Application of Artificial Neural Network to Somatotype Determination
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.1.1. Anthropometric Measurement
2.1.2. Measurement Techniques
2.1.3. Body Composition Analysis
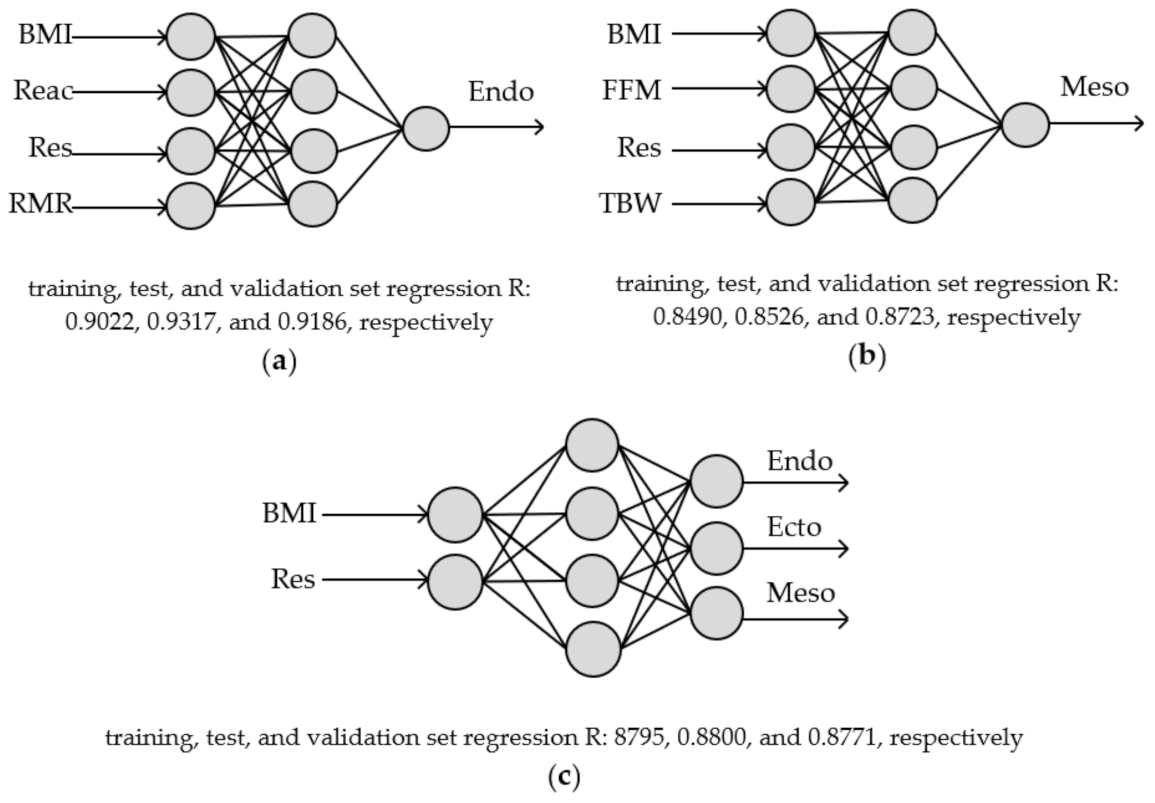
2.2. Somatotypes Modelling—Artificial Neural Network (ANN)
2.3. Sensitivity Analysis of the ANN
2.4. Statistical Analysis
BIA and Heath–Carter Method Compliance
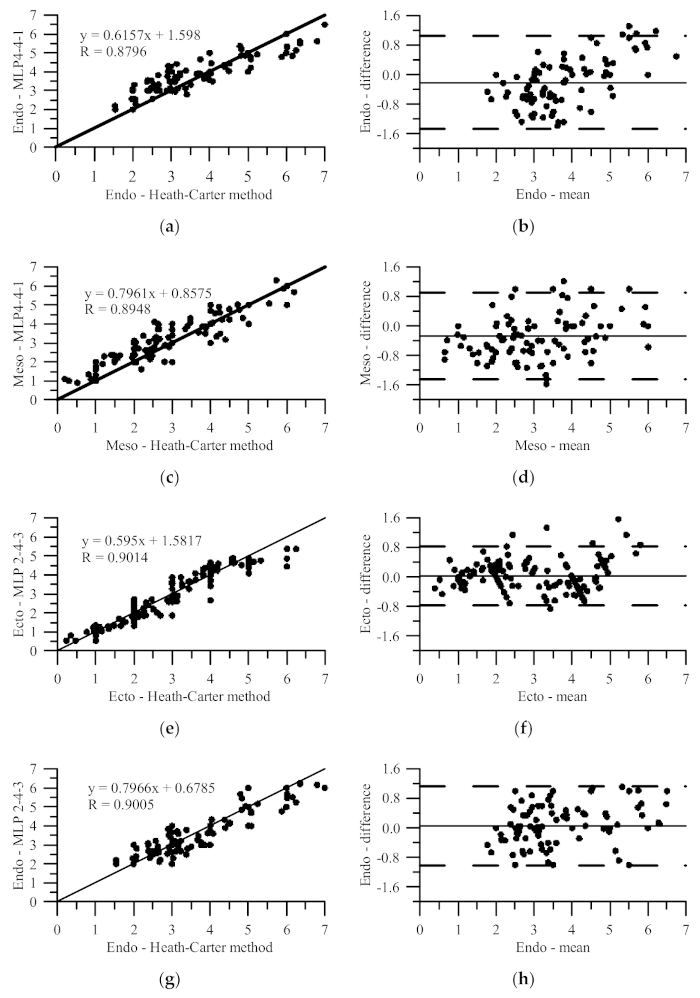
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

| Formula [References] | Age | R | RMSE | χ2 |
|---|---|---|---|---|
| [22] | 7–18 | 0.671 | 1.589 | 3.869 |
| [53,54] | 5–17 | 0.644 | 1.860 | 5.301 |
| [23] | 16–40 | 0.790 | 2.029 | 6.304 |
| [17] | 16–61 | 0.789 | 2.201 | 7.423 |
| [54] | 7–17 | 0.360 | 1.361 | 2.839 |
| [22] | 7–18 | 0.733 | 1.836 | 5.162 |
| [53,54] | 5–17 | 0.635 | 2.354 | 8.422 |
| [23] | 16–40 | 0.888 | 1.079 | 1.785 |
| [17] | 16–61 | 0.822 | 1.421 | 3.095 |
| [54] | 7–17 | 0.594 | 2.349 | 8.455 |
References
- Ryan-Stewart, H.; Faulkner, J.; Jobson, S. The influence of somatotype on anaerobic performance. PLoS ONE 2018, 13, e0197761. [Google Scholar] [CrossRef]
- Malina, R.M.; Bouchard, C.; Bar-Or, O. Growth, Maturation, and Physical Activity, 2nd ed.; Human Kinetics: Champaign, IL, USA, 2004; ISBN 978-0-88011-882-8. [Google Scholar]
- Somatotyping, C.L. Anthropometrica: A Textbook of Body Measurement for Sports and Health Courses; Norton, K., Olds, T., Eds.; University of New South Wales Press: Sydney, Australia, 1996; pp. 147–170. ISBN 978-0-86840-223-9. [Google Scholar]
- Yang, L.-T.; Wang, N.; Li, Z.-X.; Liu, C.; He, X.; Zhang, J.-F.; Han, H.; Wen, Y.-F.; Qian, Y.-H.; Xi, H.-J. Study on the adult physique with the Heath-Carter anthropometric somatotype in the Han of Xi’an, China. Anat. Sci. Int. 2016, 91, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Hermassi, S.; Sellami, M.; Fieseler, G.; Bouhafs, E.G.; Hayes, L.D.; Schwesig, R. Differences in body fat, body mass index, and physical performance of specific field tests in 10-to-12-year-old school-aged team handball players. Appl. Sci. 2020, 10, 9022. [Google Scholar] [CrossRef]
- Gutnik, B.; Zuoza, A.; Zuozienė, I.; Alekrinskis, A.; Nash, D.; Scherbina, S. Body physique and dominant somatotype in elite and low-profile athletes with different specializations. Medicina 2015, 51, 247–252. [Google Scholar] [CrossRef]
- Marta, C.C.; Marinho, D.A.; Barbosa, T.M.; Carneiro, A.L.; Izquierdo, M.; Marques, M.C. Effects of body fat and dominant somatotype on explosive strength and aerobic capacity trainability in prepubescent children. J. Strength Cond. Res. 2013, 27, 3233–3244. [Google Scholar] [CrossRef]
- Noh, J.-W.; Kim, J.-H.; Kim, M.-Y.; Lee, J.-U.; Lee, L.-K.; Park, B.-S.; Yang, S.-M.; Jeon, H.-J.; Lee, W.-D.; Kwak, T.-Y.; et al. Somatotype analysis of elite boxing athletes compared with nonathletes for sports physiotherapy. J. Phys. Ther. Sci. 2014, 26, 1231–1235. [Google Scholar] [CrossRef]
- Tóth, T.; Michalíková, M.; Bednarčíková, L.; Živčák, J.; Kneppo, P. Somatotypes in sport. Acta Mech. Autom. 2014, 8, 27–32. [Google Scholar] [CrossRef]
- Koleva, M.; Nacheva, A.; Boev, M. Somatotype, nutrition, and obesity. Rev. Environ. Health 2000, 15, 389–398. [Google Scholar] [CrossRef]
- Koleva, M.; Nacheva, A.; Boev, M. Somatotype and disease prevalence in adults. Rev. Environ. Health 2002, 17, 65–84. [Google Scholar] [CrossRef]
- Baltadjiev, A.G. Somatotype characteristics of female patients with type 2 diabetes mellitus. Folia Med. 2013, 55, 64–69. [Google Scholar] [CrossRef]
- Almeida, A.H.S.; Santos, S.A.G.; Castro, P.J.P.; Rizzo, J.A.; Batista, G.R. Somatotype analysis of physically active individuals. J. Sports Med. Phys. Fit. 2013, 53, 268–273. [Google Scholar]
- Carter, J.E.L. The Heath-Carter Anthropometric Somatotype—Instruction Manual; Department of Exercise and Nutritional Sciences San Diego State University: San Diego, CA, USA, 2002. [Google Scholar]
- Fornetti, W.C.; Pivarnik, J.M.; Foley, J.M.; Fiechtner, J.J. Reliability and validity of body composition measures in female athletes. J. Appl. Physiol. 1999, 87, 1114–1122. [Google Scholar] [CrossRef] [PubMed]
- Pastuszak, A.; Gajewski, J.; Buśko, K. The Impact of skinfolds measurement on somatotype determination in Heath-Carter method. PLoS ONE 2019, 14, e0222100. [Google Scholar] [CrossRef] [PubMed]
- Rudnev, S.G.; Negasheva, M.A.; Godina, E.Z. Assessment of the Heath-Carter somatotype in adults using bioelectrical impedance analysis. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2019; Volume 1272, p. 012001. [Google Scholar] [CrossRef]
- Khalil, S.; Mohktar, M.; Ibrahim, F. The theory and fundamentals of bioimpedance analysis in clinical status monitoring and diagnosis of diseases. Sensors 2014, 14, 10895–10928. [Google Scholar] [CrossRef] [PubMed]
- Roubenoff, R.; Dallal, G.E.; Wilson, P.W. Predicting body fatness: The body mass index vs estimation by bioelectrical impedance. Am. J. Public Health 1995, 85, 726–728. [Google Scholar] [CrossRef] [PubMed]
- Heitmann, B.L. Impedance: A valid method in assessment of body composition? Eur. J. Clin. Nutr. 1994, 48, 228–240. [Google Scholar]
- Böhm, A.; Heitmann, B.L. The use of bioelectrical impedance analysis for body composition in epidemiological studies. Eur. J. Clin. Nutr. 2013, 67, S79–S85. [Google Scholar] [CrossRef]
- Kolesnikov, V.A.; Rudnev, S.G.; Nikolaev, D.V.; Anisimova, A.V.; Godina, E.Z. On a new protocol of the Heath-Carter somatotype assessment using software for body composition bioimpedance analyzer. Vestn. Mosk. Univ. Ser. 23 Antropol. 2016, 4, 4–13. [Google Scholar]
- Sindeyeva, L.V.; Rudnev, S.G. Characteristic of age- and sex-related variability of the Heath-Carter somatotype in adults and possibility of its bioimpedance assessment (as exemplified by Russian population of Eastern Siberia). Morfologiya 2017, 151, 77–87. [Google Scholar]
- Attanasio, S.; Forte, S.M.; Restante, G.; Gabelloni, M.; Guglielmi, G.; Neri, E. Artificial intelligence, radiomics and other horizons in body composition assessment. Quant. Imaging Med. Surg. 2020, 10, 1650–1660. [Google Scholar] [CrossRef] [PubMed]
- Xhumari, E.; Manika, P. Application of Artificial Neural Networks in Medicine, CEUR Workshop Proccedings, Tirana, Albania, 18–19 November 2016; Kika, A., Hoxha, E., Eds.; CEUR: Tirana, Albania, 2016; Volume 1746, pp. 155–157.
- Shahid, N.; Rappon, T.; Berta, W. Applications of artificial neural networks in health care organizational decision-making: A scoping review. PLoS ONE 2019, 14, e0212356. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, F.; Faisal, T.; Mohamad Salim, M.I.; Taib, M.N. Non-invasive diagnosis of risk in dengue patients using bioelectrical impedance analysis and artificial neural network. Med. Biol. Eng. Comput. 2010, 48, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Faisal, T.; Ibrahim, F.; Taib, M.N. A noninvasive intelligent approach for predicting the risk in dengue patients. Expert Syst. Appl. 2010, 37, 2175–2181. [Google Scholar] [CrossRef]
- Mohktar, M.S.; Ibrahim, F.; Ismail, N.A. Non-invasive approach to predict the cholesterol level in blood using bioimpedance and neural network techniques. Biomed. Eng. Appl. Basis Commun. 2013, 25, 1350046. [Google Scholar] [CrossRef]
- Linder, R.; Mohamed, E.I.; De Lorenzo, A.; Pöppl, S.J. The capabilities of artificial neural networks in body composition research. Acta Diabetol. 2003, 40, s9–s14. [Google Scholar] [CrossRef]
- Chiu, J.-S.; Chen, C.-A.; Lee, C.-H.; Li, Y.-C.; Lin, Y.-F.; Wang, Y.-F.; Yu, F.-C. Neural network technology to predict intracellular water volume: Neural network technology. Int. J. Clin. Pract. 2006, 60, 1231–1238. [Google Scholar] [CrossRef]
- Chiu, J.-S.; Chong, C.-F.; Lin, Y.-F.; Wu, C.-C.; Wang, Y.-F.; Li, Y.-C. Applying an artificial neural network to predict total body water in hemodialysis patients. Am. J. Nephrol. 2005, 25, 507–513. [Google Scholar] [CrossRef]
- Hsieh, K.-C.; Chen, Y.-J.; Lu, H.-K.; Lee, L.-C.; Huang, Y.-C.; Chen, Y.-Y. The novel application of artificial neural network on Bioelectrical Impedance Analysis to assess the body composition in elderly. Nutr. J. 2013, 12, 21. [Google Scholar] [CrossRef]
- Liu, T.-P.; Kao, M.-F.; Jang, T.-R.; Wang, C.-W.; Chuang, C.-L.; Chen, J.; Chen, Y.-Y.; Hsieh, K.-C. New application of bioelectrical impedance analysis by the back propagation artificial neural network mathematically predictive model of tissue composition in the lower limbs of elderly people. Int. J. Gerontol. 2012, 6, 20–26. [Google Scholar] [CrossRef][Green Version]
- Liu, T.-P.; Kao, M.-F.; Jang, T.-R.; Wang, C.-W.; Chuang, C.-L.; Chen, J.; Chen, Y.-Y.; Hsieh, K.-C. Corrigendum to “New application of bioelectrical impedance analysis by the back propagation artificial neural network mathematically predictive model of tissue composition in the lower limbs of elderly people” [Int. J. Gerontol. 2012, 6, 20–26]. Int. J. Gerontol. 2015, 9, 133. [Google Scholar] [CrossRef]
- Chao, Y.-S.; Wu, H.-C.; Wu, C.-J.; Chen, W.-C. Stages of biological development across age: An analysis of Canadian health measure survey 2007–2011. Front. Public Health 2018, 5, 355. [Google Scholar] [CrossRef] [PubMed]
- Duquet, W.; Carter, J.E.L.; Eston, R.; Reilly, T. Somatotyping. In Kinanthropometry and Exercise Physiology Laboratory Manual: Tests, Procedures and Data, Volume One: Anthropometry; Routledge: Abingdon, UK, 2009; pp. 54–72. [Google Scholar]
- Koo, T.K.; Li, M.Y. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.E.L.; Heath, B.H. Somatotyping-Development and Applications; Cambridge University Press: New York, NY, USA, 1990; ISBN 978-0-521-35951-1. [Google Scholar]
- Kwon, B.; Lee, S. Ensemble learning for skeleton-based body mass index classification. Appl. Sci. 2020, 10, 7812. [Google Scholar] [CrossRef]
- WHO. The Challenge of Obesity in the WHO European Region and the Strategies for Response: Summary; Branca, F., Nikogosian, H., Lobstein, T., World Health Organization, Eds.; World Health Organization, Regional Office for Europe: Copenhagen, Denmark, 2007; ISBN 978-92-890-1388-8. [Google Scholar]
- Winiczenko, R.; Górnicki, K.; Kaleta, A.; Janaszek-Mańkowska, M. Optimisation of ANN Topology for predicting the rehydrated apple cubes colour change using RSM and GA. Neural Comput. Appl. 2018, 30, 1795–1809. [Google Scholar] [CrossRef] [PubMed]
- Górnicki, K.; Kaleta, A.; Trajer, J. Modelling of dried apple rehydration indices using ANN. Int. Agrophys. 2019, 33, 285–296. [Google Scholar] [CrossRef]
- Nadi, F.; Górnicki, K.; Winiczenko, R. A novel optimization algorithm for Echium amoenum petals drying. Appl. Sci. 2020, 10, 8387. [Google Scholar] [CrossRef]
- Górnicki, K.; Winiczenko, R.; Kaleta, A.; Choińska, A. Evaluation of models for the dew point temperature determination. Tech. Sci. 2017, 20, 241–257. [Google Scholar] [CrossRef]
- Kaleta, A.; Górnicki, K.; Winiczenko, R.; Chojnacka, A. Evaluation of drying models of apple (var. Ligol) dried in a fluidized bed dryer. Energy Convers. Manag. 2013, 67, 179–185. [Google Scholar] [CrossRef]
- Bland, M.J.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 327, 307–310. [Google Scholar] [CrossRef]
- Krzykała, M.; Karpowicz, M.; Strzelczyk, R.; Pluta, B.; Podciechowska, K.; Karpowicz, K. Morphological asymmetry, sex and dominant somatotype among Polish youth. PLoS ONE 2020, 15, e0238706. [Google Scholar] [CrossRef] [PubMed]
- Marangoz, I.; Var, S.M. The comparison of somatotype structures in students studying at different departments of physical education. J. Educ. Train. Stud. 2018, 6, 108. [Google Scholar] [CrossRef][Green Version]
- Bulbulian, R. The influence of somatotype on anthropometric prediction of body composition in young women. Med. Sci. Sports Exerc. 1984, 16, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Marra, M.; Cioffi, I.; Sammarco, R.; Santarpia, L.; Contaldo, F.; Scalfi, L.; Pasanisi, F. Are raw BIA variables useful for predicting resting energy expenditure in adults with obesity? Nutrients 2019, 11, 216. [Google Scholar] [CrossRef] [PubMed]
- Francisco, R.; Matias, C.N.; Santos, D.A.; Campa, F.; Minderico, C.S.; Rocha, P.; Heymsfield, S.B.; Lukaski, H.; Sardinha, L.B.; Silva, A.M. The predictive role of raw bioelectrical impedance parameters in water compartments and fluid distribution assessed by dilution techniques in athletes. Int. J. Environ. Res. Publ. Health 2020, 17, 759. [Google Scholar] [CrossRef]
- Rudnev, S.C.; Tseytlin, G.Y.; Vashura, A.Y.; Lukina, S.S.; Rumyantsev, A.G. Somatotype of children and adolescents with oncological diseases in remission and possibility of its bioimpedance assessment. Pediatria 2017, 96, 186–193. [Google Scholar] [CrossRef]
- Anisimova, A.V.; Godina, E.Z.; Nikolaev, D.V.; Rudnev, S.G. Evaluation of the Heath-Carter somatotype revisited: New bioimpedance equations for children and adolescents. In Proceedings of the II Latin American Conference on Bioimpedance, Proceedings of the IFMBE, Montevideo, Uruguay, 30 September–2 October 2015; Simini, F., Bertemes-Filho, P., Eds.; Springer: Singapore, 2016; Volume 54, pp. 80–83. [Google Scholar]
| Parameter | Unit | Statistical Parameters | ||
|---|---|---|---|---|
| Mean ± SD (Range) | Coefficient of Variation | Skewness Coefficient | ||
| Age | year | 22.9 ± 1.70 (19–29) | 0.07 | 0.45 |
| Anthropometric measurement | ||||
| Body weight | kg | 59.57 ± 7.80 (40–78) | 0.13 | 0.09 |
| Body height | cm | 166.97 ± 5.91 (151–179) | 0.04 | −0.59 |
| Triceps skinfold | mm | 13.09 ± 4.82 (6–28) | 0.37 | 0.96 |
| Subscapular skinfold | mm | 11.80 ± 4.77 (5–29) | 0.40 | 1.59 |
| Supraspinale skinfold | mm | 10.64 ± 4.62 (4.5–23) | 0.43 | 0.91 |
| Medial calf skinfold | mm | 11.87 ± 6.14 (1–35.5) | 0.52 | 1.11 |
| Biepicondylar breadth of the humerus | cm | 6.19 ± 0.39 (5.5–7) | 0.06 | 1.08 |
| Biepicondylar breadth of the femur | cm | 7.64 ± 0.99 (4–9) | 0.13 | −0.81 |
| Upper arm girth | cm | 26.39 ± 2.56 (20–33) | 0.10 | 0.10 |
| Calf girth | cm | 35.91 ± 2.67 (30–41) | 0.07 | 0.19 |
| Body composition (BIA) | ||||
| FFM | % | 74.12 ± 7.10 (57.5–91) | 0.10 | −0.36 |
| FM | % | 25.88 ± 7.10 (8.9–42.5) | 0.28 | 0.36 |
| TBW | % | 53.01 ± 5.17 (43–73) | 0.10 | 1.11 |
| Reac | Ω | 157.56 ± 43.93 (86–282) | 0.28 | 0.88 |
| Res | Ω | 620.64 ± 116.45 (194–881) | 0.19 | −1.47 |
| RMR | kcal | 1546.64 ± 61.35 (1373–1719) | 0.04 | −0.16 |
| Indices in used formulas | ||||
| BMI | kg/m2 | 21.32 ± 2.70 (16–28) | 0.13 | 0.45 |
| FMi = FM/H2 | kg/m2 | 9.33 ± 2.73 (3.5–16.0) | 0.30 | 0.47 |
| FFMi = FFM/H2 | kg/m2 | 16.77 ± 2.26 (14.17–25.0) | 0.14 | 1.72 |
| Somatotype and ANN Form | Statistics | ANN | Omitted Parameter | |||||
|---|---|---|---|---|---|---|---|---|
| BMI | FFM | Res | Reac | RMR | TBW | |||
| MLP 6-5-1 | ||||||||
| Endo | R | 0.9350 | 0.8271 | 0.9136 | 0.8335 | 0.7820 | 0.8672 | 0.9275 |
| RMSE | 0.4529 | 0.7144 | 0.5340 | 0.7077 | 0.8379 | 0.6558 | 0.5128 | |
| χ2 | 0.4764 | 1.1854 | 0.6622 | 1.1633 | 1.6305 | 0.9988 | 0.6107 | |
| Meso | R | 0.8909 | −0.1128 | 0.7981 | 0.7715 | 0.8572 | 0.8195 | 0.5187 |
| RMSE | 0.6063 | 1.5257 | 1.0626 | 0.8620 | 0.7013 | 0.7734 | 1.2268 | |
| χ2 | 0.8536 | 5.4063 | 2.6222 | 1.7259 | 1.1421 | 1.3894 | 3.4957 | |
| MLP 6-4-3 | ||||||||
| Endo, Ecto, and Meso | R | 0.9060 | 0.1554 | 0.8751 | 0.7742 | 0.8928 | 0.8795 | 0.8967 |
| RMSE | 0.5906 | 1.5158 | 0.7095 | 0.8997 | 0.6287 | 0.6628 | 0.6334 | |
| χ2 | 0.4257 | 2.8039 | 0.6143 | 0.9878 | 0.4824 | 0.5361 | 0.4895 | |
| Endo | R | 0.8860 | −0.3453 | 0.8705 | 0.4759 | 0.8607 | 0.8717 | 0.8796 |
| RMSE | 0.5886 | 1.9957 | 0.8216 | 1.2928 | 0.6483 | 0.6221 | 0.6379 | |
| χ2 | 0.7558 | 8.6900 | 1.4729 | 3.6465 | 0.9170 | 0.8443 | 0.8879 | |
| Ecto | R | 0.9463 | 0.8377 | 0.9404 | 0.9381 | 0.9454 | 0.9120 | 0.9447 |
| RMSE | 0.4809 | 1.0731 | 0.5577 | 0.5159 | 0.4896 | 0.6102 | 0.5106 | |
| χ2 | 0.5046 | 2.5123 | 0.6786 | 0.5807 | 0.5231 | 0.8124 | 0.5687 | |
| Meso | R | 0.8597 | 0.1987 | 0.8472 | 0.8535 | 0.8412 | 0.8290 | 0.8442 |
| RMSE | 0.6846 | 1.3261 | 0.72402 | 0.7006 | 0.7252 | 0.7473 | 0.7320 | |
| χ2 | 1.0227 | 3.8368 | 1.1436 | 1.0710 | 1.1474 | 1.2185 | 1.1690 | |
| MLP 4-4-1 | ||||||||
| Endo | R | 0.8687 | 0.4680 | - | 0.7178 | 0.7926 | 0.8366 | - |
| RMSE | 0.6562 | 1.1365 | - | 0.9502 | 0.8898 | 0.7361 | - | |
| χ2 | 0.6596 | 1.9787 | - | 1.3830 | 1.2128 | 0.8298 | - | |
| Meso | R | 0.8293 | 0.6050 | 0.5464 | 0.8066 | - | - | 0.7535 |
| RMSE | 0.7546 | 1.0929 | 1.1304 | 0.8250 | - | - | 1.1365 | |
| χ2 | 0.8723 | 1.8298 | 1.9574 | 1.0426 | - | - | 1.9787 | |
| MLP 2-4-3 | ||||||||
| Endo, Ecto, and Meso | R | 0.8796 | 0.2569 | - | 0.8314 | - | - | - |
| RMSE | 0.6703 | 1.3463 | - | 0.7745 | - | - | - | |
| χ2 | 0.5135 | 2.0715 | - | 0.6855 | - | - | - | |
| Endo | R | 0.8777 | 0.2924 | - | 0.7316 | - | - | - |
| RMSE | 0.6091 | 1.2550 | - | 0.8637 | - | - | - | |
| χ2 | 0.5937 | 2.5200 | - | 1.1936 | - | - | - | |
| Ecto | R | 0.9092 | 0.0436 | - | 0.9078 | - | - | - |
| RMSE | 0.6195 | 1.4715 | - | 0.6230 | - | - | - | |
| χ2 | 0.6140 | 3.4644 | - | 0.6211 | - | - | - | |
| Meso | R | 0.8165 | 0.2323 | - | 0.7918 | - | - | - |
| RMSE | 0.7702 | 1.3029 | - | 0.8157 | - | - | - | |
| χ2 | 0.9492 | 2.7160 | - | 1.0645 | - | - | - | |
| Somatotypes and ANN Form | No. | Weights and Biases | ||||||
|---|---|---|---|---|---|---|---|---|
| MLP 4-4-1 | i | D1i | D2i | D3i | D4i | D5i | Wi | Wb |
| Endo | 1 | −2.5196 | 3.3665 | −0.1069 | 0.2737 | −2.0654 | −0.0124 | 0.6713 |
| 2 | −1.6057 | 0.6506 | −1.8208 | 0.2305 | 1.2134 | −0.8628 | ||
| 3 | 0.7439 | −1.2159 | −0.8333 | 0.0953 | 2.2781 | −0.1618 | ||
| 4 | 2.2264 | 2.1972 | 1.2505 | 2.5316 | 3.9790 | −0.5781 | ||
| Meso | 1 | 1.8162 | 1.4232 | −1.8635 | −0.2445 | −0.7219 | 1.4688 | −0.2921 |
| 2 | 0.5244 | −2.2078 | −0.5086 | 1.9776 | 1.0502 | 1.3685 | ||
| 3 | −1.3144 | 0.0394 | 0.8123 | −0.6986 | −0.6232 | 1.0849 | ||
| 4 | −0.9115 | 0.1162 | −3.0057 | 0.6690 | −1.8619 | −1.1589 | ||
| MLP 2-4-3 | D1i | D2i | D3i | WEndo i | WEcto i | WMeso i | WbEndo | |
| Endo, Ecto, and Meso | 1 | 1.5266 | −0.0379 | 0.2213 | −1.1164 | −0.1439 | −0.0378 | −0.1789 |
| 2 | 2.0005 | 1.5189 | −0.7945 | 0.0018 | ||||
| 3 | 3.1287 | −5.8295 | −2.1418 | −0.1264 | ||||
| 4 | −2.0762 | 1.5257 | 0.8236 | −0.2852 | ||||
| Somatotype | Heath-Carter Method Mean ± SD | ANN MLP 4-4-1 Mean ± SD | ANN MLP 2-4-3 Mean ± SD | p-Value |
|---|---|---|---|---|
| Endo | 3.63 ± 1.28 a | 3.83 ± 0.87 a | 3.55 ± 1.12 a | ns |
| Meso | 2.86 ± 1.34 b | 3.19 ± 1.17 b | 2.94 ± 1.11 b | ns |
| Ecto | 2.89 ± 1.35 c | -1 | 2.90 ± 1.39 c | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drywień, M.; Górnicki, K.; Górnicka, M. Application of Artificial Neural Network to Somatotype Determination. Appl. Sci. 2021, 11, 1365. https://doi.org/10.3390/app11041365
Drywień M, Górnicki K, Górnicka M. Application of Artificial Neural Network to Somatotype Determination. Applied Sciences. 2021; 11(4):1365. https://doi.org/10.3390/app11041365
Chicago/Turabian StyleDrywień, Małgorzata, Krzysztof Górnicki, and Magdalena Górnicka. 2021. "Application of Artificial Neural Network to Somatotype Determination" Applied Sciences 11, no. 4: 1365. https://doi.org/10.3390/app11041365
APA StyleDrywień, M., Górnicki, K., & Górnicka, M. (2021). Application of Artificial Neural Network to Somatotype Determination. Applied Sciences, 11(4), 1365. https://doi.org/10.3390/app11041365








