In Situ Assembly of Gold Nanoparticles in the Presence of Poly-DADMAC Resulting in Hierarchical and Highly Fractal Nanostructures
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
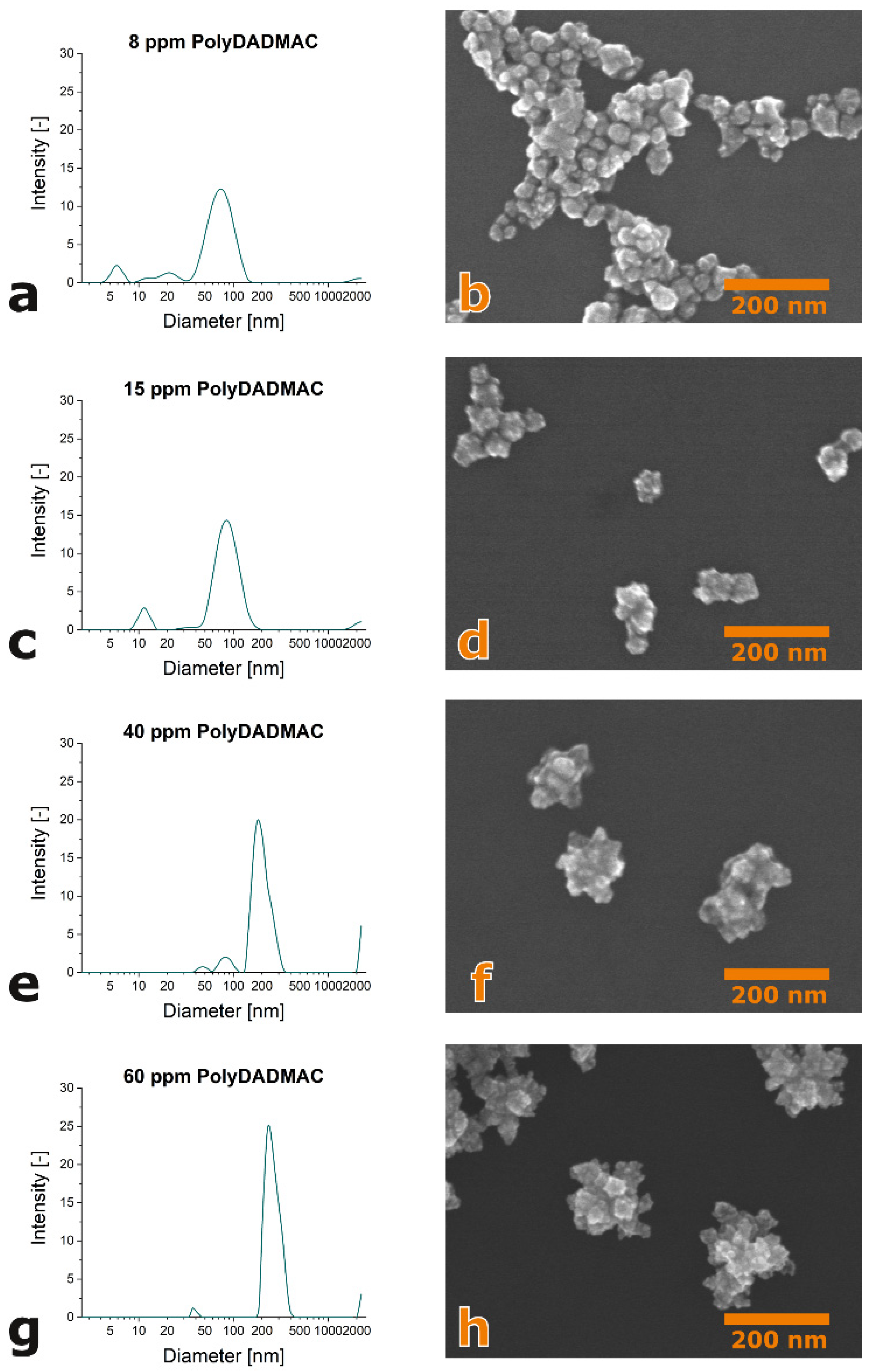
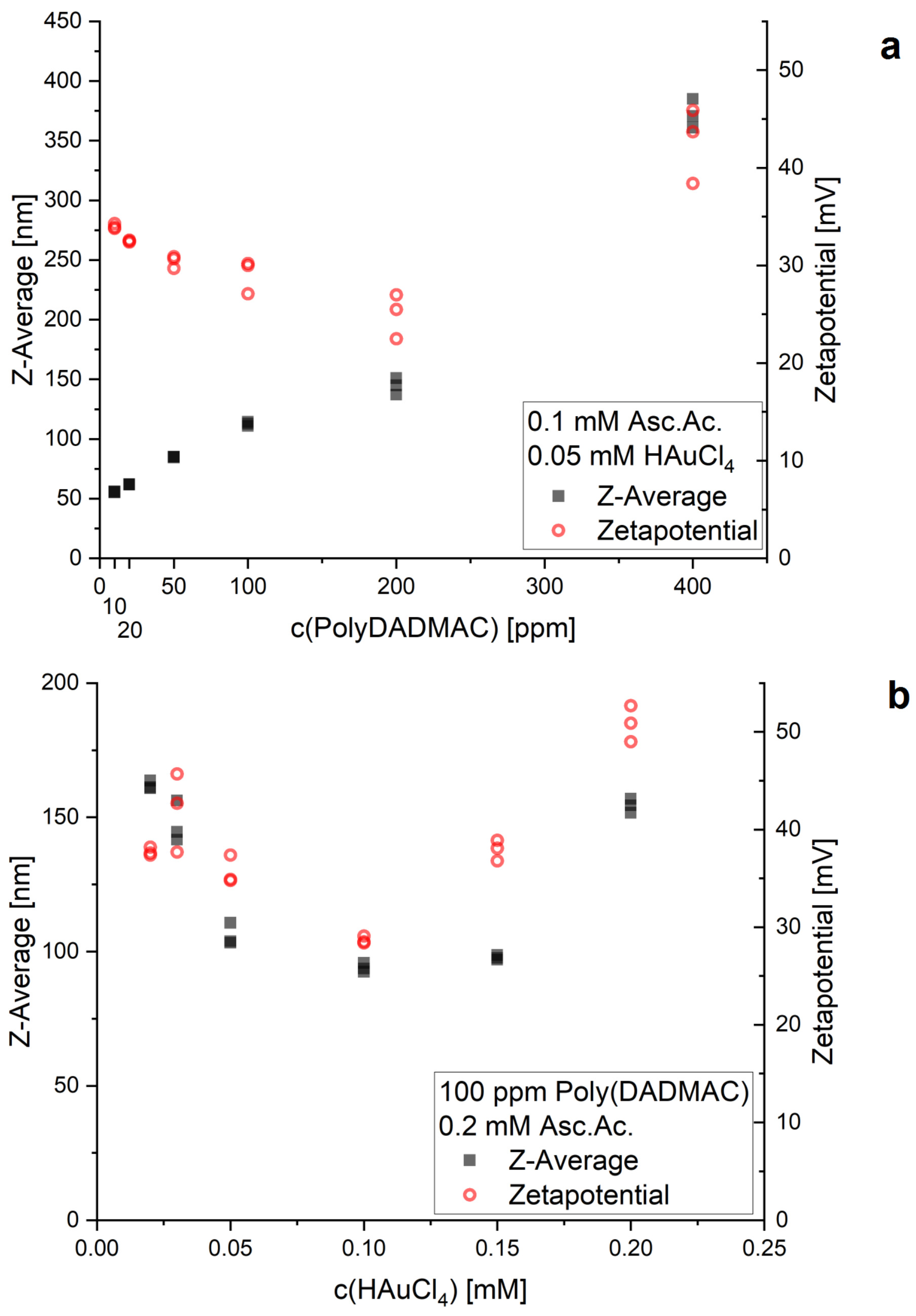
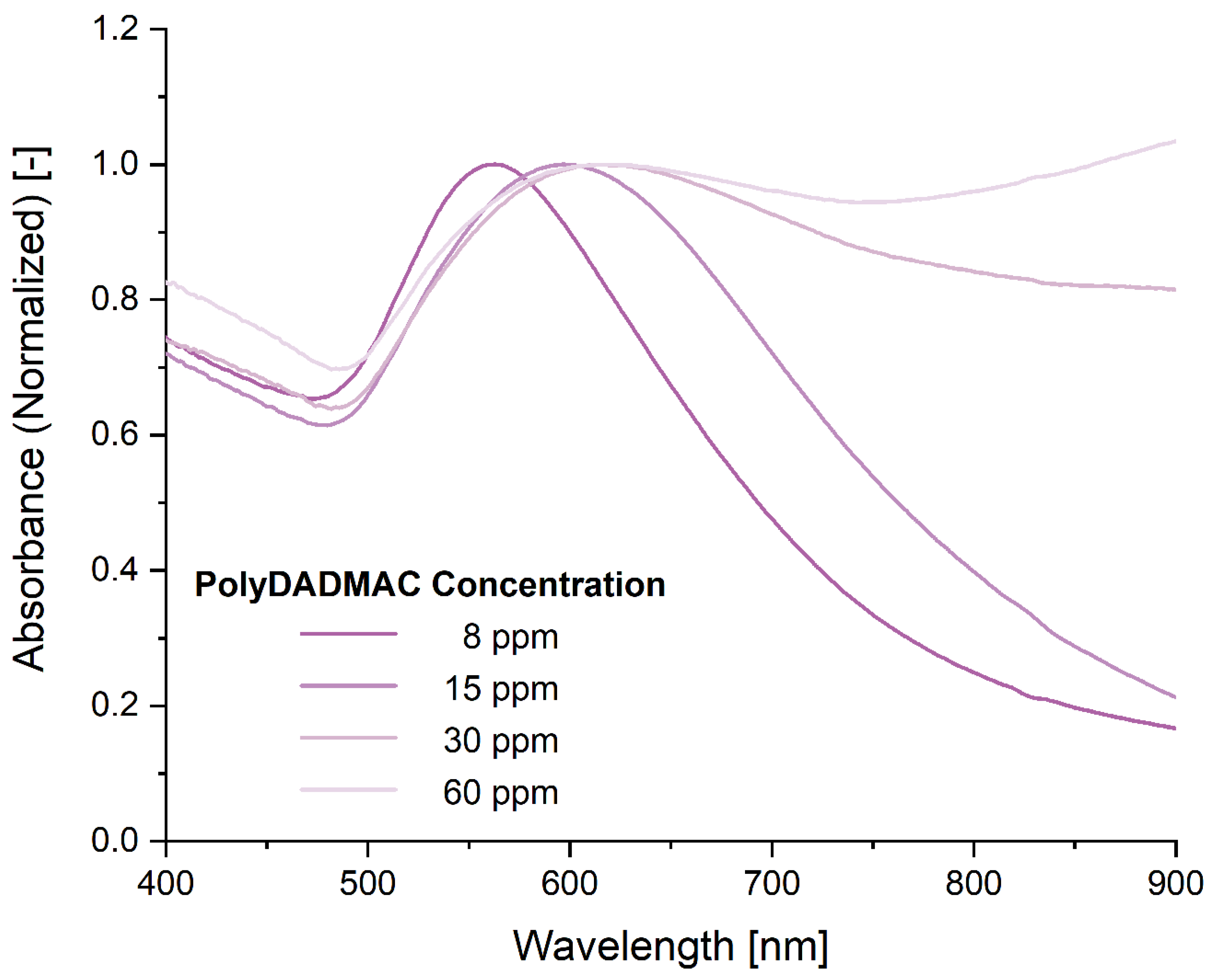
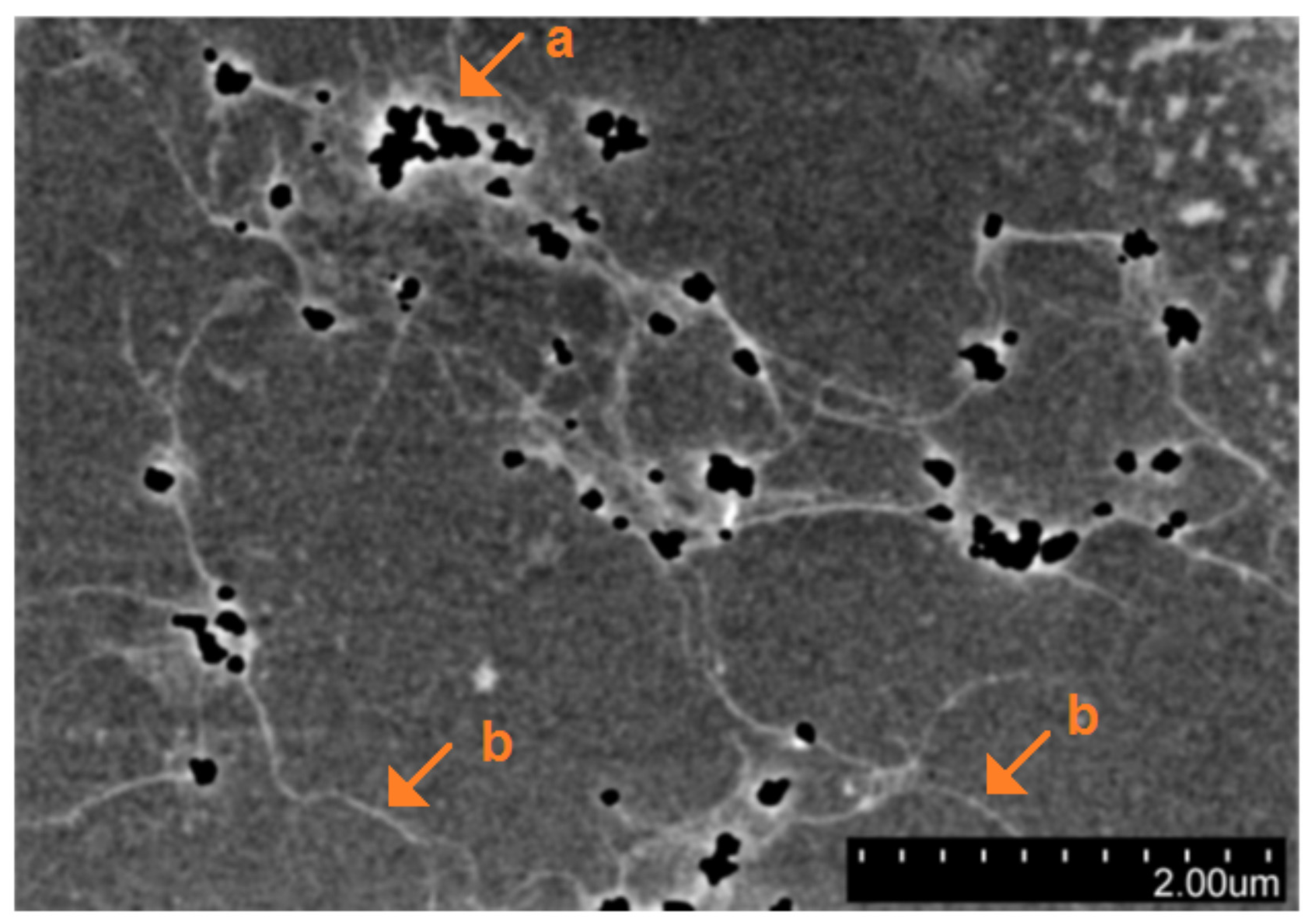
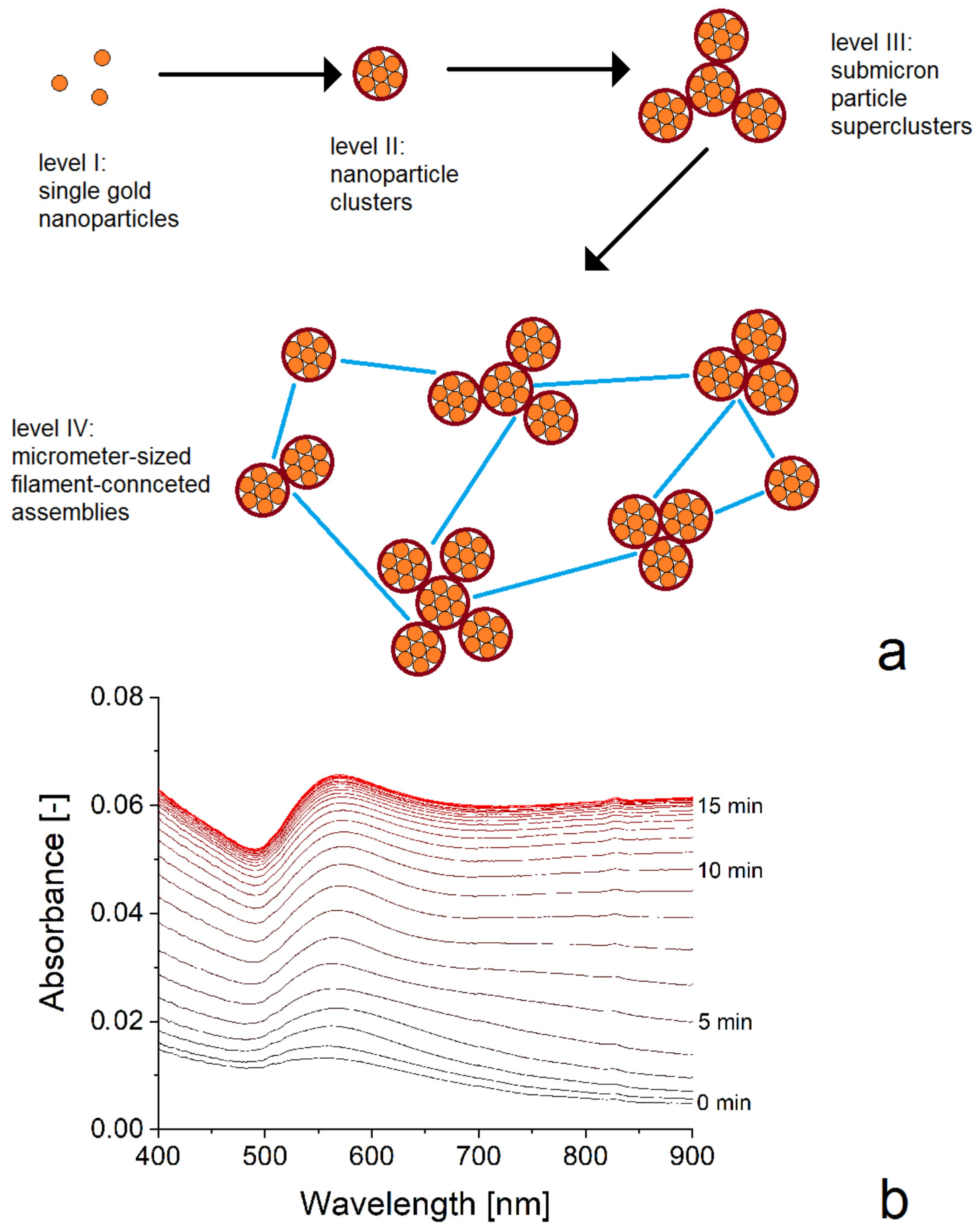
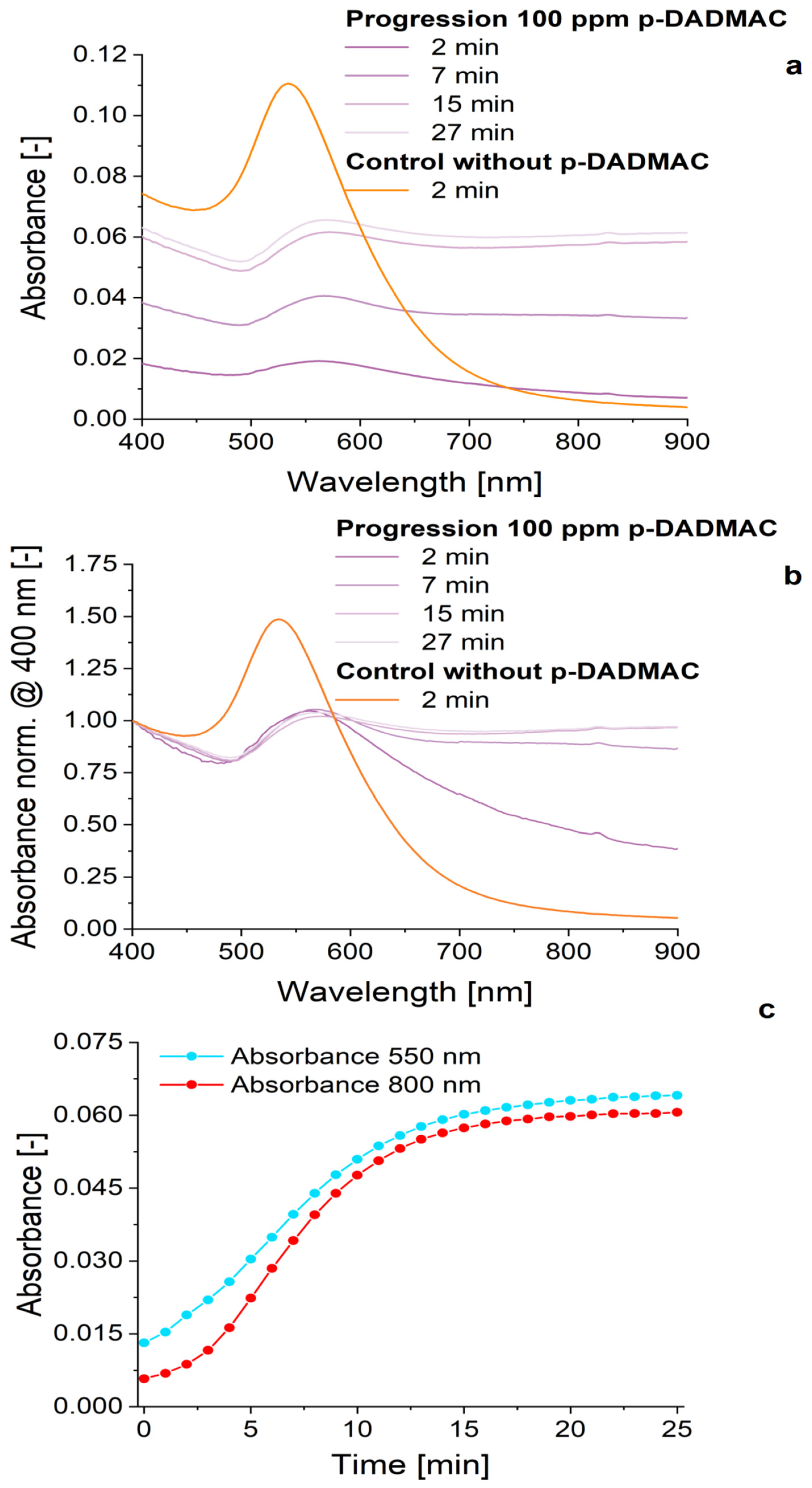
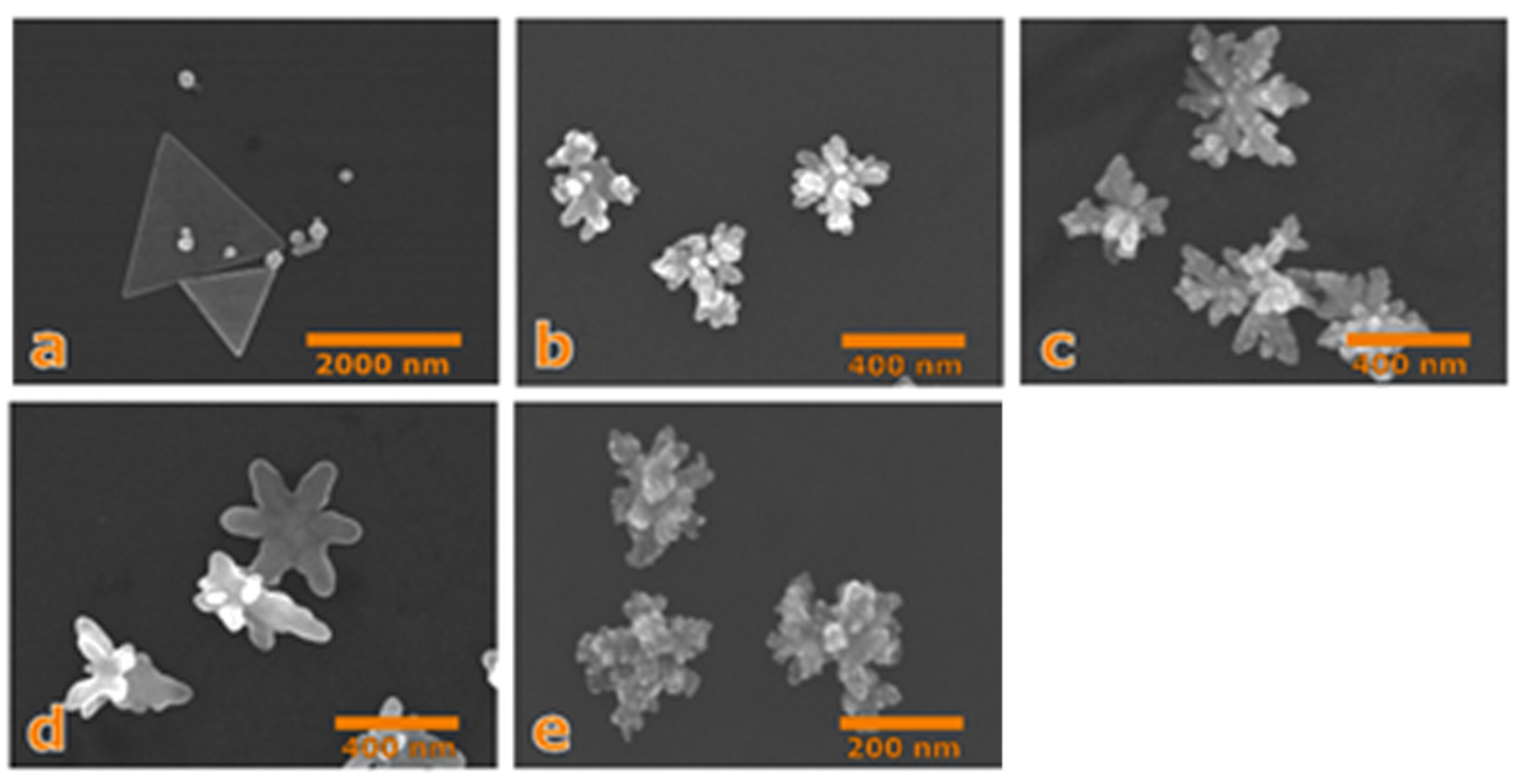
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Homberger, M.; Simon, U. On the application potential of gold nanoparticles in nanoelectronics and biomedicine. Philos. Trans. R. Soc. A-Math. Phys. Eng. Sci. 2010, 368, 1405–1453. [Google Scholar] [CrossRef] [PubMed]
- Link, S.; El-Sayed, M.A. Spectral properties and relaxation dynamics of surface plasmon electronic oscillations in gold and silver nanodots and nanorods. J. Phys. Chem. B 1999, 103, 8410–8426. [Google Scholar] [CrossRef]
- Reichert, J.; Csaki, A.; Kohler, J.M.; Fritzsche, W. Chip-based optical detection of DNA hybridization by means of nanobead labeling. Anal. Chem. 2000, 72, 6025–6029. [Google Scholar] [CrossRef] [PubMed]
- Csaki, A.; Kaplanek, P.; Moller, R.; Fritzsche, W. The optical detection of individual DNA-conjugated gold nanoparticle labels after metal enhancement. Nanotechnology 2003, 14, 1262–1268. [Google Scholar] [CrossRef] [PubMed]
- Bunz, U.H.F.; Rotello, V.M. Gold Nanoparticle-Fluorophore Complexes: Sensitive and Discerning “Noses” for Biosystems Sensing. Angew. Chem. Int. Edit. 2010, 49, 3268–3279. [Google Scholar] [CrossRef] [PubMed]
- Hwang, W.S.; Truong, P.L.; Sim, S.J. Size-dependent plasmonic responses of single gold nanoparticles for analysis of biorecognition. Anal. Biochem. 2012, 421, 213–218. [Google Scholar] [CrossRef]
- Jia, H.; Fang, C.; Zhu, X.-M.; Ruan, Q.; Wang, Y.-X.J.; Wang, J. Synthesis of Absorption-Dominant Small Gold Nanorods and Their Plasmonic Properties. Langmuir 2015, 31, 7418–7426. [Google Scholar] [CrossRef]
- Murphy, C.J.; San, T.K.; Gole, A.M.; Orendorff, C.J.; Gao, J.X.; Gou, L.; Hunyadi, S.E.; Li, T. Anisotropic metal nanoparticles: Synthesis, assembly, and optical applications. J. Phys. Chem. B 2005, 109, 13857–13870. [Google Scholar] [CrossRef]
- González, A.L.; Noguez, C. Influence of Morphology on the Optical Properties of Metal Nanoparticles. J. Comput. Theor. Nanosci. 2007, 4, 231–238. [Google Scholar] [CrossRef]
- Penn, S.G.; He, L.; Natan, M.J. Nanoparticles for bioanalysis. Curr. Opin. Chem. Biol. 2003, 7, 609–615. [Google Scholar] [CrossRef]
- Haes, A.J.; Zou, S.L.; Schatz, G.C.; van Duyne, R.P. Nanoscale optical biosensor: Short range distance dependence of the localized surface plasmon resonance of noble metal nanoparticles. J. Phys. Chem. B 2004, 108, 6961–6968. [Google Scholar] [CrossRef]
- Gumbi, B.; Ngila, J.C.; Ndungu, P.G. Gold nanoparticles for the quantification of very Low levels of poly-diallyldimethylammonium chloride in river water. Anal. Methods 2014, 6, 6963–6972. [Google Scholar] [CrossRef]
- Magubane, S.E.; Ntlhoro, S.; Sabela, M.; Kanchi, S.; Mlambo, M.; Onwubu, S.C.; Mdluli, P.S.; Inamuddin; Asiri, A.M. Novel on-site residual screening of poly-diallyldimethylammonium chloride in treated potable water using gold nanoparticle based lovibond color filters. J. Taiwan Inst. Chem. Eng. 2019, 101, 159–166. [Google Scholar] [CrossRef]
- Capek, I. Noble Metal Nanoparticles: Preparation, Composite Nanostructures, Biodecoration and Collective Properties; Springer: Berlin, Germany, 2017; pp. 211–316. [Google Scholar]
- Pelras, T.; Mahon, C.S.; Nonappa, O.I.; Gröschel, A.H.; Müllner, M. Polymer nanowires with highly precise internal morphology and topography. JACS 2018, 140, 12736–12740. [Google Scholar] [CrossRef] [PubMed]
- Moulin, E.; Armao IV, J.J.; Guiseppone, N. Triarylamine-based supramolecular polymers: Structures, dynamics, and functions. Acc. Chem. Res. 2019, 52, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, H.; Cheng, M.; Li, C.; Faller, R.; Sun, S.; Hu, S. Controllable multigeometry nanoparticles via cooperative assembly by amphiphilic diblock copolymer blends with asymmetric architectures. ACS Nano 2018, 12, 1413–1419. [Google Scholar] [CrossRef]
- Smith, J.D.; Scanlan, M.M.; Chen, A.N.; Ashberry, H.M.; Skrabalak, S.E. Kinetically controlled sequential seeded growth: A general route to crystals with different hierarchies. ACS Nano 2020, 14, 15953–15961. [Google Scholar] [CrossRef] [PubMed]
- Lunn, D.J.; Finnegan, J.R.; Manners, I. Self-assembly of patchy nanoparticles: A versatile approach to functional hierarchical materials. Chem. Sci. 2015, 6, 3663–3673. [Google Scholar] [CrossRef]
- Maji, A.; Beg, M.; Das, S.; Sahoo, N.K.; Jha, P.K.; Islam, M.M.; Hossain, M. Binding interaction study on human serum albumin with bactericidal gold nanoparticles synthesized from a leaf extract of Musa balbisiana: Amultispectroscopic approach. Luminescence 2019, 34, 6. [Google Scholar] [CrossRef]
- Lengert, E.; Saveleva, M.; Abalymov, A.; Atkin, V.; Wuytens, P.C.; Kamyshinsky, R.; Vasiliev, A.L.; Gorin, D.A.; Sukhorukov, G.; Skirtach, A.; et al. Silver alginate hydrogel micro and nanocontainers for thernostics: Synthesis, encapsulation, remote release, and detection. ACS Appl. Mat. Interfaces 2017, 9, 21949–21958. [Google Scholar] [CrossRef]
- Brasili, F.; Capocefalo, A.; Palmieri, D.; Capitani, F.; Chiessi, E.; Paradossi, G.; Bordi, F.; Domenici, F. Assembling patchy plasmonic nanoparticles with aggregation-dependent antibacterial activity. J. Colloid Interface Sci. 2020, 580, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Cozzoli, P.D.; Manna, L. Synthetic Strategies to Size and Shape Controlled Nanocrystals and Nanocrystal Heterostructures. In Bio-Applications of Nanoparticles; Chan, W.C.W., Ed.; Springer: New York, NY, USA, 2007; pp. 1–17. [Google Scholar]
- Meyer, M.; Le Ru, E.C.; Etchegoin, P.G. Self-limiting aggregation leads to long-lived metastable clusters in colloidal solutions. J. Phys. Chem. B 2006, 110, 6040–6047. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.; Soler-Illia, G.; Ribot, F.; Lalot, T.; Mayer, C.R.; Cabuil, V. Designed hybrid organic-inorganic nanocomposites from functional nanobuilding blocks. Chem. Mat. 2001, 13, 3061–3083. [Google Scholar] [CrossRef]
- Cozzoli, P.D.; Pellegrino, T.; Manna, L. Synthesis, properties and perspectives of hybrid nanocrystal structures. Chem. Soc. Rev. 2006, 35, 1195–1208. [Google Scholar] [CrossRef]
- Zeng, C. Precision at the nanoscale: On the structure and property evolution of gold nanoclusters. Pure Appl. Chem. 2018, 90, 1409–1427. [Google Scholar] [CrossRef]
- Lofton, C.; Sigmund, W. Mechanisms controlling crystal habits of gold and silver colloids. Adv. Funct. Mater. 2005, 15, 1197–1208. [Google Scholar] [CrossRef]
- Koehler, J.M.; Visaveliya, N.; Knauer, A. Controlling formation and assembling of nanoparticles by control of electrical charging, polarization, and electrochemical potential. Nanotechnol. Rev. 2014, 3, 553–568. [Google Scholar] [CrossRef]
- Koehler, J.M.; Romanus, H.; Huebner, U.; Wagner, J. Formation of star-like and core-shell AuAg nanoparticles during two- and three-step preparation in batch and in microfluidic systems. J. Nanomater. 2007. [Google Scholar] [CrossRef]
- Koehler, J.M.; Kluitmann, J.; Knauer, A. Metal Nano Networks by Potential-Controlled In Situ Assembling of Gold/Silver Nanoparticles. ChemistryOpen 2019, 8, 1369–1374. [Google Scholar] [CrossRef]
- Visaveliya, N.; Knauer, A.; Yu, W.; Serra, C.A.; Koehler, J.M. Microflow-assisted assembling of multi-scale polymer particles by controlling surface properties and interactions. Eur. Polym. J. 2016, 80, 256–267. [Google Scholar] [CrossRef]
- Kohler, J.M.; Wagner, J.; Albert, J.; Mayer, G.; Hubner, U. Formation of gold nano-particles and nano-particle aggregates in static micromixers in the presence of bovine serum albumin (BSA). Chem. Ing. Tech. 2005, 77, 867–873. [Google Scholar]
- Visaveliya, N.; Knauer, A.; Köhler, J.M. Application of polyionic macromolecules in micro flow syntheses of nanoparticles. Macromol. Chem. Phys. 2017, 218, 1600371. [Google Scholar] [CrossRef]
- Wang, X.; Yang, D.-P.; Huang, P.; Li, M.; Li, C.; Chen, D.; Cui, D. Hierarchically assembled Au microspheres and sea urchin-like architectures: Formation mechanism and SERS study. Nanoscale 2012, 4, 7766–7772. [Google Scholar] [CrossRef] [PubMed]
- Höller, R.P.; Dulle, M.; Thomä, S.; Mayer, M.; Steiner, A.M.; Förster, S.; Fery, A.; Kuttner, C.; Chanana, M. Protein-assisted assembly of modular 3D plasmonic raspberry-like core/satellite nanoclusters: Correlation of structure and optical properties. ACS Nano 2016, 10, 5740–5750. [Google Scholar] [CrossRef] [PubMed]
- Köhler, J.M.; Wagner, J.; Albert, J. Formation of isolated and clustered Au nanoparticles in the presence of polyelectrolyte molecules using a flow-through Si chip reactor. J. Mater. Chem. 2005, 15, 1924–1930. [Google Scholar] [CrossRef]
- Köhler, J.M.; Wagner, J.; Albert, J.; Mayer, G.; Hübner, U. Bildung von Goldnanopartikeln und Nanopartikelaggregaten in statischen Mikromischern in Gegenwart von Rinderserumalbumin. Chem. Ing. Tech. 2005, 77, 867–873. [Google Scholar] [CrossRef]
- Aherne, D.; Ledwith, D.M.; Gara, M.; Kelly, J.M. Optical Properties and Growth Aspects of Silver Nanoprisms Produced by a Highly Reproducible and Rapid Synthesis at Room Temperature. Adv. Funct. Mater. 2008, 18, 2005–2016. [Google Scholar] [CrossRef]
- Knauer, A.; Visaveliya, N.; Koehler, J.M. Spontaneous transformation of polyelectrolyte-stabilized silver nanoprisms by interaction with thiocyanate. J. Colloid Interface Sci. 2013, 394, 78–84. [Google Scholar] [CrossRef]
- Jana, N.R.; Gearheart, L.; Murphy, C.J. Evidence for Seed-Mediated Nucleation in the Chemical Reduction of Gold Salts to Gold Nanoparticles. Chem. Mat. 2001, 13, 2313–2322. [Google Scholar] [CrossRef]
- Wagner, J.; Köhler, J.M. Continuous Synthesis of Gold Nanoparticles in a Microreactor. Nano Lett. 2005, 5, 685–691. [Google Scholar] [CrossRef]
- Senthil Kumar, P.; Pastoriza-Santos, I.; Rodriguez-Gonzalez, B.; de Abajo, G.; Javier, F.; Liz-Marzan, L.M. High-yield synthesis and optical response of gold nanostars. Nanotechnology 2008, 19, 015606. [Google Scholar] [CrossRef] [PubMed]
- Khoury, C.G.; Vo-Dinh, T. Gold Nanostars for Surface-Enhanced Raman Scattering: Synthesis, Characterization and Optimization. J. Phys. Chem. C 2008, 112, 18849–18859. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Qi, L. Surfactant-assisted, shape-controlled synthesis of gold nanocrystals. Nanoscale 2011, 3, 1383–1396. [Google Scholar] [CrossRef] [PubMed]
- Polte, J.; Ahner, T.T.; Delissen, F.; Sokolov, S.; Emmerling, F.; Thuenemann, A.F.; Kraehnert, R. Mechanism of Gold Nanoparticle Formation in the Classical Citrate Synthesis Method Derived from Coupled In Situ XANES and SAXS Evaluation. J. Am. Chem. Soc. 2010, 132, 1296–1301. [Google Scholar] [CrossRef]
- Viswanath, B.; Kundu, P.; HaIder, A.; Ravishankar, N. Mechanistic Aspects of Shape Selection and Symmetry Breaking during Nanostructure Growth by Wet Chemical Methods. J. Phys. Chem. C 2009, 113, 16866–16883. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Köhler, J.M.; Kluitmann, J. In Situ Assembly of Gold Nanoparticles in the Presence of Poly-DADMAC Resulting in Hierarchical and Highly Fractal Nanostructures. Appl. Sci. 2021, 11, 1191. https://doi.org/10.3390/app11031191
Köhler JM, Kluitmann J. In Situ Assembly of Gold Nanoparticles in the Presence of Poly-DADMAC Resulting in Hierarchical and Highly Fractal Nanostructures. Applied Sciences. 2021; 11(3):1191. https://doi.org/10.3390/app11031191
Chicago/Turabian StyleKöhler, J. Michael, and Jonas Kluitmann. 2021. "In Situ Assembly of Gold Nanoparticles in the Presence of Poly-DADMAC Resulting in Hierarchical and Highly Fractal Nanostructures" Applied Sciences 11, no. 3: 1191. https://doi.org/10.3390/app11031191
APA StyleKöhler, J. M., & Kluitmann, J. (2021). In Situ Assembly of Gold Nanoparticles in the Presence of Poly-DADMAC Resulting in Hierarchical and Highly Fractal Nanostructures. Applied Sciences, 11(3), 1191. https://doi.org/10.3390/app11031191






