Smart Fibrous Structures Produced by Electrospinning Using the Combined Effect of PCL/Graphene Nanoplatelets
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production of Electrospun PCL Membranes
2.2.1. Preparation and Optimization of the PCL Polymeric Solutions
2.2.2. Electrospinning Process
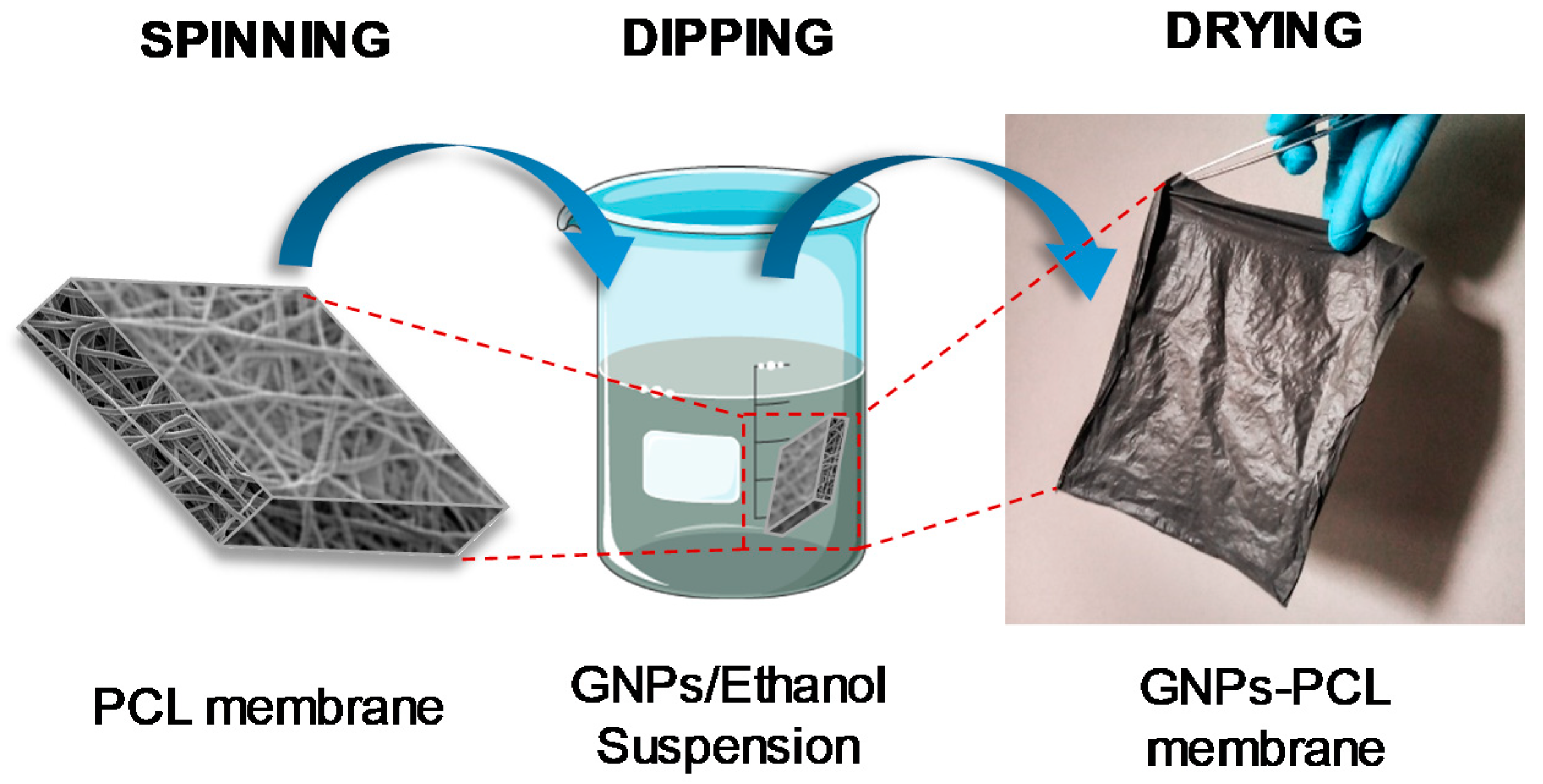
2.2.3. Preparation of the GNPs-PCL Membranes
2.3. GNPs-PCL Membranes Characterization
2.3.1. Field Emission Scanning Electron Microscopy (FESEM)
2.3.2. CIELAB Color Coordinates
2.3.3. Thermogravimetric Analysis (TGA)
2.3.4. Raman Spectroscopy
2.4. Functional Properties Evaluation
2.4.1. Electrical Conductivity
2.4.2. Piezoresistive Behavior
3. Results
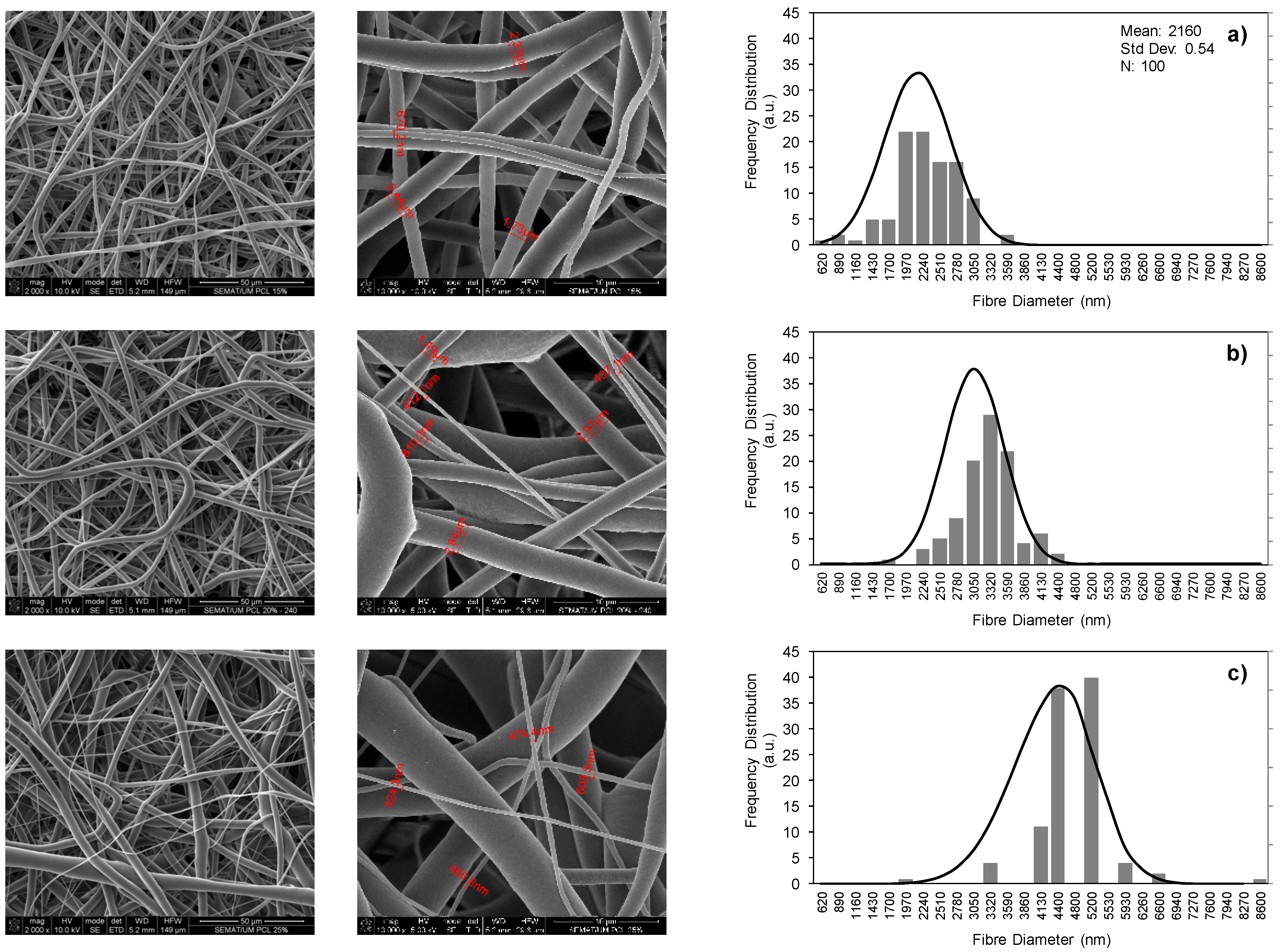
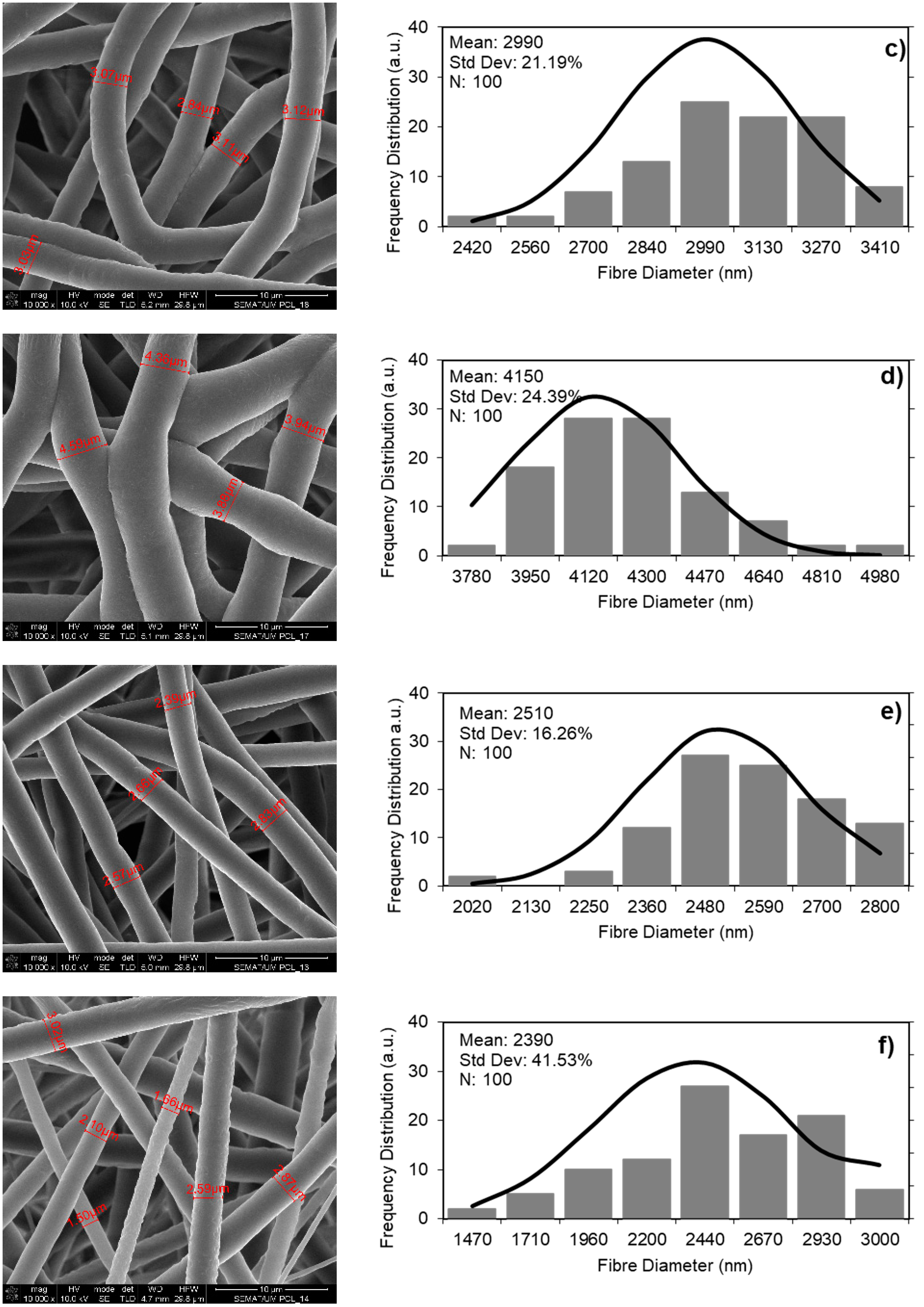
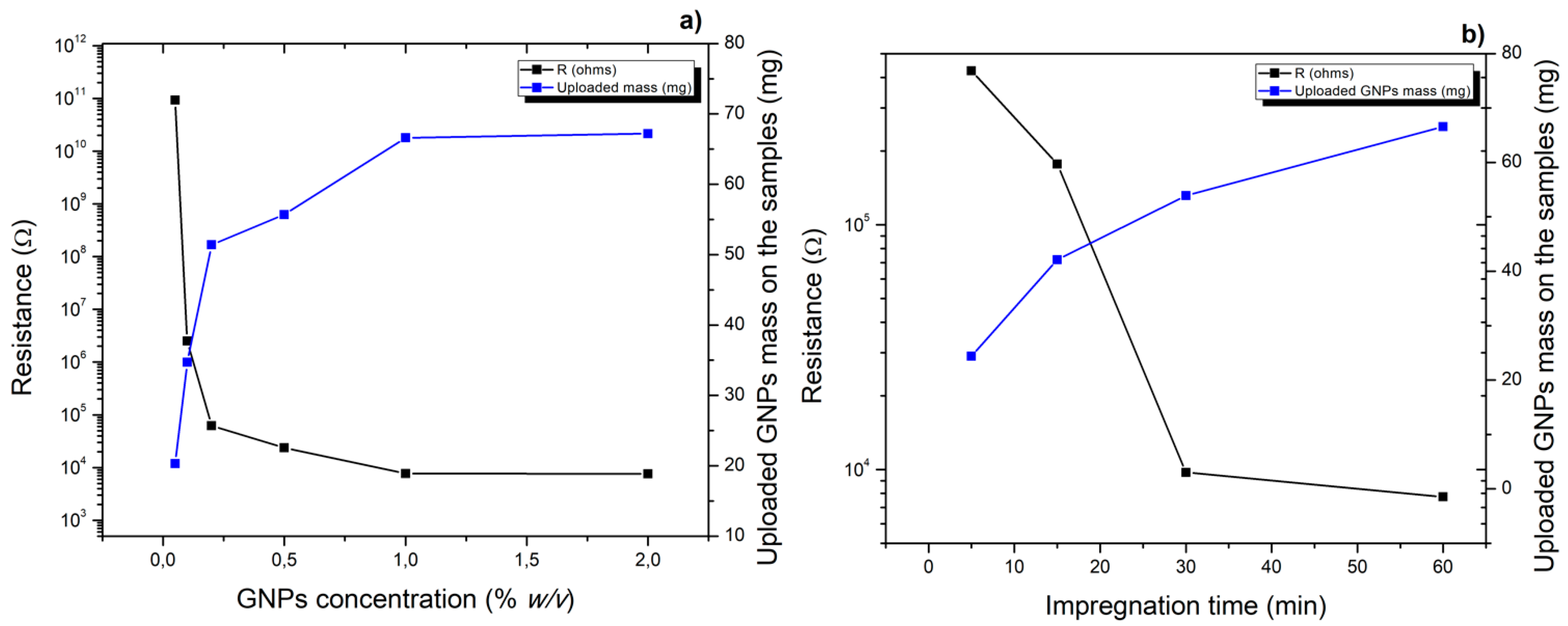
3.1. PCL Polymer Solution, Microfibers and GNPs Impregnation Optimization
3.2. Thermal Analysis
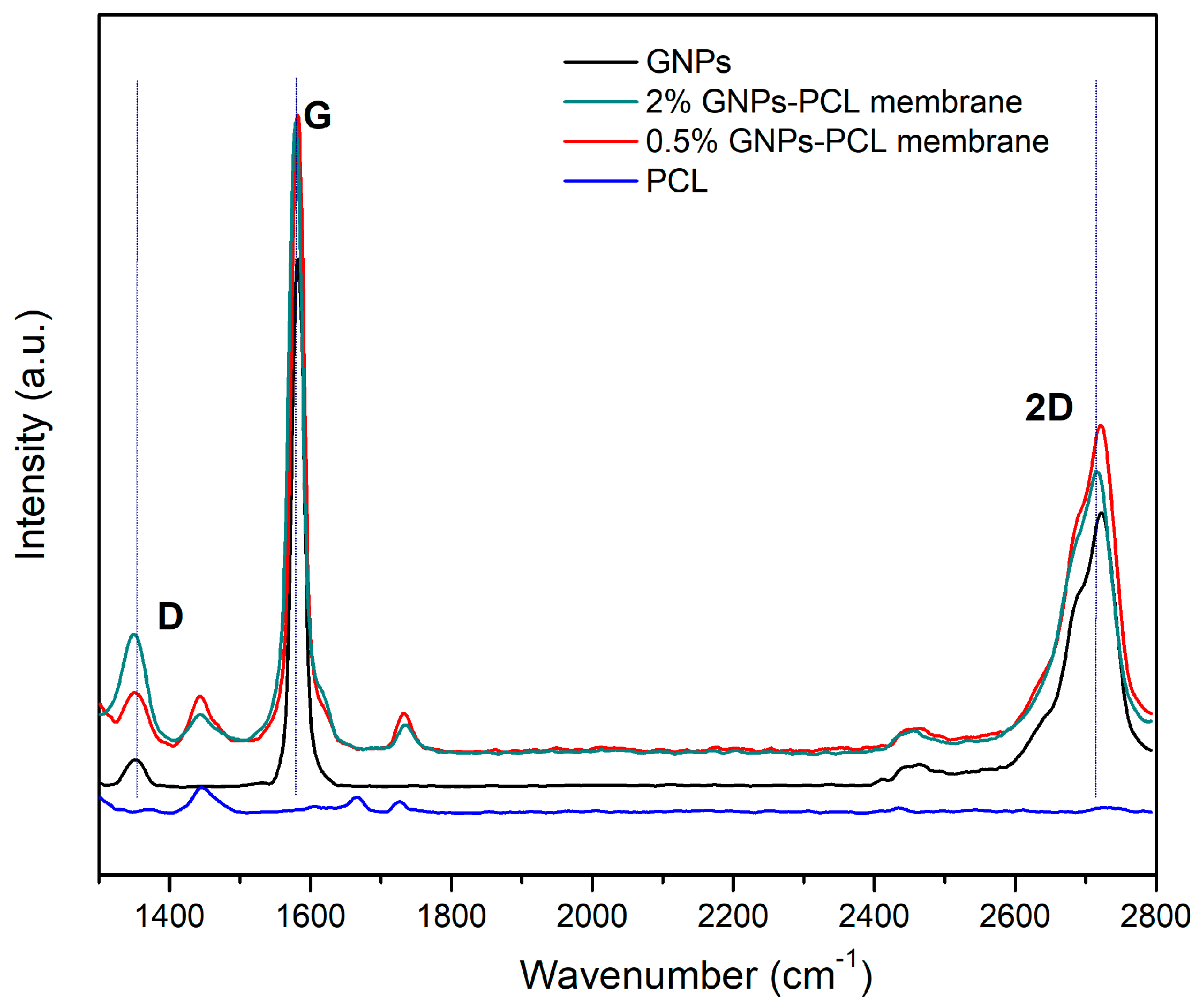
3.3. Raman Spectroscopy
3.4. Functional Properties Evaluation
3.4.1. Electrical Conductivity
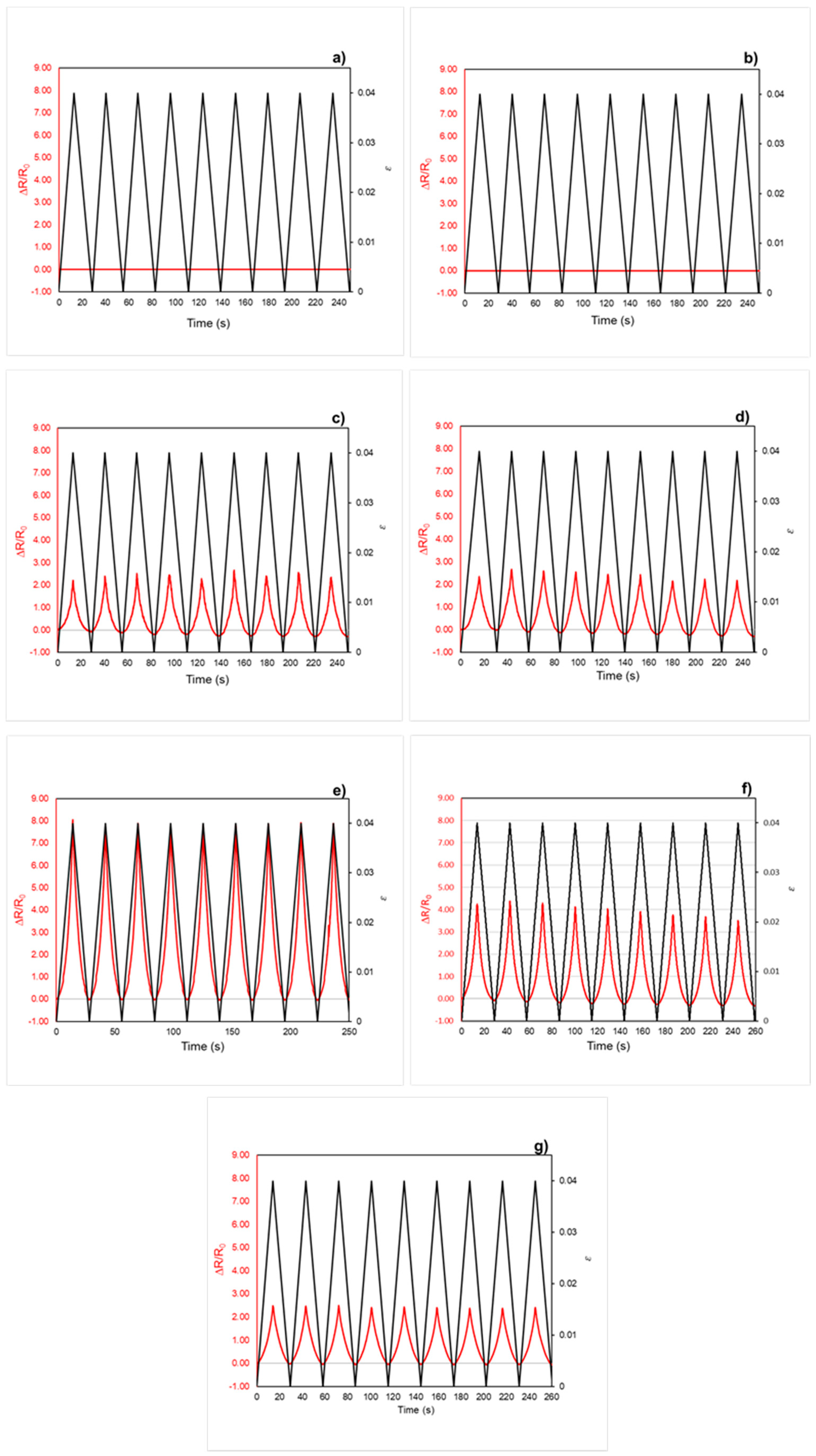
3.4.2. Piezoresistive Behavior
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferreira, D.P.; Costa, S.M.; Felgueiras, H.P.; Fangueiro, R. Smart and Sustainable Materials for Military Applications Based on Natural Fibres and Silver Nanoparticles. Key Eng. Mater. 2019, 812, 66–74. [Google Scholar] [CrossRef]
- Costa, J.C.; Spina, F.; Lugoda, P.; Garcia-Garcia, L.; Roggen, D.; Münzenrieder, N. Flexible Sensors—From Materials to Applications. Technologies 2019, 7, 35. [Google Scholar] [CrossRef]
- Shi, H.; Zhao, H.; Liu, Y.; Gao, W.; Dou, S.C. Systematic analysis of a military wearable device based on a multi-level fusion framework: Research directions. Sensors 2019, 19, 2651. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.M.; Ferreira, D.P.; Ferreira, A.; Vaz, F.; Fangueiro, R. Multifunctional Flax Fibres Based on the Combined Effect of Silver and Zinc Oxide (Ag/ZnO) Nanostructures. Nanomaterials 2018, 8, 1069. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Shu, L.; Li, Q.; Chen, S.; Wang, F.; Tao, X.-M. Fiber-Based Wearable Electronics: A Review of Materials, Fabrication, Devices, and Applications. Adv. Mater. 2014, 26, 5310–5336. [Google Scholar] [CrossRef]
- Pereira, P.; Ferreira, D.P.; Araújo, J.C.; Ferreira, A.; Fangueiro, R. The Potential of Graphene Nanoplatelets in the Development of Smart and Multifunctional Ecocomposites. Polymers 2020, 12, 2189. [Google Scholar] [CrossRef]
- Araújo, J.C.; Ferreira, D.P.; Teixeira, P.; Fangueiro, R. In-Situ synthesis of CaO and SiO2 nanoparticles onto jute fabrics: Exploring the multifunctionality. Cellulose 2020. [Google Scholar] [CrossRef]
- Muñoz, V.; Buffa, F.; Molinari, F.; Hermida, L.G.; García, J.J.; Abraham, G.A. Electrospun ethylcellulose-based nanofibrous mats with insect-repellent activity. Mater. Lett. 2019, 253, 289–292. [Google Scholar] [CrossRef]
- Ojha, S. Structure-Property Relationship of Electrospun Fibers. In Electrospun Nanofibers; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; ISBN 9780081009116. [Google Scholar]
- Wang, X.; Zhao, H.; Turng, L.S.; Li, Q. Crystalline morphology of electrospun poly(ε-caprolactone) (PCL) nanofibers. Ind. Eng. Chem. Res. 2013, 52, 4939–4949. [Google Scholar] [CrossRef]
- Mochane, M.J.; Motsoeneng, T.S.; Sadiku, E.R.; Mokhena, T.C.; Sefadi, J.S. Morphology and properties of electrospun PCL and its composites for medical applications: A mini review. Appl. Sci. 2019, 9, 2205. [Google Scholar] [CrossRef]
- Yew, C.H.T.; Azari, P.; Choi, J.R.; Muhamad, F.; Pingguan-Murphy, B. Electrospun polycaprolactone nanofibers as a reaction membrane for lateral flow assay. Polymers 2018, 10, 1387. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Jasper, S.; Zhang, X. Chemical Characterization of Electrospun Nanofibers. In Electrospun Nanofibers; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; ISBN 9780081009116. [Google Scholar]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Li, B.; Xu, L.; Qiao, Y.; Wang, F.; Xia, Q.; Lu, Z. A sandwich-structured piezoresistive sensor with electrospun nanofiber mats as supporting, sensing, and packaging layers. Polymers 2018, 10, 575. [Google Scholar] [CrossRef] [PubMed]
- Fiorillo, A.S.; Critello, C.D.; Pullano, A.S. Theory, technology and applications of piezoresistive sensors: A review. Sensors Actuators A Phys. 2018, 281, 156–175. [Google Scholar] [CrossRef]
- Flynn, G. Atomic Scale Imaging of the Electronic Structure and Chemistry of Graphene and its Precursors on Metal Surfaces. Final Tech. Rep. Submitt. Dep. Energy 2014, 1, 1–13. [Google Scholar]
- Cataldi, P.; Athanassiou, A.; Bayer, I.S. Graphene Nanoplatelets-Based Advanced Materials and Recent Progress in Sustainable Applications. Appl. Sci. 2018, 8, 1438. [Google Scholar] [CrossRef]
- Bahiraei, M.; Heshmatian, S. Graphene family nanofluids: A critical review and future research directions. Energy Convers. Manag. 2019, 196, 1222–1256. [Google Scholar] [CrossRef]
- Milovanović, S.P.; Peeters, F.M. Strained graphene structures: From valleytronics to pressure sensing. NATO Sci. Peace Secur. Ser. A Chem. Biol. 2018, 3–17. [Google Scholar] [CrossRef]
- Baloda, S.; Ansari, Z.A.; Singh, S.; Gupta, N. Development and Analysis of Graphene Nanoplatelets (GNP) Based Flexible Strain Sensor for Health Monitoring Applications. IEEE Sens. J. 2020. [Google Scholar] [CrossRef]
- Sabzi, M.; Jiang, L.; Liu, F.; Ghasemi, I.; Atai, M. Graphene nanoplatelets as poly(lactic acid) modifier: Linear rheological behavior and electrical conductivity. J. Mater. Chem. A 2013, 1, 8253–8261. [Google Scholar] [CrossRef]
- Lu, S.; Tian, C.; Wang, X.; Zhang, L.; Du, K.; Ma, K.; Xu, T. Strain sensing behaviors of GnPs/epoxy sensor and health monitoring for composite materials under monotonic tensile and cyclic deformation. Compos. Sci. Technol. 2018, 158, 94–100. [Google Scholar] [CrossRef]
- Moriche, R.; Jiménez-Suárez, A.; Sánchez, M.; Prolongo, S.G.; Ureña, A. High sensitive damage sensors based on the use of functionalized graphene nanoplatelets coated fabrics as reinforcement in multiscale composite materials. Compos. Part B Eng. 2018, 149, 31–37. [Google Scholar] [CrossRef]
- Souri, H.; Bhattacharyya, D. Wearable strain sensors based on electrically conductive natural fiber yarns. Mater. Des. 2018, 154, 217–227. [Google Scholar] [CrossRef]
- Sagitha, P.; Reshmi, C.R.; Sundaran, S.P.; Sujith, A. Recent advances in post-modification strategies of polymeric electrospun membranes. Eur. Polym. J. 2018, 105, 227–249. [Google Scholar] [CrossRef]
- Ekram, B.; Abdel-Hady, B.M.; El-Kady, A.M.; Amr, S.M.; Waley, A.I.; Guirguis, O.W. Optimum parameters for the production of nano-scale electrospun polycaprolactone to be used as a biomedical material. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8. [Google Scholar] [CrossRef]
- Guarino, V.; Gentile, G.; Sorrentino, L.; Ambrosio, L. Polycaprolactone: Synthesis, Properties, and Applications. In Encyclopedia of Polymer Science and Technology, 4th ed.; Mark, H., Ed.; John Wiley & Sons: New Jersey, NJ, USA, 2017; ISBN 0471440264. [Google Scholar]
- Safarova, V.; Gregr, J. Electrical Conductivity Measurement of Fibers and Yarns. In Proceedings of the 7th International Conference, TEXSCI, Liberec, Czech Republic, 6–8 September 2010; pp. 2–9. [Google Scholar]
- Singh, Y. Electrical Resistivity Measurements: A Review. Int. J. Mod. Phys. Conf. Ser. 2013, 22, 745–756. [Google Scholar] [CrossRef]
- Fotheringham, S.; Wgener, M.; Longley, P.; Goodchild, M.; Maguire, D. Lecture 9: Piezoresistivity. Univ. Victoria Dept Mech. Eng. 2019, 466, 1–13. [Google Scholar]
- Mondal, S. Review on Nanocellulose Polymer Nanocomposites. Polym. Plast. Technol. Eng. 2018, 57, 1377–1391. [Google Scholar] [CrossRef]
- Rong, D.; Chen, P.; Yang, Y.; Li, Q.; Wan, W.; Fang, X.; Zhang, J.; Han, Z.; Tian, J.; Ouyang, J. Fabrication of Gelatin/PCL Electrospun Fiber Mat with Bone Powder and the Study of Its Biocompatibility. J. Funct. Biomater. 2016, 7, 6. [Google Scholar] [CrossRef]
- Bellani, C.F.; Pollet, E.; Hebraud, A.; Pereira, F.V.; Schlatter, G.; Avérous, L.; Bretas, R.E.S.; Branciforti, M.C. Morphological, thermal, and mechanical properties of poly(ε-caprolactone)/poly(ε-caprolactone)-grafted-cellulose nanocrystals mats produced by electrospinning. J. Appl. Polym. Sci. 2016, 133, 4–11. [Google Scholar] [CrossRef]
- Croisier, F.; Duwez, A.S.; Jérôme, C.; Léonard, A.F.; Van Der Werf, K.O.; Dijkstra, P.J.; Bennink, M.L. Mechanical testing of electrospun PCL fibers. Acta Biomater. 2012, 8, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Roso, M.; Sundarrajan, S.; Pliszka, D.; Ramakrishna, S.; Modesti, M. Multifunctional membranes based on spinning technologies: The synergy of nanofibers and nanoparticles. Nanotechnology 2008, 19. [Google Scholar] [CrossRef] [PubMed]
- Metwally, S.; Ferraris, S.; Spriano, S.; Krysiak, Z.J.; Kaniuk, Ł.; Marzec, M.M.; Kim, S.K.; Szewczyk, P.K.; Gruszczyński, A.; Wytrwal-Sarna, M.; et al. Surface potential and roughness controlled cell adhesion and collagen formation in electrospun PCL fibers for bone regeneration. Mater. Des. 2020, 194. [Google Scholar] [CrossRef]
- Wang, B.; Li, H.; Li, L.; Chen, P.; Wang, Z.; Gu, Q. Electrostatic adsorption method for preparing electrically conducting ultrahigh molecular weight polyethylene/graphene nanosheets composites with a segregated network. Compos. Sci. Technol. 2013, 89, 180–185. [Google Scholar] [CrossRef]
- Vogel, C.; Siesler, H.W. Thermal degradation of poly(ε-caprolactone), poly(L-lactic acid) and their blends with poly(3-hydroxy-butyrate) studied by TGA/FT-IR spectroscopy. Macromol. Symp. 2008, 265, 183–194. [Google Scholar] [CrossRef]
- Gao, R.; Hu, N.; Yang, Z.; Zhu, Q.; Chai, J.; Su, Y.; Zhang, L.; Zhang, Y. Paper-like graphene-Ag composite films with enhanced mechanical and electrical properties. Nanoscale Res. Lett. 2013, 8, 32. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, M.; Kumar, A.; Singh, R.; Kashyap, R.; Rani, S.; Kumar, D. Surface modification of Graphene Oxide using Esterification. Mater. Today Proc. 2019, 18, 1556–1561. [Google Scholar] [CrossRef]
- Hu, N.; Gao, R.; Wang, Y.; Wang, Y.; Chai, J.; Yang, Z.; Kong, E.S.-W.; Zhang, Y. The preparation and characterization of non-covalently functionalized graphene. J. Nanosci. Nanotechnol. 2012, 12, 99–104. [Google Scholar] [CrossRef]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron-phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Munir, K.S.; Qian, M.; Li, Y.; Oldfield, D.T.; Kingshott, P.; Zhu, D.M.; Wen, C. Quantitative Analyses of MWCNT-Ti Powder Mixtures using Raman Spectroscopy: The Influence of Milling Parameters on Nanostructural Evolution. Adv. Eng. Mater. 2015, 17, 1660–1669. [Google Scholar] [CrossRef]
- Sadasivuni, K.K.; Ponnamma, D.; Kim, J.; Thomas, S. Electrical Properties of Graphene Polymer Nanocomposites. In Graphene-Based Polymer Nanocomposites in Electronics; Springer: Cham, Switzerland, 2015; pp. 25–47. [Google Scholar] [CrossRef]







| PCL | 0.05% GNPs-PCL | 0.1% GNPs-PCL | 0.2% GNPs-PCL | 0.5% GNPs-PCL | 1% GNPs-PCL | 2% GNPs-PCL | |
|---|---|---|---|---|---|---|---|
| Lightness Parameter | L* | L* | L* | L* | L* | L* | L* |
| Point 1 | 68.34 | 55.32 | 48.29 | 44.19 | 42.34 | 40.25 | 39.68 |
| Point 2 | 67.69 | 55.57 | 48.93 | 44.41 | 41.35 | 40.55 | 39.49 |
| Point 3 | 66.84 | 56.30 | 47.66 | 43.49 | 41.06 | 40.03 | 38.52 |
| Point 4 | 67.81 | 54.38 | 49.42 | 42.79 | 41.91 | 39.76 | 38.63 |
| Point 5 | 69.06 | 56.14 | 48.83 | 43.47 | 40.81 | 40.57 | 39.00 |
| Mean | 67.95 | 55.54 | 48.63 | 43.67 | 41.50 | 40.23 | 39.06 |
| Standard deviation | 0.8 | 0.8 | 0.7 | 0.6 | 0.6 | 0.3 | 0.5 |
| Electrical Conductivity (S/m) | ||||||
|---|---|---|---|---|---|---|
| Sample | Water Treatment Cycle (0) | Water Treatment Cycle (1) | Water Treatment Cycle (2) | Water Treatment Cycle (3) | Water Treatment Cycle (4) | Water Treatment Cycle (5) |
| 0.05%GNPs-PCL | 6.00 × 10−9 | 6.67 × 10−9 | 6.15 × 10−9 | 1.00 × 10−8 | 1.06 × 10−8 | 6.43 × 10−9 |
| 0.1%GNPs-PCL | 5.75 × 10−2 | 1.17 × 10−2 | 1.05 × 10−2 | 3.75 × 10−4 | 1.85 × 10−4 | 2.40 × 10−4 |
| 0.2%GNPs-PCL | 0.29 | 0.24 | 0.21 | 0.02 | 0.01 | 0.010 |
| 0.5%GNPs-PCL | 1.61 | 0.21 | 0.09 | 0.03 | 0.03 | 0.025 |
| 1%GNPs-PCL | 3.01 | 0.56 | 0.43 | 0.19 | 0.13 | 0.078 |
| 2%GNPs-PCL | 3.17 | 0.68 | 0.74 | 0.25 | 0.11 | 0.08 |
| Sample | GF | Error |
|---|---|---|
| 0.1%GNPs-PCL | 0.74 | 0.01 |
| 0.2%GNPs-PCL | 1.17 | 0.02 |
| 0.5%GNPs-PCL | 3.20 | 0.03 |
| 1%GNPs-PCL | 1.29 | 0.02 |
| 2%GNPs-PCL | 1.21 | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Francavilla, P.; Ferreira, D.P.; Araújo, J.C.; Fangueiro, R. Smart Fibrous Structures Produced by Electrospinning Using the Combined Effect of PCL/Graphene Nanoplatelets. Appl. Sci. 2021, 11, 1124. https://doi.org/10.3390/app11031124
Francavilla P, Ferreira DP, Araújo JC, Fangueiro R. Smart Fibrous Structures Produced by Electrospinning Using the Combined Effect of PCL/Graphene Nanoplatelets. Applied Sciences. 2021; 11(3):1124. https://doi.org/10.3390/app11031124
Chicago/Turabian StyleFrancavilla, Paola, Diana P. Ferreira, Joana C. Araújo, and Raul Fangueiro. 2021. "Smart Fibrous Structures Produced by Electrospinning Using the Combined Effect of PCL/Graphene Nanoplatelets" Applied Sciences 11, no. 3: 1124. https://doi.org/10.3390/app11031124
APA StyleFrancavilla, P., Ferreira, D. P., Araújo, J. C., & Fangueiro, R. (2021). Smart Fibrous Structures Produced by Electrospinning Using the Combined Effect of PCL/Graphene Nanoplatelets. Applied Sciences, 11(3), 1124. https://doi.org/10.3390/app11031124








