Influence of Post-Flowering Climate Conditions on Anthocyanin Profile of Strawberry Cultivars Grown from North to South Europe
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Sites, Plant Material and Cultivation
2.2. Sample Preparation for Anthocyanin Analysis
2.3. HPLC Analysis of Anthocyanins
2.4. Electrospray Ionization (ESI)-MS Identification of Anthocyanins
2.5. Software Used for Statistical Analysis
2.6. Data Arrangement for PCA
3. Results and Discussion
3.1. Harvest Season and Environmental Characterization of the Growing Locations
3.2. Effect of Genotype on Total and Individual Anthocyanins
3.3. Anthocyanins are Affected by Location
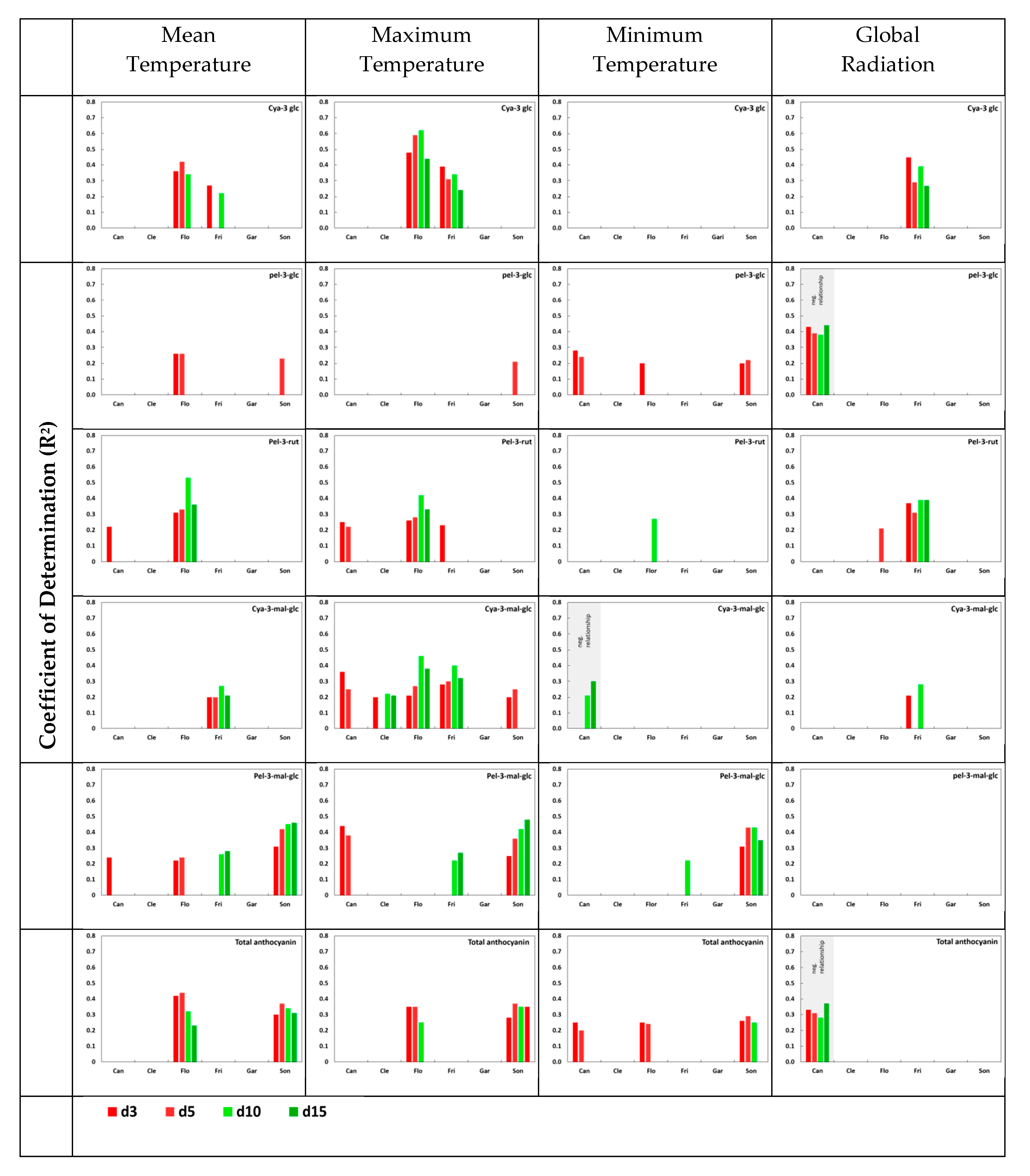
3.4. Impact of Temperature and Global Radiation on Cultivar-Specific Anthocyanin Profiles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nile, S.H.; Park, S.W. Edible berries: Bioactive components and their effect on human health. Nutrition 2014, 30, 134–144. [Google Scholar] [CrossRef]
- Giampieri, F.; Tulipani, S.; Alvarez-Suarez, J.M.; Quiles, J.L.; Mezzetti, B.; Battino, M. The strawberry: Composition, nutritional quality, and impact on human health. Nutrition 2012, 28, 9–19. [Google Scholar] [CrossRef]
- Giampieri, F.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Alvarez-Suarez, J.M.; Afrin, S.; Bompadre, S.; Quiles, J.L.; Mezzetti, B.; Battino, M. Strawberry as a health promoter: An evidence based review. Food Funct. 2015, 6, 1386–1398. [Google Scholar] [CrossRef]
- Afrin, S.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Reboredo-Rodriguez, P.; Mezzetti, B.; Varela-López, A.; Giampieri, F.; Battino, M. Promising health benefits of the strawberry: A focus on clinical studies. J. Agric. Food Chem. 2016, 64, 4435–4449. [Google Scholar] [CrossRef] [PubMed]
- Tena, N.; Martín, J.; Asuero, A.G. State of the art of anthocyanins: Antioxidant activity, sources, bioavailability, and therapeutic effect in human health. Antioxidants 2020, 9, 451. [Google Scholar] [CrossRef] [PubMed]
- Giampieri, F.; Alvarez-Suarez, J.M.; Mazzoni, L.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Gonzàlez-Paramàs, A.M.; Santos-Buelga, C.; Quiles, J.L.; Bompadre, S.; Mezzetti, B.; et al. An anthocyanin-rich strawberry extract protects against oxidative stress damage and improves mitochondrial functionality in human dermal fibroblasts exposed to an oxidizing agent. Food Funct. 2014, 5, 1939–1948. [Google Scholar] [CrossRef] [PubMed]
- Kuntz, S.; Rudloff, S.; Asseburg, H.; Borsch, C.M.; Fröhling, B.; Unger, F.; Dold, S.; Spengler, B.; Römpp, A.; Kunz, C. Uptake and bioavailability of anthocyanins and phenolic acids from grape/blueberry juice and smoothie in vitro and in vivo. Br. J. Nutr. 2015, 113, 1044–1055. [Google Scholar] [CrossRef] [PubMed]
- Treutter, D. Significance of flavonoids in plant resistance and enhancement of their biosynthesis. Plant. Biol. 2005, 7, 581–591. [Google Scholar] [CrossRef]
- Wang, S.Y.; Zheng, W.; Galletta, G.J. Cultural system affects fruit quality and antioxidant capacity in strawberries. J. Agric. Food Chem. 2002, 50, 6534–6542. [Google Scholar] [CrossRef]
- Tulipani, S.; Mezzetti, B.; Capocasa, F.; Bompadre, S.; Beekwilder, J.; De Vos, C.H.R.; Capanoglu, E.; Bovy, A.; Battino, M. Antioxidants, phenolic compounds, and nutritional quality of different strawberry genotypes. J. Agric. Food Chem. 2008, 56, 696–704. [Google Scholar] [CrossRef]
- Aaby, K.; Mazur, S.; Nes, A.; Skrede, G. Phenolic compounds in strawberry (Fragaria × ananassa Duch.) fruits: Composition in 27 cultivars and changes during ripening. Food Chem. 2012, 132, 86–97. [Google Scholar] [CrossRef]
- Ariza, M.; Martínez-Ferri, E.; Domínguez, P.; Medina, J.J.; Miranda, L.; Soria, C. Effects of harvest time on functional compounds and fruit antioxidant capacity in ten strawberry cultivars. J. Berry Res. 2015, 5, 71–80. [Google Scholar] [CrossRef]
- Cocco, C.; Magnani, S.; Maltoni, M.L.; Quacquarelli, I.; Cacchi, M.; Antunes, M.L.C.; D’Antuono, F.; Faedi, W.; Baruzzi, G. Effects of site and genotype on strawberry fruits quality traits and bioactive compounds. J. Berry Res. 2015, 5, 145–155. [Google Scholar] [CrossRef]
- Cervantes, L.; Ariza, M.T.; Miranda, L.; Lozano, D.; Medina, J.J.; Soria, C.; Martínez-Ferri, E. Stability of fruit quality traits of different strawberry varieties under variable environmental conditions. Agronomy 2020, 10, 1242. [Google Scholar] [CrossRef]
- Jaakola, L.; Hohtola, A. Effect of latitude on flavonoid biosynthesis in plants. Plant Cell Environ. 2010, 33, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Jaakola, L. New insights into the regulation of anthocyanin biosynthesis in fruits. Trends Plant. Sci. 2013, 18, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Zoratti, L.; Karpinnen, K.; Escobar, A.L.; Häggman, H.; Jaakola, L. Light-controlled flavonoid biosynthesis in fruits. Front. Plant. Sci. 2014, 5, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Zheng, W. Effect of plant growth temperature on antioxidant capacity in strawberry. J. Agric. Food Chem. 2001, 49, 4977–4982. [Google Scholar] [CrossRef]
- Josuttis, M.; Dietrich, H.; Patz, C.-D.; Krüger, E. Effects of air and soil temperatures on the chemical composition of fruit and agronomic performance in strawberry (Fragaria × ananassa Duch.). J. Hortic. Sci. Biotechnol. 2011, 86, 415–421. [Google Scholar] [CrossRef]
- Balasooriya, B.L.H.N.; Dassanayake, K.; Ajlouni, S. High temperature effects on strawberry fruit quality and antioxidant contents. Acta Hortic. 2020, 1278, 225–234. [Google Scholar] [CrossRef]
- Josuttis, M.; Carlen, C.; Crespo, P.; Nestby, R.; Dietrich, H.; Krüger, E. A comparison of bioactive compounds of strawberry fruit from Europe affected by genotype and latitude. J. Berry Res. 2012, 2, 73–95. [Google Scholar] [CrossRef]
- Anttonnen, M.J.; Hoppula, K.J.; Nestby, R.; Verheul, M.J.; Karjalainen, R.O. Influence of fertilization, mulch color, early forcing, fruit order, planting date, shading, growing environment and genotype, on the content of selected phenolics in strawberry (Fragria × ananassa Duch.) fruits. J. Agric. Food Chem. 2006, 54, 2614–2620. [Google Scholar] [CrossRef] [PubMed]
- Josuttis, M.; Dietrich, H.; Treuter, D.; Will, F.; Linnemannstöns, L.; Krüger, E. Solar UVB response of bioactives in strawberry (Fragaria × ananassa Duch.): A comparison of protected and open-field cultivation. J. Agric. Food Chem. 2010, 58, 12692–12702. [Google Scholar] [CrossRef] [PubMed]
- Ordidge, M.; García-Macías, P.; Battey, N.; Gordon, M.H.; Hadley, P.; John, P.; Lovegrove, J.A.; Vysini, E.; Wagstaffe, A. Phenolic contents of lettuce, strawberry, raspberry, and blueberry crops cultivated under plastic films varying in ultraviolet transparency. Food Chem. 2010, 119, 1224–1227. [Google Scholar] [CrossRef]
- Heide, O.M.; Sønsteby, A. Climate-photothermographs, a tool for ecophysiological assessment of effects of climate warming in crop plants: Examples with three berry crops. J. Berry Res. 2020, 411–418. [Google Scholar] [CrossRef]
- Tsormpatsidis, E.; Ordrige, M.; Henbest, R.G.C.; Wagstaffe, A.; Battey, N.H.; Hadley, P. Harvesting fruit of equivalent chronological age and fruit position shows individual effect of UV radiation on aspects of the strawberry ripening process. Environ. Exp. Botany. 2011, 74, 178–185. [Google Scholar] [CrossRef]
- Zhang, Y.; Leiyu, J.; Li, Y.; Chen, Q.; Ye, Y.; Zhang, Y.; Luo, Y.; Sun, B.; Wang, X.; Tang, H. Effect of red and blue light on anthocyanin accumulation and differential gene expression in strawberry (Fragaria × ananassa). Molecules 2018, 23, 820. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, W.; Peng, X.; Sun, B.; Wang, X.; Tang, H. Characterization of anthocyanin and proanthocyanidin biosynthesis in two strawberry genotypes during fruit development in response to different light qualities. J. Photochem. Photobiol. B 2018, 186, 225–231. [Google Scholar] [CrossRef]
- Bro, R.; Smilde, A.K. Principal component analysis. Anal Methods 2014, 6, 2812–2831. [Google Scholar] [CrossRef]
- Bueniída, B.; Gil, M.I.; Tudela, J.A.; Gady, A.L.; Medina, J.J.; Soria, C.; López, J.M.; Tomás-Barberán, F. HPLC-MS analysis of proanthocyanidin oligomers and other phenolics in 15 strawberry cultivars. J. Agric. Food Chem. 2010, 58, 3916–3926. [Google Scholar] [CrossRef]
- Crespo, P.; Bordonaba, J.G.; Terry, L.A.; Carlen, C. Characterisation of major taste and health related compounds of four strawberry genotypes grown at different Swiss production sites. Food Chem. 2010, 122, 16–24. [Google Scholar] [CrossRef]
- Palmieri, L.; Masuero, D.; Martinatti, P.; Baratto, G.; Martens, S.; Vrhovsek, U. Genotype-by-environment effect on bioactive compounds in strawberry (Fragaria × ananassa Duch.). J. Sci. Food Agric. 2017, 97, 4180–4189. [Google Scholar] [CrossRef] [PubMed]
- Behn, H.; Schurr, U.; Ulbrich, A.; Noga, G. Development-dependent UV-B responses in red oak leaf lettuce (Lactuca sativa L.): Physiological mechanisms and significance for hardening. Eur. J. Hortic. Sci. 2011, 76, 33–44. [Google Scholar]
- Awad, M.A.; Wagenmakers, P.; de Jager, A. Effects of light on flavonoid and chlorogenic acid levels in skin of ‘Jonagold’ apples. Sci. Hortic. 2001, 88, 289–298. [Google Scholar] [CrossRef]
- Ubi, B.E.; Honda, C.; Bessho, H.; Kondo, S.; Wada, M.; Kobayashi, S.; Moriguchi, T. Expression analysis of anthocyanin biosynthetic genes in apple skin: Effect of UV-B and temperature. Plant. Sci. 2006, 170, 571–578. [Google Scholar] [CrossRef]
- Zhao, Y.; Dong, W.; Wang, K.; Zhang, B.; Allan, A.C.; Lin-Wang, K.; Chen, K.; Xu, C. Differential sensitivity of fruit pigmentation to ultraviolet light between two peach cultivar. Front. Plant. Sci. 2017, 8, 1552. [Google Scholar] [CrossRef]
- Matsushita, K.; Sakayori, T.; Ikeda, S. The effect of high air temperature on anthocyanin and the expression of its biosynthetic genes in strawberry ‘Sachinoka’. Environ. Control. Biol. 2016, 54, 101–107. [Google Scholar] [CrossRef]
- Carbonne, F.; Preuss, A.; de Vos, R.C.H.; D’Amico, E.; Perrotta, G.; Bovy, A.G.; Martens, S.; Rosati, C. Developmental, genetic and environmental factors affect the expression of flavonoid genes, enzymes and metabolites in strawberry fruits. Plant. Cell Environ. 2009, 32, 1117–1131. [Google Scholar] [CrossRef]
- Guerrero-Chavez, G.; Scampicchio, M.; Andreotti, C. Influence of the site altitude on strawberry phenolic composition and quality. Sci. Hortic. 2015, 192, 21–28. [Google Scholar] [CrossRef]
- Lätti, A.K.; Riihinen, K.; Kainulainen, P.S. Analysis of anthocyanin variation in wild populations of bilberry (Vaccinium myrtillus L.) in Finland. J. Agric. Food Chem. 2008, 56, 190–196. [Google Scholar] [CrossRef]
- Lätti, A.K.; Jaakola, L.; Riihinen, K.; Kainulainen, P.S. Anthocyanin and flavanol variation in bog bilberries (Vaccinium uliginosum L.) in Finland. J. Agric. Food Chem. 2010, 58, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Åkerström, A.; Jaakola, L.; Båäng, U.; Jäderlund, A. Effects of latitude-related factors and geographical origin on anthocyanidin concentrations in fruits of Vaccinium myrtillus L. (Bilberries). J. Agric. Food Chem. 2010, 58, 11939–11945. [Google Scholar] [CrossRef] [PubMed]
- Ferreyra, R.M.; Viña, S.Z.; Mudridge, A.; Chaves, A.R. Growth and ripening season effects on antioxidant capacity of strawberry cultivar Selva. Sci. Hortic. 2007, 112, 27–32. [Google Scholar] [CrossRef]
- Halbwirth, H.; Puhl, I.; Haas, U.; Jezik, K.; Treutter, D.; Stich, K. Two-phase flavonoid formation in developing strawberry (Fragaria× ananassa Duch.) fruit. J. Agric. Food. Chem. 2006, 54, 1479–1485. [Google Scholar] [CrossRef]
- Fait, A.; Hanhineva, K.; Beleggia, R.; Dai, N.; Rogachey, I.; Nikiforova, V.J.; Fernie, A.R.; Aharoni, A. Reconfiguration of the achene and receptacle metabolic networks during strawberry fruit development. Plant. Physiol. 2008, 148, 730–750. [Google Scholar] [CrossRef]
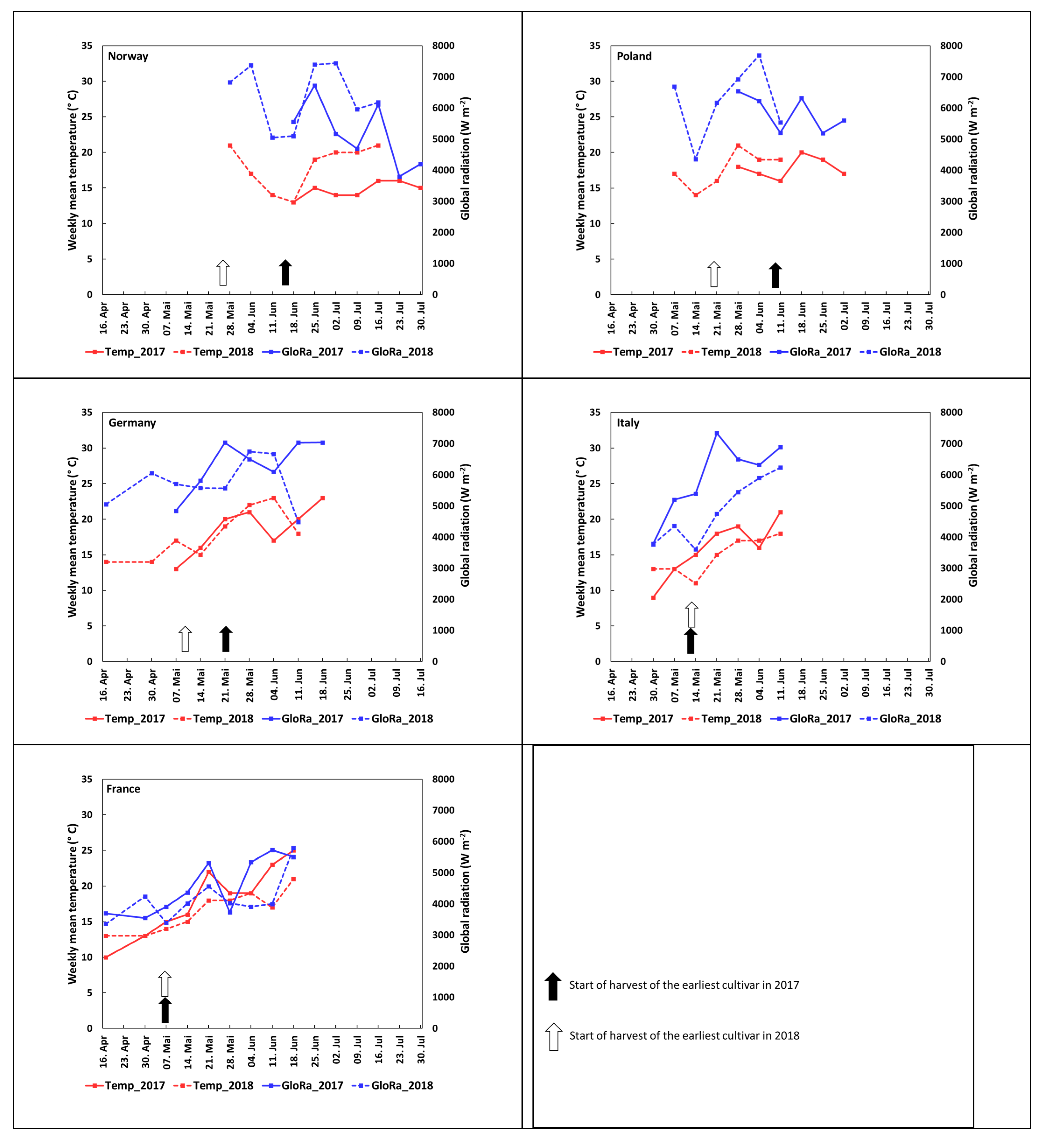



| NIBIO Norway | INHORT Poland | HGU Germany | Sant’Orsola Italy | INVENIO France | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Latitude | 60°40′ N | 51o95′ N | 49°59′ N | 46°4′ N | 44°85′ N | |||||
| Altitude (m a.s.l.) | 262 | 252 | 95 | 925 | 145 | |||||
| Yearly mean temperature (°C) (a) | 5.0 | 7.9 | 9.9 | 11.3 | 12.9 | |||||
| Soil type | Loam | Pseudopodsol with light clay | Sandy loam | Soilless culture | Soilless culture | |||||
| pH of the soil/substrate | 5.7–6.2 | 6.5–7.0 | 6.5–7.0 | 5.5–6.0 | 6.1 | |||||
| Cultivation type | open field | open field | open field | tunnel | tunnel | |||||
| 2017 | 2018 | 2017 | 2018 | 2017 | 2018 | 2017 | 2018 | 2017 | 2018 | |
| Yearly mean temperature (°C) (a) | 5.0 | 5.7 | 9.1 | 9.8 | 11.3 | 12.4 | 15.6 | 15.1 | 13.2 | 13.8 |
| Start of flowering (b) | 06.06. | 21.05. | 16.05 | 30.04. | 04.04. (d) | 16.04. | 04.04. | 11.04. | 04.04. | 07.04. |
| Start of harvest (b) | 07.07. | 17.06. | 13.06. | 22.05. | 29.05. | 14.05. | 19.05. | 17.05. | 09.05. | 09.05. |
| End of harvest (c) | 16.08. | 21.07. | 07.07. | 19.06. | 16.06. | 18.06. | 12.06. | 13.06. | 22.06. | 22.06. |
| Day length at start of harvest (h) (b) | 18:40 | 19:09 | 16:43 | 16:04 | 15:56 | 15:20 | 14:30 | 14:30 | 14.36 | 14:36 |
| Total Anthocyanins HPLC (mg kg−1 Fresh Weight) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Cultivar | ||||||||
| Location | Year | Can | Cle | Flo | Fri | Gar | Son | Yearly Mean per Location |
| Norway | 2017 | 212.6 ± 25.6 | 284.6 ± 30.5 | 374.5 ± 30.4 | 453.4 ± 42.4 | 263.1 ± 58.2 | 191.6 ± 16.2 | 296.6 B |
| 2018 | 133.8 ± 33.8 | 195.2 ± 48.7 | 405.5 ± 43.8 | 451.6 ± 68.8 | 198.9 ± 25.8 | 145.6 ± 30.4 | 255.1 B | |
| mean | 173.2 ab | 239.9 c | 390.0 d | 452.5 e | 231.0 bc | 168.6 a | ||
| Poland | 2017 | 245.9 ± 53.0 | 310.1 ± 34.3 | 359.1 ± 89.4 | 438.0 ± 58.8 | 235.0 ± 34.9 | 210.2 ± 27.2 | 299.7 B |
| 2018 | 225.0 ± 40.8 | 249.6 ± 22.4 | 448.5 ± 68.5 | 397.9 ± 74.6 | 217.6 ± 40.1 | 222.6 ± 35.4 | 293.5 B | |
| mean | 235.5 a | 279.9 a | 403.8 b | 417.9 b | 226.3 a | 216.4 a | ||
| Germany | 2017 | 196.5 ± 22.3 | 285.5 ± 46.5 | 437.3 ± 57.7 | 429.2 ± 21.1 | 225.5 ± 44.4 | 199.4 ± 44.2 | 295.6 B |
| 2018 | 223.6 ± 31.3 | 307.7 ± 21.1 | 507.9 ± 26.5 | 385.9 ± 27.9 | 273.5 ± 19.9 | 217.7 ± 51.1 | 319.4 B | |
| mean | 211.0 a | 296.6 b | 472.6 d | 407.6 c | 249.5 ab | 208.6 a | ||
| Italy | 2017 | - | 363.0 ± 27.2 | - | 432.4 ± 52.7 | 326.4 ± 19.4 | 209.2 ± 17.7 | 332.8 B |
| 2018 | - | 276.6 ± 44.9 | - | 302.2 ± 56.2 | 166.9 ± 22.3 | 124.7 ± 34.4 | 217.6 A | |
| mean | - | 319.8 bc | - | 367.4 c | 246.6 b | 167.0 a | ||
| France | 2017 | 315.5 ± 23.2 | 218.9 ± 63.8 | 455.2 ± 81.3 | 446.7 ± 44.3 | 261.5 ± 39.3 | 212.0 ± 48.3 | 318.3 B |
| 2018 | 306.9 ± 45.2 | 365.6 ± 61.3 | 420.9 ± 66.3 | 342.8 ± 64.3 | 221.2 ± 34.4 | 198.6 ± 49.2 | 309.2 B | |
| mean | 311.2 b | 292.3 b | 438.0 c | 394.4 c | 241.3 ab | 205.3 a | ||
| Cultivar mean over all locations | 232.5 b | 285.7 c | 426.1 d | 408.0 d | 239.0 b | 193.1 a | ||
| Significance | Cultivar | Location | Year | Cultivar x location | Cultivar x year | Location x year | ||
| *** | ns | * | ns | ns | * | |||
| Cyanidin 3-Glucoside (mg kg−1 Fresh Weight) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Cultivar | ||||||||
| Location | Year | Can | Cle | Flo | Fri | Gar | Son | Yearly Mean per Location |
| Norway | 2017 | 9.6 ± 2.6 | 3.6 ± 1.5 | 9.4 ± 2.8 | 14.1 ± 2.4 | 3.2 ± 0.7 | 5.7 ± 1.7 | 7.6 A |
| 2018 | 12.7 ± 3 | 7.4 ± 5.2 | 14.3 ± 2.2 | 13.2 ± 1.1 | 4.4 ± 1.2 | 6.0 ± 2.3 | 9.6 A | |
| mean | 11.2 b | 5.5 a | 11.8 b | 13.7 b | 3.8 a | 5.8 a | ||
| Poland | 2017 | 9.7 ± 1.7 | 3.2 ± 0.6 | 12.7 ± 4.5 | 15.0 ± 3.3 | 3.3 ± 0.5 | 6.3 ± 2.6 | 8.4 A |
| 2018 | 29.2 ± 2.6 | 8.8 ± 1.9 | 47.8 ± 6.7 | 27.8 ± 4.1 | 9.2 ± 2.1 | 13.4 ± 5.9 | 22.7 B | |
| mean | 19.4 bc | 6.0 a | 30.3 c | 21.4 bc | 6.2 a | 9.9 ab | ||
| Germany | 2017 | 10.7 ± 2.4 | 2.5 ± 0.6 | 17.4 ± 3.6 | 12.8 ± 1.2 | 2.5 ± 1.3 | 4.4 ± 1.6 | 8.4 A |
| 2018 | 12.9 ± 4.8 | 5.0 ± 4.8 | 26.4 ± 4.4 | 16.9 ± 2.0 | 4.0 ± 0.5 | 6.0 ± 2.4 | 11.9 A | |
| mean | 11.8 b | 3.7 a | 21.9 c | 14.8 b | 3.3 a | 5.2 a | ||
| Italy | 2017 | - | 1.6 ± 0.3 | - | 11.9 ± 4.6 | 2.5 ± 0.5 | 2.8 ± 0.5 | 4.7 B |
| 2018 | - | 1.6 ± 0.7 | - | 2.4 ± 1.5 | 1.5 ± 0.7 | 2.7 ± 1.8 | 2.0 A | |
| mean | - | 1.6 a | - | 7.0 b | 2.0 a | 2.7 a | ||
| France | 2017 | 11.3 ± 1.5 | 1.3 ± 0.1 | 19.0 ± 14.5 | 12.5 ± 1.7 | 1.7 ± 0.3 | 2.6 ± 0.7 | 8.0 A |
| 2018 | 10.7 ± 3.0 | 1.8 ± 0.4 | 16.9 ± 5.8 | 9.0 ± 2.1 | 2.7 ± 0.6 | 3.1 ± 1.3 | 7.4 A | |
| mean | 11.0 b | 1.6 a | 18.0 c | 10.7 b | 2.2 a | 2.8 a | ||
| Cultivar mean over all locations | 13.3 b | 3.7 a | 20.5 c | 13.5 b | 3.5 a | 5.3 a | ||
| Significance | Cultivar | Location r | Year | Cultivar x location | Cultivar x year | Location x year | ||
| *** | *** | *** | ns | ns | *** | |||
| - | Pelargonidin-3-glucoside (mg kg−1 Fresh Weight) | |||||||
|---|---|---|---|---|---|---|---|---|
| Cultivar | ||||||||
| Location | Year | Can | Cle | Flo | Fri | Gar | Son | Yearly Mean per Location |
| Norway | 2017 | 184.3 ± 23.9 | 228.4 ± 23.1 | 330.3 ± 26.7 | 328.1 ± 36.7 | 178.97 ± 39.8 | 145.4 ± 13.9 | 232.6 A |
| 2018 | 108.8 ± 27.7 | 146.5 ± 41.2 | 346.0 ± 35.6 | 340.1 ± 39.2 | 133.67 ± 16.2 | 95.4 ± 48.9 | 195.1 A | |
| mean | 146.6 ab | 187.4 b | 338.1 c | 334.1 c | 156.3 ab | 120.4 a | ||
| Poland | 2017 | 209.9 ± 48.0 | 246.2 ± 24.0 | 306.0 ± 85 | 306.5 ± 37.4 | 160.4 ± 24.1 | 154.3 ± 17.2 | 230.6 A |
| 2018 | 162.2 ± 15.2 | 177.9 ± 18.5 | 333.4 ± 54.6 | 253.2 ± 50.8 | 131.6 ± 25.5 | 146.7 ± 21.3 | 200.8 A | |
| mean | 186.0 ab | 212.1 b | 319.7 c | 279.9 c | 146.0 a | 150.5 a | ||
| Germany | 2017 | 164.5 ± 17.6 | 216.4 ± 35.3 | 382.3 ± 49.8 | 292.4 ± 12.7 | 149.8 ± 24.3 | 140.7 ± 26.6 | 224.3 A |
| 2018 | 189.5 ± 21.6 | 233.2 ± 15.7 | 420.8 ± 16.8 | 268.1 ± 18.8 | 183.8 ± 9.0 | 158.4 ± 37.6 | 242.1 A | |
| mean | 177.0 a | 224.8 b | 401.5 d | 280.3 c | 166.8 a | 149.5 a | ||
| Italy | 2017 | - | 288.5 ± 22.4 | - | 306.2 ± 41.7 | 222.2 ± 19.9 | 160.1 ± 15.8 | 244.3 B |
| 2018 | - | 200.8 ± 33.4 | - | 221.0 ± 39.5 | 102.1 ± 17.5 | 91.4 ± 24.0 | 153.8 A | |
| mean | - | 244.7 b | - | 263.8 b | 162.1 a | 125.8 a | ||
| France | 2017 | 279.5 ± 21.6 | 171.1 ± 46.4 | 395.0 ± 50.6 | 321.8 ± 33.1 | 176.0 ± 27.8 | 159.9 ± 36.1 | 250.5 B |
| 2018 | 271.0 ± 39.5 | 274.0 ± 53.1 | 345.7 ± 55.5 | 225.2 ± 42.7 | 125.5 ± 31.6 | 140.7 ± 33.6 | 230.4 A | |
| mean | 275.2 b | 222.6 b | 370.3 c | 273.5 b | 150.8 a | 150.3 a | ||
| Cultivar mean over all locations | 196.2 b | 218.3 b | 357.4 d | 286.3 c | 156.4 a | 139.3 a | ||
| Significance | Cultivar | Location | Year | Cultivar x location | Cultivar x year | Location x Year | ||
| *** | ns | *** | ns | ns | * | |||
| Pelargonidin-3-rutinoside (mg kg−1 Fresh Weight) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Cultivar | ||||||||
| Location | Year | Can | Cle | Flo | Fri | Gar | Son | Yearly Mean per Location |
| Norway | 2017 | 18.2 ± 2.5 | 5.3 ± 1.1 | 1.4 ± 0.2 | 27.0 ± 3.7 | 0.9 ± 0.5 | 7.3 ± 1.1 | 10.0 A |
| 2018 | 11.9 ± 3.4 | 2.5 ± 1.5 | 1.4 ± 0.2 | 23.3 ± 11.9 | 0.5 ± 0.1 | 19.2 ± 3.2 | 9.8 A | |
| mean | 15.1 cd | 3.9 abc | 1.4 ab | 25.2 d | 0.7 a | 13.2 bcd | ||
| Poland | 2017 | 26.2 ± 4.4 | 7.1 ± 0.4 | 2.1 ± 1.7 | 25.2 ± 3.2 | 0.9 ± 0.3 | 5.9 ± 0.9 | 11.2 A |
| 2018 | 28.3 ± 5.7 | 9.1 ± 1.1 | 3.7 ± 0.3 | 25.9 ± 4.8 | 3.0 ± 0.2 | 8.5 ± 0.6 | 13.1 A | |
| mean | 27.3 c | 8.1 b | 2.9 a | 25.5 c | 2.0 a | 7.2 b | ||
| Germany | 2017 | 21.0 ± 3.6 | 6.7 ± 0.7 | 1.4 ± 0.3 | 25.4 ± 1.8 | 0.9 ± 0.5 | 6.4 ± 1.9 | 10.3 A |
| 2018 | 21.2 ± 9.6 | 9.6 ± 6.8 | 6.2 ± 1.5 | 20.9 ± 3.6 | 2.3 ± 0.7 | 6.8 ± 2.5 | 11.2 A | |
| mean | 21.1 c | 8.2 b | 3.8 ab | 23.1 c | 1.6 a | 6.6 b | ||
| Italy | 2017 | - | 8.1 ± 0.9 | - | 24.0 ± 3.8 | 2.0 ± 0.2 | 5.5 ± 0.5 | 9.9 B |
| 2018 | - | 7.1 ± 1.2 | - | 7.1 ± 2.5 | 1.3 ± 0.1 | 2.6 ± 1.2 | 4.5 A | |
| mean | - | 7.6 b | - | 15.6 c | 1.6 a | 4.1 ab | ||
| France | 2017 | 23.6 ± 3.2 | 4.5 ± 1.6 | 2.0 ± 0.1 | 20.4 ± 3.7 | 1.2 ± 0.4 | 4.8 ± 1.8 | 9.4 A |
| 2018 | 24.3 ± 3.1 | 7.2 ± 1.3 | 1.8 ± 0.4 | 14.1 ± 3.8 | 1.8 ± 0.9 | 3.6 ± 1.5 | 8.8 A | |
| mean | 23.9 d | 5.8 b | 1.9 a | 17.2 c | 1.5 a | 4.2 ab | ||
| Cultivar mean over all locations | 21.8 c | 6.7 b | 2.5 a | 21.3 c | 1.5 a | 7.0 b | ||
| Significance | Cultivar | Location | Year | Cultivar x location | Cultivar x year | Location x year | ||
| *** | ns | ns | ** | * | ns | |||
| Cyanidin-3-(6-O-malonyl)-glucoside (mg kg−1 Fresh Weight) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Cultivar | ||||||||
| Location | Year | Can | Cle | Flo | Fri | Gar | Son | Yearly Mean per Location |
| Norway | 2017 | 0.1 ± 0.2 | 0.7 ± 0.2 | 0.8 ± 0.2 | 3.3 ± 1.5 | 1.4 ± 0.6 | 1.2 ± 0.5 | 1.3 A |
| 2018 | 0.2 ± 0.1 | 1.5 ± 0.9 | 2.6 ± 0.5 | 2.8 ± 0.4 | 2.3 ± 0.5 | 0.8 ± 0.7 | 1.7 A | |
| mean | 0.2 a | 1.1 b | 1.7 b | 3.0 c | 1.8 b | 1.0 ab | ||
| Poland | 2017 | 0.0 ± 0.0 | 0.7 ± 0.2 | 1.6 ± 0.8 | 3.5 ± 1.7 | 1.2 ± 0.5 | 1.4 ± 0.9 | 1.4 A |
| 2018 | 2.5 ± 0.0 | 4.4 ± 0.4 | 11.0 ± 1.6 | 8.6 ± 1.2 | 5.6 ± 1.2 | 5.6 ± 1.0 | 6.3 B | |
| mean | 1.2 a | 2.5 a | 6.3 b | 6.1 b | 3.4 ab | 3.5 ab | ||
| Germany | 2017 | 0.0 ± 0.0 | 0.7 ± 0.2 | 2.3 ± 0.6 | 3.8 ± 0.3 | 1.2 ± 1.1 | 1.3 ± 0.7 | 1.6 A |
| 2018 | 0.0 ± 0.0 | 0.8 ± 0.4 | 3.5 ± 0.7 | 4.1 ± 0.9 | 1.7 ± 0.2 | 1.6 ± 0.8 | 2.0 A | |
| mean | 0.0 a | 0.8 b | 2.9 c | 4.0 d | 1.5 b | 1.5 b | ||
| Italy | 2017 | - | 0.7 ± 0.1 | - | 2.6 ± 1.4 | 0.9 ± 0.4 | 0.8 ± 0.1 | 1.2 B |
| 2018 | - | 0.7 ± 0.2 | - | 0.7 ± 0.4 | 0.7 ± 0.3 | 0.7 ± 0.4 | 0.7 A | |
| mean | - | 0.7 a | - | 1.7 b | 0.8 a | 0.8 a | ||
| France | 2017 | 0.1 ± 0.2 | 0.2 ± 0.1 | 1.6 ± 0.9 | 3.1 ± 0.3 | 0.5 ± 0.2 | 0.7 ± 0.2 | 1.0 A |
| 2018 | 0.3 ± 0.1 | 0.6 ± 0.2 | 3.3 ± 1.4 | 3.1 ± 0.6 | 1.7 ± 0.4 | 1.0 ± 0.4 | 1.7 B | |
| mean | 0.2 a | 0.4 ab | 2.5 c | 3.1 c | 1.1 b | 0.8 ab | ||
| Cultivar mean over all locations | 0.4 a | 1.1 ab | 3.4 c | 3.6 c | 1.7 b | 1.5 b | ||
| Significance | Cultivar | Location | Year | Cultivar x location | Cultivar x year | Location x year | ||
| *** | *** | *** | ns | * | *** | |||
| Pelargonidin-3-(6-O-malonyl)-glucoside (mg kg−1 Fresh Weight) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Cultivar | ||||||||
| location | Year | Can | Cle | Flo | Fri | Gar | Son | Yearly Mean per Location |
| Norway | 2017 | 0.4 ± 0.6 | 46.7 ± 7.7 | 32.6 ± 5.9 | 80.9 ± 10.3 | 78.7 ± 19.1 | 32.0 ± 5.3 | 45.2 A |
| 2018 | 0.2 ± 0.1 | 37.3 ± 8.6 | 41.3 ± 7.9 | 72.2 ± 24.5 | 58.0 ± 9.3 | 24.2 ± 5.5 | 38.9 A | |
| mean | 0.3 a | 42.0 b | 36.9 b | 76.5 c | 68.3 c | 28.1 b | ||
| Poland | 2017 | 0.2 ± 0.4 | 52.9 ± 10.4 | 36.6 ± 8.0 | 87.8 ± 17.9 | 69.2 ± 11.8 | 42.4 ± 7.5 | 48.2 A |
| 2018 | 2.8 ± 0.3 | 49.4 ± 3 | 52.5 ± 9.9 | 82.4 ± 16.1 | 68.1 ± 12.7 | 48.4 ± 8.2 | 50.6 A | |
| mean | 1.5 a | 51.2 b | 44.5 b | 85.1 d | 68.6 c | 45.4 b | ||
| Germany | 2017 | 0.4 ± 0.3 | 59.2 ± 12.8 | 33.9 ± 16.7 | 94.8 ± 10.5 | 71.1 ± 18.8 | 46.7 ± 14.4 | 51.0 A |
| 2018 | 0.0 ± 0.0 | 59.2 ± 6.4 | 51.1 ± 8.5 | 75.9 ± 12.8 | 81.6 ± 10.6 | 44.9 ± 11.8 | 52.1 A | |
| mean | 0.2 a | 59.2 c | 42.5 b | 85.4 d | 76.4 d | 45.8 b | ||
| Italy | 2017 | - | 64.1 ± 11.0 | - | 87.8 ± 13.0 | 98.8 ± 7.7 | 40.0 ± 5.9 | 72.6 B |
| 2018 | - | 66.3 ± 13 | - | 71.5 ± 13.3 | 61.3 ± 8.8 | 27.3 ± 7.4 | 56.6 A | |
| mean | - | 65.2 b | - | 79.6 b | 80.1 b | 33.6 a | ||
| France | 2017 | 1.1 ± 1.5 | 41.8 ± 16.0 | 37.7 ± 29.4 | 88.8 ± 14.3 | 82.1 ± 13.2 | 44.2 ± 11.8 | 49.3 A |
| 2018 | 0.7 ± 0.3 | 82.0 ± 14.8 | 53.1 ± 10.1 | 90.9 ± 17.8 | 89.4 ± 24.4 | 50.3 ± 15.6 | 61.1 A | |
| mean | 0.9 a | 61.9 b | 45.4 b | 89.9 c | 85.8 c | 47.2 b | ||
| Cultivar mean over all locations | 0.7 a | 55.9 c | 42.3 b | 83.3 d | 75.8 d | 40.2 b | ||
| significance | Cultivar | Location | Year | Cultivar x location | Cultivar x year | Location x Year | ||
| *** | *** | ns | ns | ns | * | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krüger, E.; Will, F.; Kumar, K.; Celejewska, K.; Chartier, P.; Masny, A.; Mott, D.; Petit, A.; Savini, G.; Sønsteby, A. Influence of Post-Flowering Climate Conditions on Anthocyanin Profile of Strawberry Cultivars Grown from North to South Europe. Appl. Sci. 2021, 11, 1326. https://doi.org/10.3390/app11031326
Krüger E, Will F, Kumar K, Celejewska K, Chartier P, Masny A, Mott D, Petit A, Savini G, Sønsteby A. Influence of Post-Flowering Climate Conditions on Anthocyanin Profile of Strawberry Cultivars Grown from North to South Europe. Applied Sciences. 2021; 11(3):1326. https://doi.org/10.3390/app11031326
Chicago/Turabian StyleKrüger, Erika, Frank Will, Keshav Kumar, Karolina Celejewska, Philippe Chartier, Agnieszka Masny, Daniela Mott, Aurélie Petit, Gianluca Savini, and Anita Sønsteby. 2021. "Influence of Post-Flowering Climate Conditions on Anthocyanin Profile of Strawberry Cultivars Grown from North to South Europe" Applied Sciences 11, no. 3: 1326. https://doi.org/10.3390/app11031326
APA StyleKrüger, E., Will, F., Kumar, K., Celejewska, K., Chartier, P., Masny, A., Mott, D., Petit, A., Savini, G., & Sønsteby, A. (2021). Influence of Post-Flowering Climate Conditions on Anthocyanin Profile of Strawberry Cultivars Grown from North to South Europe. Applied Sciences, 11(3), 1326. https://doi.org/10.3390/app11031326






