]. However, the positive effect of CLE on the integrity of colon tissue is only achieved with higher doses, such as 500 mg/kg/day.
Bloody stools are characteristic of IBD; however, they might be absent in mild diseases [37]. Therefore, to evaluate whether C. cardunculus L. has an influence on the amelioration of the bloody stools, the fecal hemoglobin was measured and analyzed. In fact, colitic mice demonstrated an elevated concentration of fecal hemoglobin; however, the CLE treatment showed the ability to significantly attenuate it. Additionally, it was possible to confirm a dose-dependent effect. Thuds, the daily administration of C. cardunculus L. can attenuate intestinal bleeding in a patient with IBD.
Regarding ALP, colitic mice showed higher concentrations of ALP in comparison to healthy mice. ALP is expressed in the intestinal tissue, more specifically, on the enterocytes, and it has a protective role of the mucosa, so in the case of colon lesions, an increase of this marker is expected [24,38,39]. ALP levels were significantly reduced by CLE treatment, suggesting that C. cardunculus L. has an important role in promoting enterocyte integrity. The usage of both doses of CLE (TNBS + CLE100 and TNBS + CLE500 groups) promoted ALP values similar to the control groups.
TNF-α is a pro-inflammatory cytokine, which is produced during the innate immune response of IBD. Colitic mice presented an elevated concentration of this cytokine, consistent with an acute model of inflammation [24,40,41,42]. After daily treatment, both CLE doses exhibited a significant reduction in TNF-α. This effect might be due to the inhibition of NF-κB activation promoted by CLE since TNF-α expression is induced by this transcription factor [11]. This finding suggests that C. cardunculus L. reduces colon inflammation, and for this reason, it can be a complementary therapeutic approach to the management of IBD.
An evaluation of renal and hepatic functions is crucial in this animal model because IBD leads to extra-intestinal manifestations, as previously mentioned, and also, the tested therapeutic approaches can affect the renal and hepatic functions [38]. Indeed, higher levels of urea, creatinine, and ALT were identified in the colitic mice. Despite the CLE treatment having no effect on the amelioration of the hepatic function, the renal biomarkers urea and creatinine decreased to similar levels in comparison to healthy mice. These findings suggest that C. cardunculus L. may induce renoprotection, but it is not able to revert the alteration of the hepatic function promoted by this aggressive chemical, TNBS.
Although the administration of CLE had demonstrated a positive influence in an IBD context, there are some limitations to be aware of. Firstly, this plant is known to be administered orally through alimentation or supplementation, and in this experiment, it was injected IP, which can produce different results by skipping, for example, digestive reactions. However, the main objective of this study was to make a proof of content, verifying whether CLE had an anti-inflammatory effect on IBD. Additionally, it was not possible to evaluate the efficacy and safety of CLE in a longer spectrum of time, which would be interesting since IBD is a chronic disease. Using this protocol, it was only possible to evaluate this extract in the acute phase characteristic of this disease. Lastly, the utilization of CLE would be as a complementary supplement of allopathic therapy, which can be prepared by infusion of cardoon leaves. It would be interesting to evaluate, in future studies, the effect of this extract in an IBD context through oral administration.
5. Conclusions
Based on the findings of this study, C. cardunculus L. presents an anti-inflammatory effect with an apparent absence of significant side effects, so this plant achieves an interesting role in future studies in the context of inflammation-related diseases, such as IBD. In fact, C. cardunculus L. may have a beneficial influence on the acute inflammatory response correspondent to an acute crisis of colitis with CD characteristics, attenuating several manifestations such as colon inflammation, intestinal bleeding, disintegration of enterocytes, and renal dysfunction. The daily IP administration of CLE demonstrated that C. cardunculus L. can become an interesting tool as a complementary therapeutic approach to the conventional medicines used in IBD. It would be of interest to evaluate the effect of this aqueous extract by oral gavage to simulate the administration in humans.
Author Contributions
V.M. and I.S. carried out the animal experimentation and wrote the manuscript with support from J.E. and P.B. E.T.-L. and R.P. conceived the original idea and supervised the project. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Directive 2010/63/EU, and approved by the Ethics Committee of Faculty of Pharmacy, University of Lisbon (protocol code 35/2014 at 5/12/2014).
Acknowledgments
H&TRC authors gratefully acknowledge the FCT/MCTES national support through the UIDB/05608/2020 and UIDP/05608/2020.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Falleh, H.; Ksouri, R.; Chaieb, K.; Karray-Bouraoui, N.; Trabelsi, N.; Boulaaba, M.; Abdelly, C. Phenolic composition of Cynara cardunculus L. organs, and their biological activities. C. R. Biol. 2008, 331, 372–379. [Google Scholar] [CrossRef]
- Sonnante, G.; Pignone, D.; Hammer, K. Domestication of artichoke and cardoon: From roman times to the genomic age. Ann. Bot. 2007, 100, 1095–1100. [Google Scholar] [CrossRef]
- Pandino, G.; Courts, F.L.; Lombardo, S.; Mauromicale, G.; Williamson, G. Caffeoylquinic acids and flavonoids in the immature inflorescence of globe artichoke, wild cardoon, and cultivated cardoon. J. Agric. Food Chem. 2010, 58, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- Rottenberg, A.; Zohary, D. The wild ancestry of the cultivated artichoke. Genet. Resour. Crop. Evol. 1996, 43, 53–58. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G.; Graziani, G.; Ritieni, A.; Cardarelli, M.; De Pascale, S. Phenolic composition, antioxidant activity and mineral profile in two seed-propagated artichoke cultivars as affected by microbial inoculants and planting time. Food Chem. 2017, 234, 10–19. [Google Scholar] [CrossRef]
- D’Antuono, I.; Garbetta, A.; Linsalata, V.; Minervini, F.; Cardinali, A. Polyphenols from artichoke heads (Cynara cardunculus (L.) subsp. scolymus Hayek): In vitro bio-accessibility, intestinal uptake and bioavailability. Food Funct. 2015, 6, 1268–1277. [Google Scholar] [CrossRef]
- Lattanzio, V.; Kroon, P.A.; Linsalata, V.; Cardinali, A. Globe artichoke: A functional food and source of nutraceutical ingredients. J. Funct. Foods 2009, 1, 131–144. [Google Scholar] [CrossRef]
- Wahba, H.E.; Sarhan, A.Z.; Salama, A.B.; Sharaf-Eldin, M.A.; Gad, H.M. Growth response and active constituents of Cynara cardunculus plants to the number of leaves harvests. Eur. J. Agron. 2016, 73, 118–123. [Google Scholar] [CrossRef]
- Ben Salem, M.; Affes, H.; Ksouda, K.; Dhouibi, R.; Sahnoun, Z.; Hammami, S.; Zeghal, K.M. Pharmacological studies of artichoke leaf extract and their health benefits. Plant. Foods Hum. Nutr. 2015, 70, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Kukić, J.; Popović, V.; Petrović, S.; Mucaji, P.; Ćirić, A.; Stojković, D.; Soković, M. Antioxidant and antimicrobial activity of Cynara cardunculus extracts. Food Chem. 2008, 107, 861–868. [Google Scholar] [CrossRef]
- Tanaka, Y.T.; Tanaka, Y.; Kojima, H.; Hamada, T.; Masutani, T.; Tsuboi, M.; Akao, Y. Cynaropicrin from Cynara scolymus L. suppresses photoaging of skin by inhibiting the transcription activity of nuclear factor-kappa B. Biorgan. Med. Chem. Lett. 2013, 23, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Miláčková, I.; Kapustová, K.; Mučaji, P.; Hošek, J. Artichoke Leaf Extract Inhibits AKR1B1 and Reduces NF-κB Activity in Human Leukemic Cells. Phytother. Res. 2017, 31, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Eljounaidi, K.; Cankar, K.; Comino, C.; Moglia, A.; Hehn, A.; Bourgaud, F.; Bouwmeester, H.J.; Menin, B.; Lanteri, S.; Beekwilder, J. Cytochrome P450s from Cynara cardunculus L. CYP71AV9 and CYP71BL5, catalyze distinct hydroxylations in the sesquiterpene lactone biosynthetic pathway. Plant Sci. 2014, 223, 59. [Google Scholar] [CrossRef]
- Tighe-Neira, R.; Alberdi, M.; Arce-Johnson, P.; Romero-Romero, J.L.; Reyes-Díaz, M.; Inostroza-Blancheteau, C. Foods with Functional Properties and their Potential Uses in Human Health. In Superfood and Functional Food—An Overview of Their Processing and Utilization; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Yadav, V.; Varum, F.; Bravo, R.; Furrer, E.; Bojic, D.; Basit, A.W. Inflammatory bowel disease: Exploring gut pathophysiology for novel therapeutic targets. Transl. Res. 2016, 176, 38–68. [Google Scholar] [CrossRef]
- Pithadia, A.B.; Jain, S. Treatment of inflammatory bowel disease (IBD). Pharmacol. Rep. 2011, 63, 629–642. [Google Scholar] [CrossRef]
- Hanauer, S.B. Inflammatory bowel disease: Epidemiology, pathogenesis, and therapeutic opportunities. Inflamm. Bowel Dis. 2006, 12, S3–S9. [Google Scholar] [CrossRef] [PubMed]
- Cominelli, F.; Arseneau, K.O.; Rodriguez-Palacios, A.; Pizarro, T.T. Uncovering pathogenic mechanisms of Inflammatory Bowel Disease using mouse models of Crohn’s Disease–like ileitis: What is the right model? Cell. Mol. Gastroenterol. Hepatol. 2017, 4, 19–32. [Google Scholar] [CrossRef]
- de Lange, K.M.; Barrett, J.C. Understanding inflammatory bowel disease via immunogenetics. J. Autoimmun. 2015, 64, 91–100. [Google Scholar] [CrossRef]
- Kaplan, G.G.; Ng, S.C. Understanding and preventing the global increase of Inflammatory Bowel Disease. Gastroenterology 2017, 152, 313–321. [Google Scholar] [CrossRef]
- Alatab, S.; Sepanlou, S.G.; Ikuta, K.; Vahedi, H.; Bisignano, C.; Safiri, S.; Sadeghi, A.; Nixon, M.R.; Abdoli, A.; Abolhassani, H.; et al. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study Lancet Gastroenterol Hepatol. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30. [Google Scholar] [CrossRef]
- Magro, F.; Langner, C.; Driessen, A.; Ensari, A.; Geboes, K.; Mantzaris, G.; Villanacci, V.; Becheanu, G.; Nunes, P.B.; Cathomas, G.; et al. European consensus on the histopathology of inflammatory bowel disease. J. Crohn’s Colitis 2013, 7, 827–851. [Google Scholar] [CrossRef]
- Conrad, K.; Roggenbuck, D.; Laass, M.W. Diagnosis and classification of ulcerative colitis. Autoimmun. Rev. 2014, 13, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Mateus, V.; Rocha, J.; Alves, P.; Mota-Filipe, H.; Sepodes, B.; Pinto, R.M.A. Anti-Inflammatory effect of erythropoietin in the TNBS-induced colitis. Basic Clin. Pharmacol. Toxicol. 2017, 120, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Randhawa, P.K.; Singh, K.; Singh, N.; Jaggi, A.S. A review on chemical-induced Inflammatory Bowel Disease models in rodents. Korean J. Physiol. Pharmacol. 2014, 18, 279. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, E.; Nguyen, D.; Low, D. Animal models of ulcerative colitis and their application in drug research. Drug Des. Devel. Ther. 2013, 7, 1341. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, B.; Moraes, L.; Magnusson, M.K.; Öhman, L. Immunopathogenesis of inflammatory bowel disease and mechanisms of biological therapies. Scand. J. Gastroenterol. 2018, 53, 379–389. [Google Scholar] [CrossRef]
- Triantafillidis, J. Current and emerging drugs for the treatment of inflammatory bowel disease. Drug Des. Devel. Ther. 2011, 5, 185. [Google Scholar] [CrossRef]
- Guo, B.; Bian, Z.; Qiu, H.; Wang, Y.-T.; Wang, Y. Biological and clinical implications of herbal medicine and natural products for the treatment of inflammatory bowel disease. Ann. N. Y. Acad. Sci. 2017, 1401, 37–48. [Google Scholar] [CrossRef]
- Do Nascimento, R.B.; Machado, A.P.F.; Galvez, J.; Cazarin, C.B.B.; Junior, M.R.M. Ulcerative colitis: Gut microbiota, immunopathogenesis and application of natural products in animal models. Life Sci. 2020, 258, 118–129. [Google Scholar] [CrossRef]
- Ceccarelli, N.; Curadi, N.M.; Picciarelli, P.; Martelloni, L.; Sbrana, C.; Giovannetti, M. Globe artichoke as a functional food. Med. J. Nutr. Metab. 2010, 3, 197–201. [Google Scholar] [CrossRef]
- Barracosa, P.; Barracosa, M.; Pires, E. Cardoon as a sustainable crop for biomass and bioactive compounds production. Chem. Biodivers. 2019, 16, e1900498. [Google Scholar] [CrossRef] [PubMed]
- Ramos, P.A.B.; Santos, S.A.O.; Guerra, Â.R.; Guerreiro, O.; Freire, C.S.R.; Rocha, S.M.; Duarte, M.F.; Silvestre, A.J.D. Phenolic composition and antioxidant activity of different morphological parts of Cynara cardunculus L. var. altilis (DC). Ind. Crop. Prod. 2014, 61, 460–471. [Google Scholar] [CrossRef]
- Sharaf EL-Deen, S.A.; Brakat, R.M.; Mohamed, A.S.E.D. Artichoke leaf extract protects liver of Schistosoma mansoni infected mice through modulation of hepatic stellate cells recruitment. Exp. Parasitol. 2017, 178, 51–59. [Google Scholar] [CrossRef]
- Kollia, E.; Markaki, P.; Zoumpoulakis, P.; Proestos, C. Antioxidant activity of Cynara scolymus L. and Cynara cardunculus L. extracts obtained by different extraction techniques. Nat. Prod. Res. 2017, 31, 1163–1167. [Google Scholar] [CrossRef]
- Martin, D.A.; Bolling, B.W. A review of the efficacy of dietary polyphenols in experimental models of inflammatory bowel diseases. Food Funct. 2015, 6, 1773–1786. [Google Scholar] [CrossRef] [PubMed]
- Kornbluth, A.; Sachar, D.B. Ulcerative colitis practice guidelines in adults: American College of Gastroenterology, Practice Parameters Committee. Am. J. Gastroenterol. 2010, 105, 501–523. [Google Scholar] [CrossRef]
- Mateus, V.; Rocha, J.; Alves, P.; Mota-Filipe, H.; Sepodes, B.; Pinto, R. Thiadiazolidinone-8 ameliorates inflammation associated with experimental colitis in mice. Pharmacology 2018, 101, 35–42. [Google Scholar] [CrossRef]
- Seibel, J.; Molzberger, A.F.; Hertrampf, T.; Laudenbach-Leschowski, U.; Diel, P. Oral treatment with genistein reduces the expression of molecular and biochemical markers of inflammation in a rat model of chronic TNBS-induced colitis. Eur. J. Nutr. 2009, 48, 213–220. [Google Scholar] [CrossRef]
- Kumar, V.S.; Rajmane, A.R.; Adil, M.; Kandhare, A.D.; Ghosh, P.; Bodhankar, S.L. Naringin ameliorates acetic acid induced colitis through modulation of endogenous oxido-nitrosative balance and DNA damage in rats. J. Biomed. Res. 2014, 28, 132–145. [Google Scholar] [CrossRef]
- Wirtz, S.; Neufert, C.; Weigmann, B.; Neurath, M.F. Chemically induced mouse models of intestinal inflammation. Nat. Protoc. 2007, 2, 541–546. [Google Scholar] [CrossRef]
- Gajendran, M.; Loganathan, P.; Catinella, A.P.; Hashash, J.G. A comprehensive review and update on Crohn’s disease. Dis. A Month 2018, 64, 20–57. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).