Polylactic Acid/Carbon Nanoparticle Composite Filaments for Sensing
Abstract
Featured Application
Abstract
1. Introduction
| Plasticizer | Filler | Processing Method | Reference |
|---|---|---|---|
| Tributyl 2-acetylcitrate | Bi0·5Sb1·5Te3 MWCNT | Melt extrusion | [17] |
| PEG (MW 200 g·mol−1) | Reduced graphene oxide | Batch mixing | [18] |
| Epoxidized soybean oil | MWCNT | Solvent casting | [14] |
| PEG (MW 950–1050 g·mol−1) | Acid treated MWCNT | Two-roll mill | [19] |
| PEG (MW 200 g·mol−1) | Graphene nanoplatelets | Batch mixing and compression molding | [20] |
| PEG (MW 2000 g·mol−1) | SWCNT | Melt extrusion and compression molding | [15] |
| Triacetin | Flax fiber | Melt extrusion | [21] |
| Poly(butylene adipate-co-terephthalate) | Rami fiber | Melt extrusion and compression molding | [22] |
| Epoxidized linseed oil | Hazelnut shell flour | Melt extrusion | [16] |
| PEG (MW 6000 g·mol−1) | CaCO3 Halloysite nanotubes LAK 301 Micro-sized talc | Melt extrusion | [23] |
| Glycerol | Bamboo (different forms) | Batch mixing | [24] |
| PEG (MW 1500 g·mol−1) | ZnO Clove essential oil | Solvent cast | [25] |
| Acetyl triethyl citrate | Montmorillonite layered silicate | Melt extrusion | [26] |
| Diglycerine tetraacetate and PEG (MW 1000 g·mol−1) | Sodium montmorillonite and organoclays | Milling and injection molding | [27] |
| PEG (MW 6000 g·mol−1) | Montmorillonite | Melt extrusion | [28] |
| PEG (MW 1000 g·mol−1) | Montmorillonite | Batch mixing and compression molding | [29] |
| PEG (MW 6000 g·mol−1) | Organoclay | Melt extrusion and microinjection molding | [30] |
| PEG (MW 400 g·mol−1) and Tributyl Citrate | Nanosized CaCO3 | Melt extrusion | [31] |
| Glycerol | Halloysite nanoclay | Solvent casting | [32] |
| Methoxy polyethylene glycol | Silica | Batch mixing | [33] |
2. Materials and Methods
2.1. MWCNT Dispersion in PPG
2.2. Coating of PLA Pellets
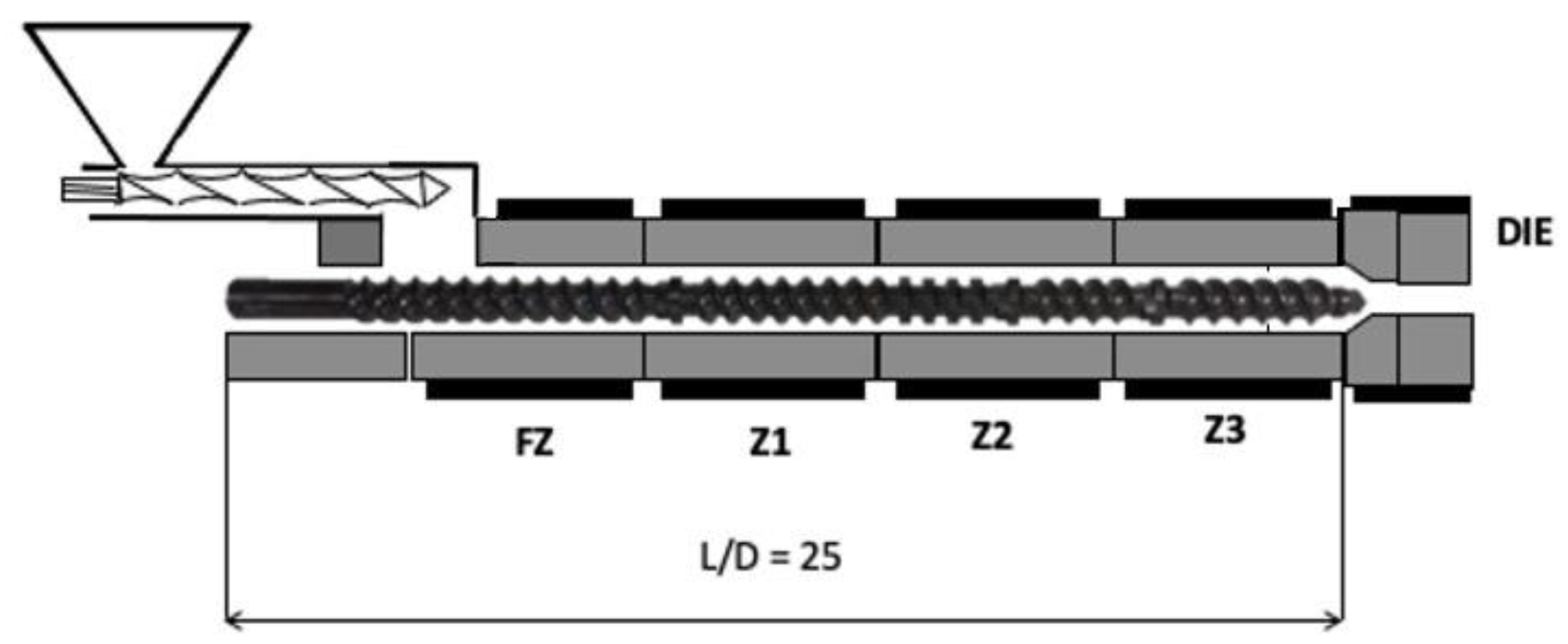
2.3. Melt Extrusion of PLA/MWCNT/PPG Composites
2.4. Characterization of PLA Composite Filaments
2.4.1. Optical Microscopy
2.4.2. Scanning Electron Microscopy
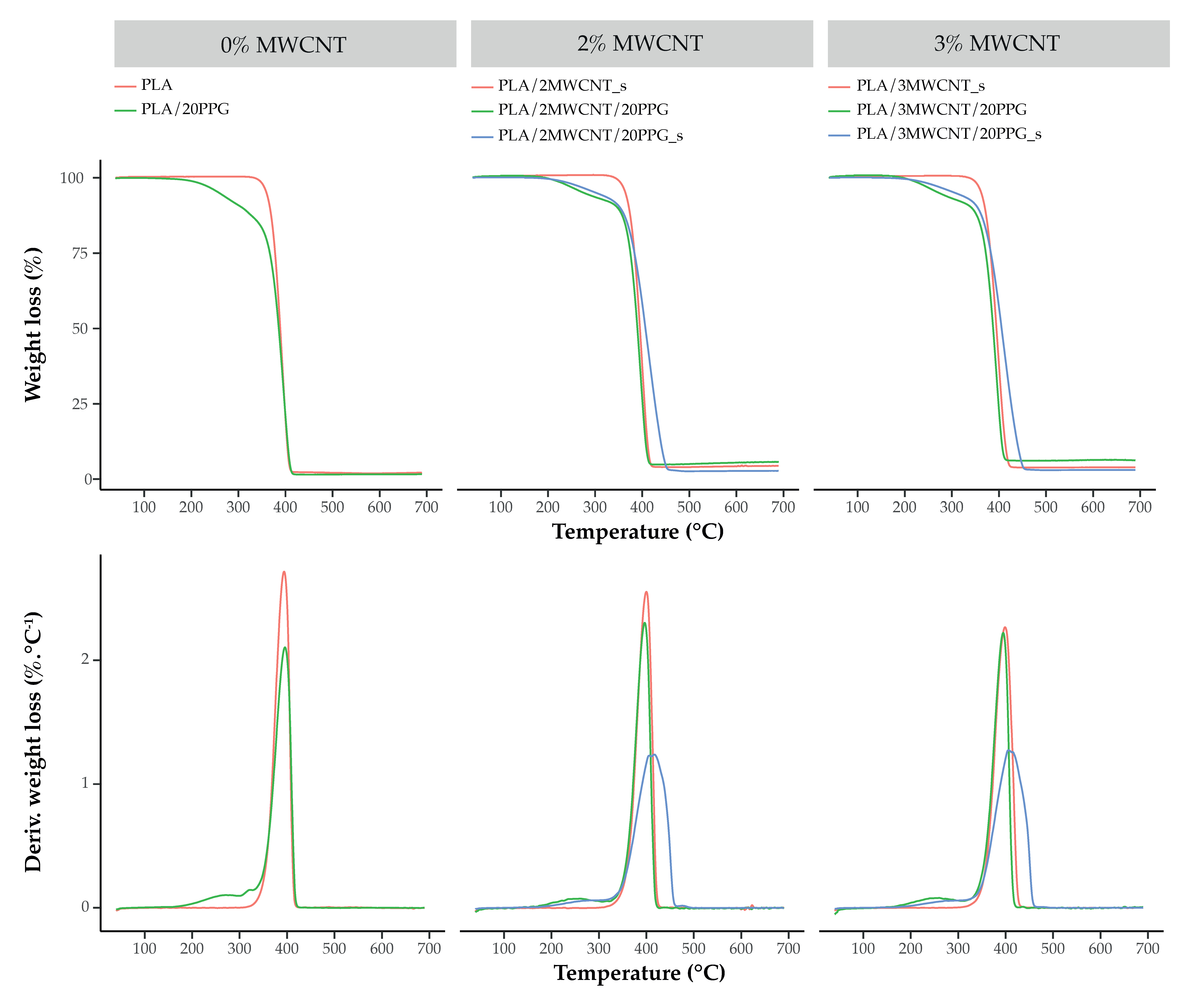
2.4.3. Thermogravimetric Analysis
2.4.4. Differential Scanning Calorimetry
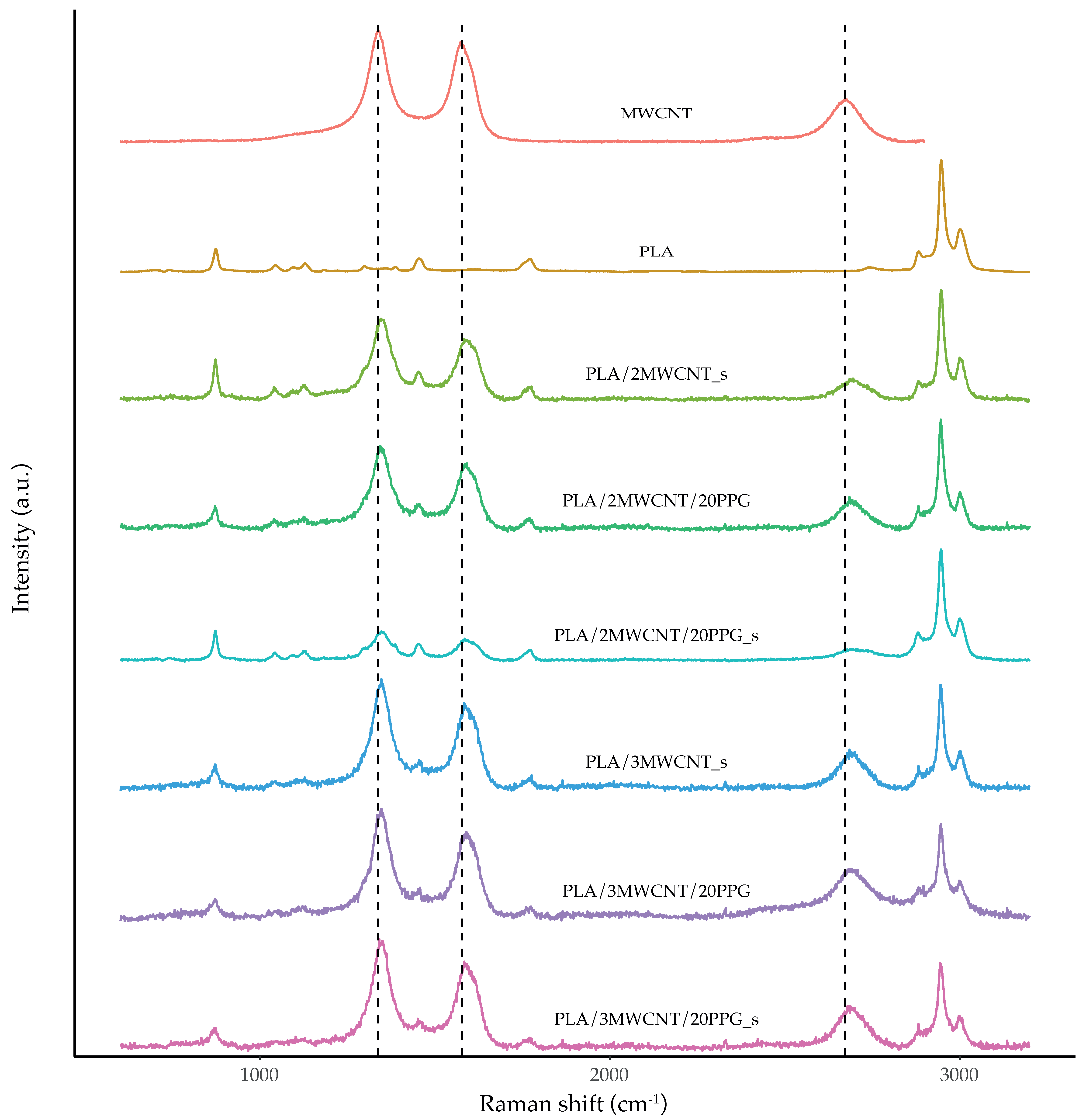
2.4.5. Raman Spectroscopy
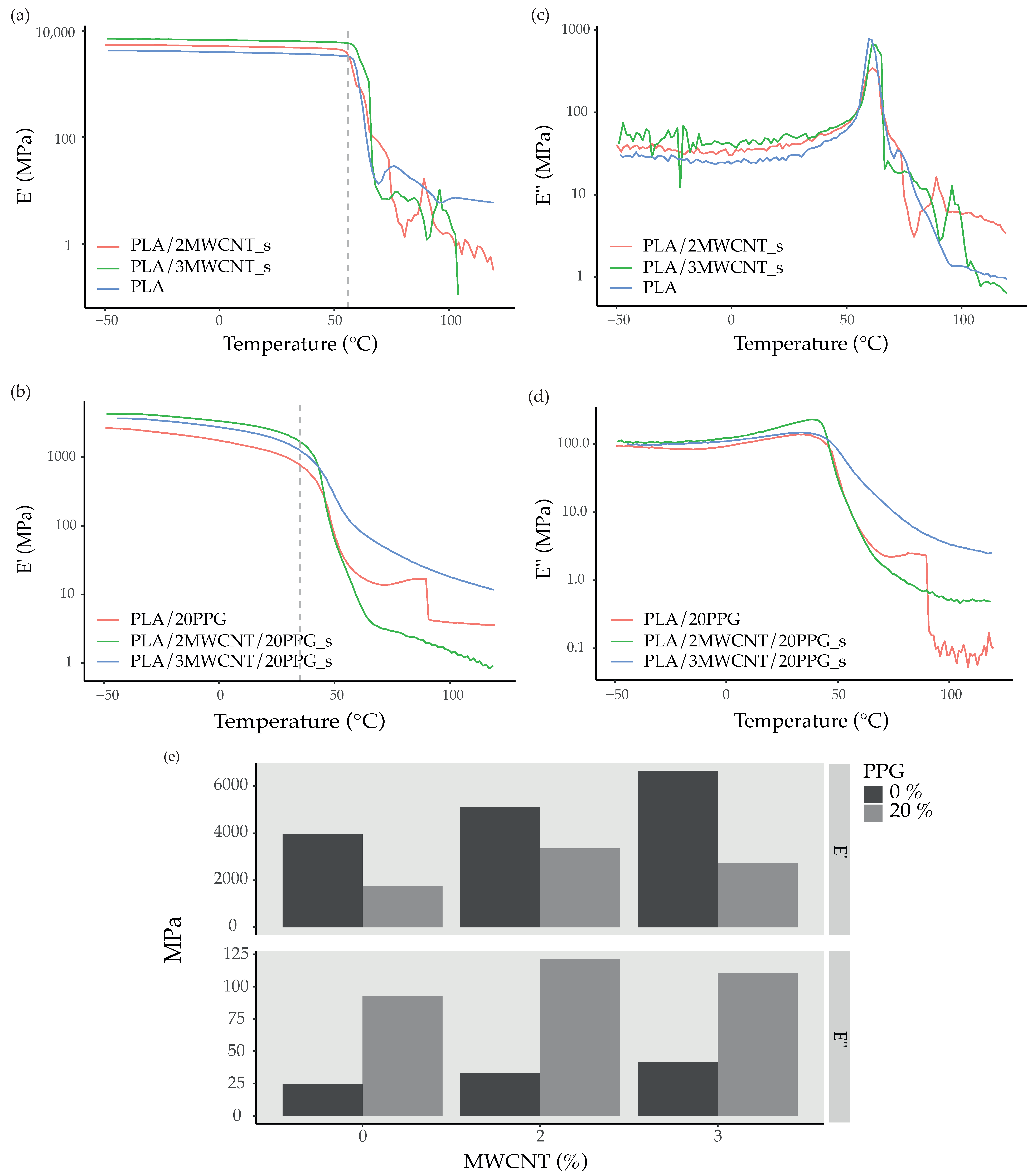
2.4.6. Dynamic Mechanical Analysis
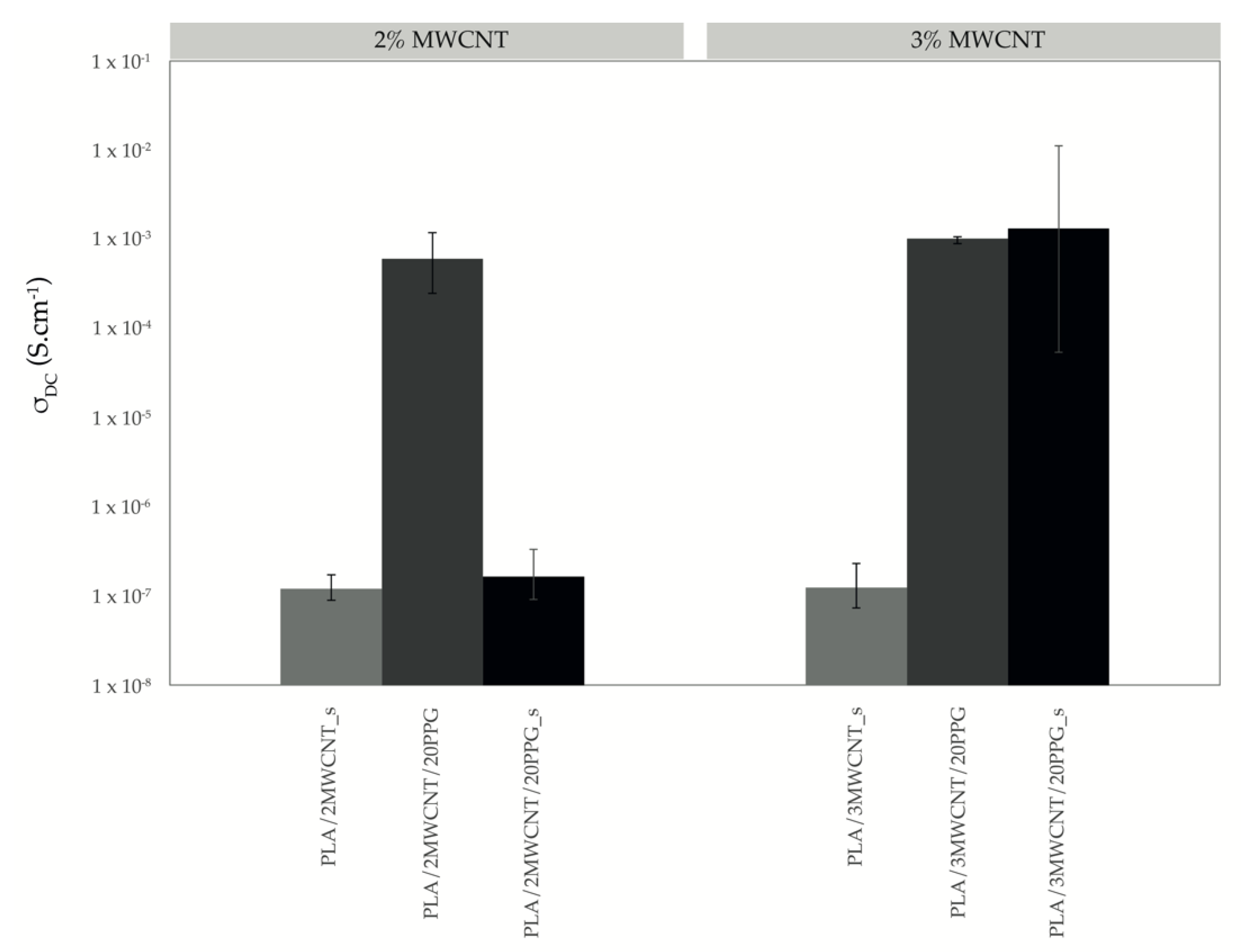
2.4.7. Resistivity Measurements
2.4.8. Vapor Sensing Measurements
2.5. Statistical Analysis and Data Visualization
3. Results
3.1. Morphology of Melt-Extruded Filaments
3.2. Raman Spectroscopy
3.3. Thermal Characterization
3.4. Dynamic Mechanical Properties
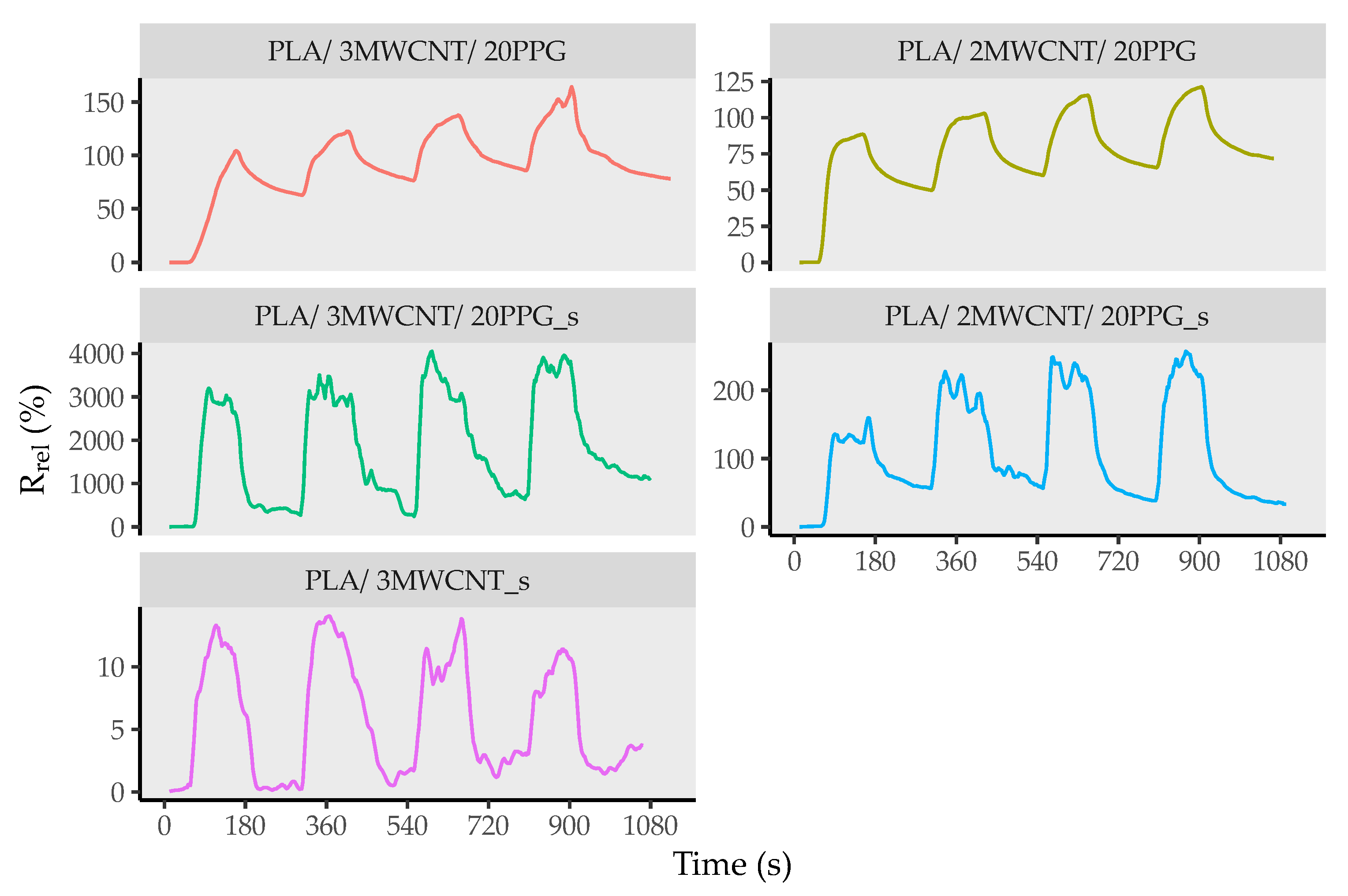
3.5. Conductivity and Sensing Ability of Composite Filaments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gupta, B.; Revagade, N.; Hilborn, J. Poly(lactic acid) fiber: An overview. Progress Polym. Sci. 2007, 32, 455–482. [Google Scholar] [CrossRef]
- Castro-Aguirre, E.; Iniguez-Franco, F.; Samsudin, H.; Fang, X.; Auras, R. Poly(lactic acid)-Mass production, processing, industrial applications, and end of life. Adv. Drug Deliv. Rev. 2016, 107, 333–366. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Son, D.; Kim, J.; Lee, Y.B.; Song, J.K.; Choi, S.; Lee, D.J.; Kim, J.H.; Lee, M.; Hyeon, T.; et al. Transparent and Stretchable Interactive Human Machine Interface Based on Patterned Graphene Heterostructures. Adv. Funct. Mater. 2015, 25, 375–383. [Google Scholar] [CrossRef]
- Reyes, C.; Somogyi, R.; Niu, S.; Cruz, M.A.; Yang, F.C.; Catenacci, M.J.; Rhodes, C.P.; Wiley, B.J. Three-Dimensional Printing of a Complete Lithium Ion Battery with Fused Filament Fabrication. ACS Appl. Energy Mater. 2018, 1, 5268–5279. [Google Scholar] [CrossRef]
- Guo, Y.; Zhong, M.J.; Fang, Z.W.; Wan, P.B.; Yu, G.H. A Wearable Transient Pressure Sensor Made with MXene Nanosheets for Sensitive Broad-Range Human-Machine Interfacing. Nano Lett. 2019, 19, 1143–1150. [Google Scholar] [CrossRef]
- De Falco, F.; Guarino, V.; Gentile, G.; Cocca, M.; Ambrogi, V.; Ambrosio, L.; Avella, M. Design of functional textile coatings via non-conventional electrofluidodynamic processes. J. Colloid Interface Sci. 2019, 541, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.C.; Li, B.T.; Xu, L.Q.; Qiao, Y.; Wang, F.; Xia, Q.Y.; Lu, Z.S. A Sandwich-Structured Piezoresistive Sensor with Electrospun Nanofiber Mats as Supporting, Sensing, and Packaging Layers. Polymers 2018, 10, 575. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.S.; Schreck, K.M.; Hillmyer, M.A. Toughening polylactide. Polym. Rev. 2008, 48, 85–108. [Google Scholar] [CrossRef]
- Goncalves, C.; Goncalves, I.C.; Magalhaes, F.D.; Pinto, A.M. Poly(lactic acid) Composites Containing Carbon-Based Nanomaterials: A Review. Polymers 2017, 9, 269. [Google Scholar] [CrossRef]
- Mascia, L.; Xanthos, M. An overview of additives and modifiers for polymer blends: Facts, deductions, and uncertainties. Adv. Polym. Technol. J. Polym. Process. Inst. 1992, 11, 237–248. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef]
- Kulinski, Z.; Piorkowska, E.; Gadzinowska, K.; Stasiak, M. Plasticization of poly(L-lactide) with poly(propylene glycol). Biomacromolecules 2006, 7, 2128–2135. [Google Scholar] [CrossRef] [PubMed]
- Piorkowska, E.; Kulinski, Z.; Galeski, A.; Masirek, R. Plasticization of semicrystalline poly(L-lactide) with poly(propylene glycol). Polymer 2006, 47, 7178–7188. [Google Scholar] [CrossRef]
- Mohan, R.; Subha, J.; Alam, J. Influence of Multiwalled Carbon Nanotubes on Biodegradable Poly(lactic acid) Nanocomposites for Electroactive Shape Memory Actuator. Adv. Polym. Technol. 2018, 37. [Google Scholar] [CrossRef]
- Sun, Y.C.; Chu, M.; Huang, M.; Hegazi, O.; Naguib, H.E. Hybrid Electroactive Shape Memory Polymer Composites with Room Temperature Deformability. Macromol. Mater. Eng. 2019, 304. [Google Scholar] [CrossRef]
- Balart, J.F.; Fombuena, V.; Fenollar, O.; Boronat, T.; Sanchez-Nacher, L. Processing and characterization of high environmental efficiency composites based on PLA and hazelnut shell flour (HSF) with biobased plasticizers derived from epoxidized linseed oil (ELO). Compos. Part B Eng. 2016, 86, 168–177. [Google Scholar] [CrossRef]
- Wang, J.Z.; Li, H.Z.; Liu, R.X.; Li, L.L.; Lin, Y.H.; Nan, C.W. Thermoelectric and mechanical properties of PLA/Bi0.5Sb1.5Te3 composite wires used for 3D printing. Compos. Sci. Technol. 2018, 157, 1–9. [Google Scholar] [CrossRef]
- Ahmad, A.F.; Ab Aziz, S.; Abbas, Z.; Obaiys, S.J.; Matori, K.A.; Zaid, M.H.M.; Raad, H.K.; Aliyu, U.S. Chemically Reduced Graphene Oxide-Reinforced Poly(Lactic Acid)/Poly(Ethylene Glycol) Nanocomposites: Preparation, Characterization, and Applications in Electromagnetic Interference Shielding. Polymers 2019, 11, 661. [Google Scholar] [CrossRef] [PubMed]
- Norazlina, H.; Hadi, A.A.; Qurni, A.U.; Amri, M.; Mashelmie, S.; Kamal, Y. Effects of multi-walled carbon nanotubes (MWCNTs) on the degradation behavior of plasticized PLA nanocomposites. Polym. Bull. 2019, 76, 1453–1469. [Google Scholar] [CrossRef]
- Chieng, B.W.; Ibrahim, N.A.; Yunus, W.; Hussein, M.Z. Poly(lactic acid)/Poly(ethylene glycol) Polymer Nanocomposites: Effects of Graphene Nanoplatelets. Polymers 2014, 6, 93–104. [Google Scholar] [CrossRef]
- Oksman, K.; Skrifvars, M.; Selin, J.F. Natural fibres as reinforcement in polylactic acid (PLA) composites. Compos. Sci. Technol. 2003, 63, 1317–1324. [Google Scholar] [CrossRef]
- Chen, D.K.; Li, J.; Ren, J. Study on sound absorption property of ramie fiber reinforced poly(L-lactic acid) composites: Morphology and properties. Compos. Part A Appl. Sci. Manuf. 2010, 41, 1012–1018. [Google Scholar] [CrossRef]
- Shi, X.T.; Zhang, G.C.; Phuong, T.V.; Lazzeri, A. Synergistic Effects of Nucleating Agents and Plasticizers on the Crystallization Behavior of Poly(lactic acid). Molecules 2015, 20, 1579–1593. [Google Scholar] [CrossRef]
- Posada, J.C.; Jaramillo, L.Y.; Cadena, E.M.; Garcia, L.A. Bio-based composites from agricultural wastes: Polylactic acid and bamboo Guadua angustifolia. J. Compos. Mater. 2016, 50, 3229–3237. [Google Scholar] [CrossRef]
- Ahmed, J.; Mulla, M.; Jacob, H.; Luciano, G.; Bini, T.B.; Almusallam, A. Polylactide/poly(epsilon-caprolactone)/zinc oxide/clove essential oil composite antimicrobial films for scrambled egg packaging. Food Packag. Shelf Life 2019, 21. [Google Scholar] [CrossRef]
- Thellen, C.; Orroth, C.; Froio, D.; Ziegler, D.; Lucciarini, J.; Farrell, R.; D’Souza, N.A.; Ratto, J.A. Influence of montmorillonite layered silicate on plasticized poly(L-lactide) blown films. Polymer 2005, 46, 11716–11727. [Google Scholar] [CrossRef]
- Shibata, M.; Someya, Y.; Orihara, M.; Miyoshi, M. Thermal and mechanical properties of plasticized poly(L-lactide) nanocomposites with organo-modified montmorillonites. J. Appl. Polym. Sci. 2006, 99, 2594–2602. [Google Scholar] [CrossRef]
- Zenkiewicz, M.; Richert, J.; Rozanski, A. Effect of blow moulding ratio on barrier properties of polylactide nanocomposite films. Polym. Test. 2010, 29, 251–257. [Google Scholar] [CrossRef]
- Gorrasi, G.; Tammaro, L.; Vittoria, V.; Paul, M.A.; Alexandre, M.; Dubois, P. Transport properties of water vapor in polylactide/montmorillonite nanocomposites. J. Macromol. Sci. Phys. 2004, B43, 565–575. [Google Scholar] [CrossRef]
- Mohapatra, A.K.; Mohanty, S.; Nayak, S.K. Properties and characterization of biodegradable poly(lactic acid) (PLA)/poly(ethylene glycol) (PEG) and PLA/PEG/organoclay: A study of crystallization kinetics, rheology, and compostability. J. Thermoplast. Compos. Mater. 2016, 29, 443–463. [Google Scholar] [CrossRef]
- Nekhamanurak, B.; Patanathabutr, P.; Hongsriphan, N. Thermal-mechanical property and fracture behaviour of plasticised PLA-CaCO3 nanocomposite. Plast. Rubber Compos. 2012, 41, 175–179. [Google Scholar] [CrossRef]
- Risyon, N.P.; Othman, S.H.; Basha, R.K.; Talib, R.A. Effect of Halloysite Nanoclay Concentration and Addition of Glycerol on Mechanical Properties of Bionanocomposite Films. Polym. Polym. Compos. 2016, 24, 795–801. [Google Scholar] [CrossRef]
- Lai, S.M.; Chen, J.R.; Han, J.L.; Yu, Y.F.; Lai, H.Y. Preparation and properties of melt-blended polylactic acid/polyethylene glycol-modified silica nanocomposites. J. Appl. Polym. Sci. 2013, 130, 496–503. [Google Scholar] [CrossRef]
- Li, Y.L.; Pötschke, P.; Pionteck, J.; Voit, B. Electrical and vapor sensing behaviors of polycarbonate composites containing hybrid carbon fillers. Eur. Polym. J. 2018, 108, 461–471. [Google Scholar] [CrossRef]
- Wickham, H.; François, R.; Henry, L.; Müller, K. dplyr: A Grammar of Data Manipulation. R Package Version 1.0.2. Available online: https://CRAN.R-project.org/package=dplyr (accessed on 9 December 2020).
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; D’Agostino McGowan, L.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the {tidyverse}. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Wickham, H. The Split-Apply-Combine Strategy for Data Analysis. J. Stat. Softw. 2011, 40, 1–29. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Wilke, C.O. cowplot: Streamlined Plot Theme and Plot Annotations for ‘ggplot2’. Available online: https://CRAN.R-project.org/package=cowplot (accessed on 12 January 2021).
- Wilke, C.O. ggridges: Ridgeline Plots in ‘ggplot2’. Available online: https://CRAN.R-project.org/package=ggridges (accessed on 9 December 2020).
- McNally, T.; Pötschke, P.; Halley, P.; Murphy, M.; Martin, D.; Bell, S.E.J.; Brennan, G.P.; Bein, D.; Lemoine, P.; Quinn, J.P. Polyethylene multiwalled carbon nanotube composites. Polymer 2005, 46, 8222–8232. [Google Scholar] [CrossRef]
- Batakliev, T.; Petrova-Doycheva, I.; Angelov, V.; Georgiev, V.; Ivanov, E.; Kotsilkova, R.; Casa, M.; Cirillo, C.; Adami, R.; Sarno, M.; et al. Effects of Graphene Nanoplatelets and Multiwall Carbon Nanotubes on the Structure and Mechanical Properties of Poly(lactic acid) Composites: A Comparative Study. Appl. Sci. 2019, 9, 469. [Google Scholar] [CrossRef]
- Bautista-Quijano, J.R.; Pötschke, P.; Brünig, H.; Heinrich, G. Strain sensing, electrical and mechanical properties of polycarbonate/multiwall carbon nanotube monofilament fibers fabricated by melt spinning. Polymer 2016, 82, 181–189. [Google Scholar] [CrossRef]
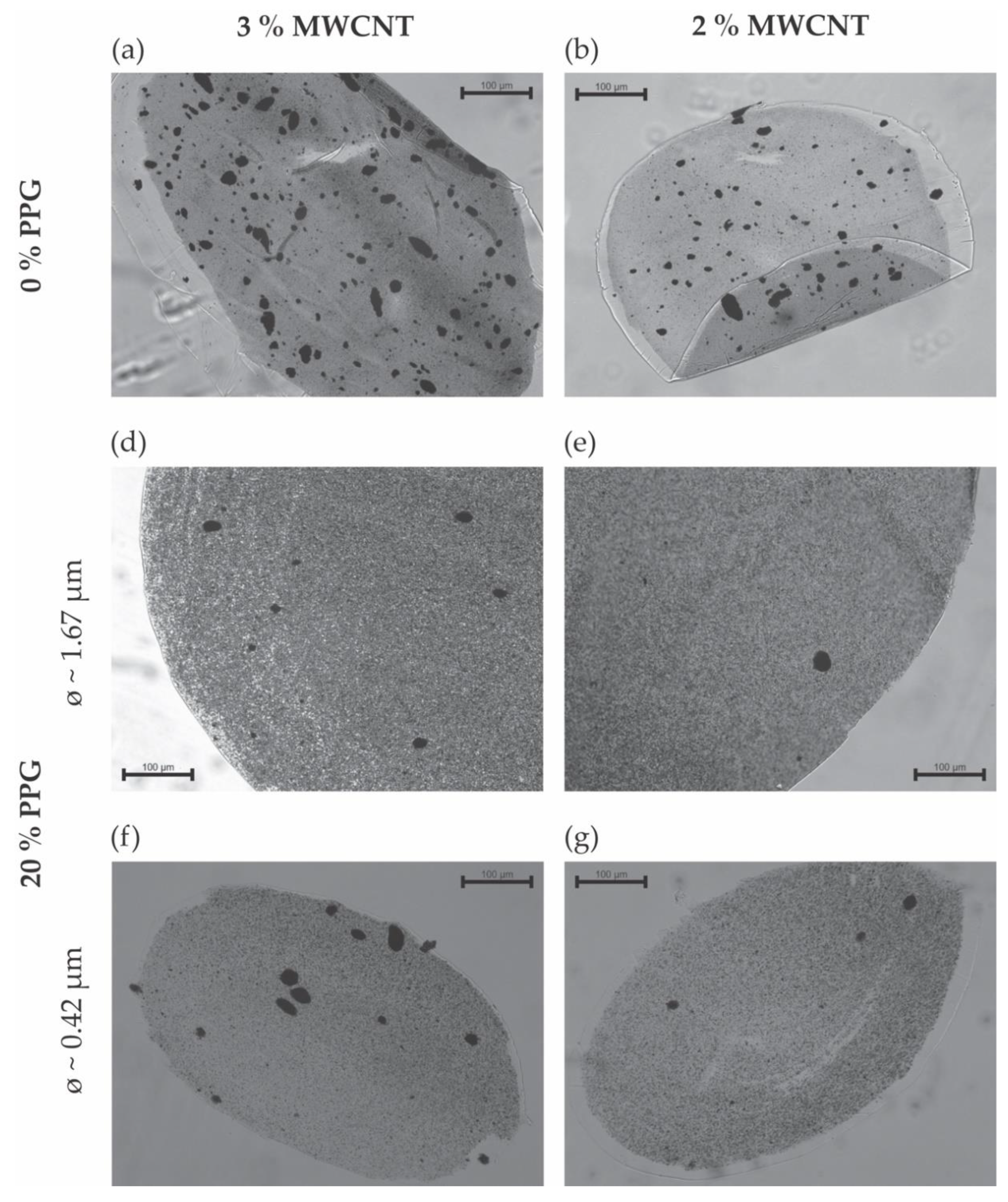
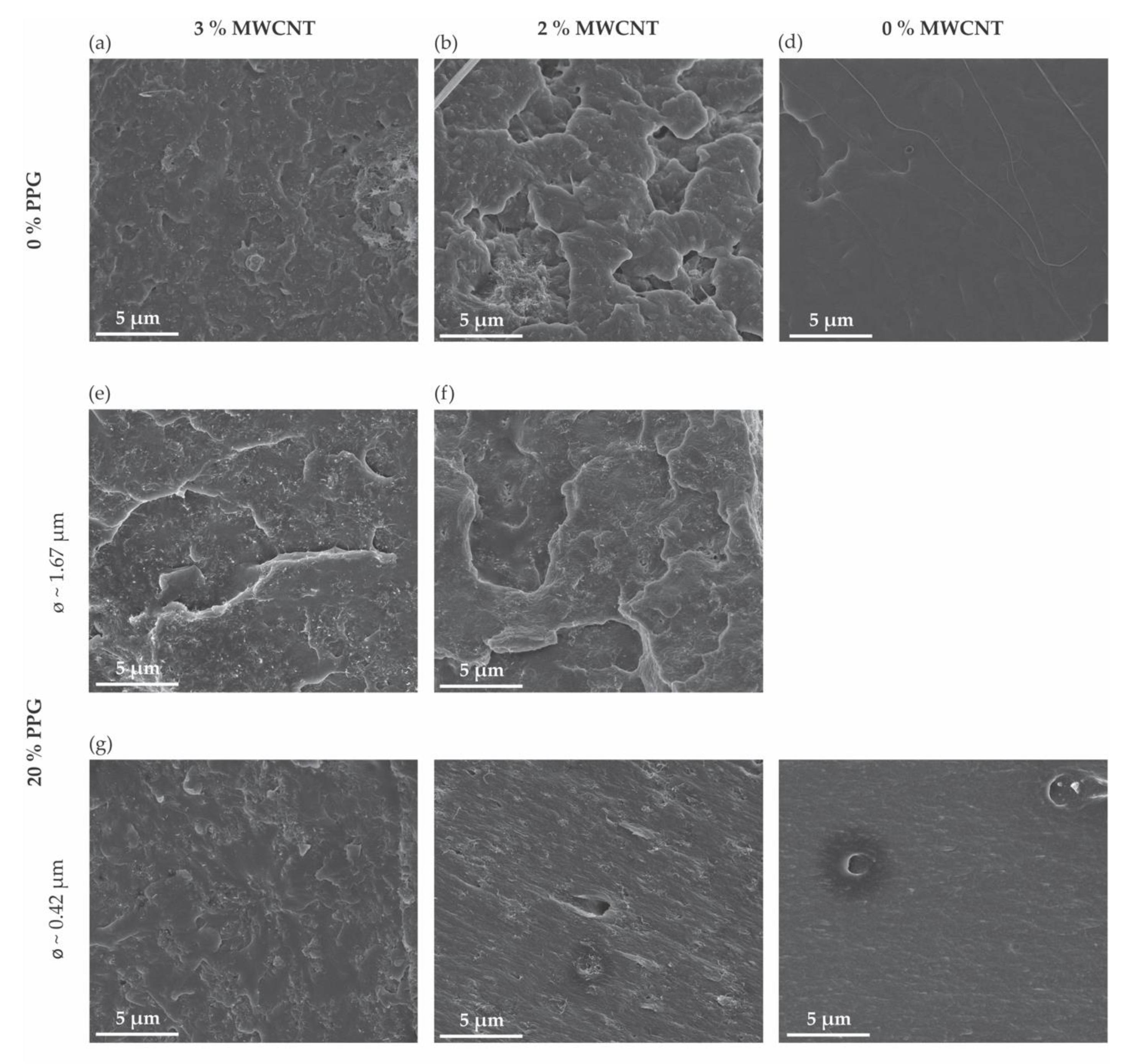

| Sample Designation | Composition | Average Diameter (mm) | |
|---|---|---|---|
| PPG (wt.%) | MWCNT (wt.%) | ||
| PLA | 0 | 0 | 1.17 |
| PLA/20PPG | 20 | 0 | 0.75 |
| PLA/2MW/20PPG | 20 | 2 | 1.64 |
| PLA/3MW/20PPG | 20 | 3 | 1.69 |
| PLA/2MW_s * | 0 | 2 | 0.50 |
| PLA/3MW_s * | 0 | 3 | 0.54 |
| PLA/2MW/20PPG_s * | 20 | 2 | 0.44 |
| PLA/3MW/20PPG_s * | 20 | 3 | 0.39 |
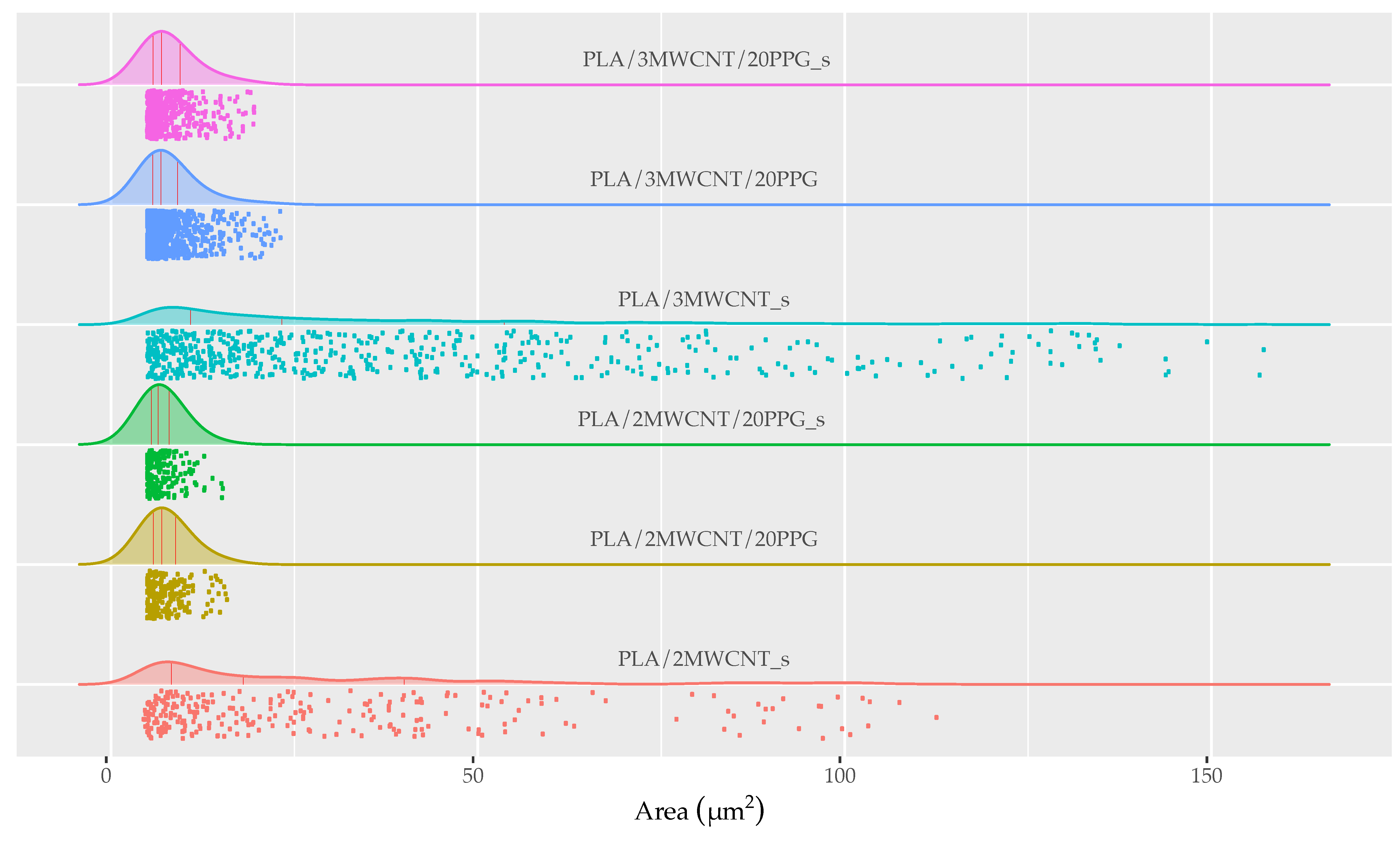
| Sample | Median (µm2) | Q1 (0.25) (µm2) | Q3 (0.75) (µm2) | Area Ratio (%) | Number of Agglomerates (mm−2) |
|---|---|---|---|---|---|
| PLA/2MWCNT_s | 18.05 | 8.25 | 39.96 | 1.74 | 356 |
| PLA/3MWCNT_s | 23.30 | 10.83 | 53.60 | 5.83 | 789 |
| PLA/2MWCNT/20PPG_s | 6.44 | 5.52 | 7.91 | 1.38 | 303 |
| PLA/3MWCNT/20PPG_s | 6.91 | 5.73 | 9.42 | 1.93 | 648 |
| PLA/2MWCNT/20PPG | 6.91 | 5.02 | 8.80 | 0.37 | 178 |
| PLA/3MWCNT/20PPG | 6.82 | 5.68 | 9.09 | 1.88 | 336 |
| Sample | Weight Loss at 350 °C (wt.%) | Temperature of PPG Vaporization (DTG Peak, °C) | Temperature at Max. Rate of PLA Degradation (DTG Peak, °C) | Residue at 700 °C (wt.%) |
|---|---|---|---|---|
| PLA | 1.4 ± 0.7 | - | 394.0 ± 3.6 | 2.8 ± 0.8 |
| PLA/20PPG | 15.3 ± 1.1 | 254.6 ± 4.8 | 395.5 ± 0.4 | 1.6 ± 0.2 |
| PLA/2MWCNT/20PPG | 9.3 ± 0.3 | 253.7 ± 3.7 | 409.6 ± 6.7 | 2.80 ± 0.04 |
| PLA/2MWCNT/20PPG_s | 11.0 ± 0.4 | 246.9 ± 7.6 | 396.0 ± 0.3 | 5.4 ± 0.2 |
| PLA/2MWCNT_s | 1.0 ± 0.4 | - | 400.6 ± 1.1 | 4.3 ± 0.9 |
| PLA/3MWCNT/20PPG | 8.9 ± 0.2 | 264.7 ± 6.6 | 409.2 ± 5.8 | 3.1 ± 0.1 |
| PLA/3MWCNT/20PPG_s | 11.6 ± 0.2 | 244.4 ± 2.0 | 395.3 ± 0.4 | 6.1 ± 0.3 |
| PLA/3MWCNT_s | 1.7 ± 0.4 | - | 396.4 ± 2.7 | 4.1 ± 0.8 |
| Sample | Tg (°C) | Tcc (°C) | TM (°C) | ΔHcc (J·g−1) | ΔHM (J·g−1) |
|---|---|---|---|---|---|
| PLA | 63.8 ± 0.3 | 95.2 ± 0.3 | 177.5 ± 0.3 | 37.9 | 49.4 |
| PLA/20PPG | 53.8 ± 4.0 | 85.9 ± 0.0 | 169.2 ± 1.3 | 14.3 | 55.5 |
| PLA/2MWCNT_s | 63.0 ± 0.2 | 83.5 ± 0.6 | 176.4 ± 0.3 | 24.7 | 48.7 |
| PLA/2MWCNT/20PPG_s | 47.3 ± 2.3 | 73.4 ± 4.0 | 171.4 ± 0.4 | 12.0 | 42.1 |
| PLA/2MWCNT/20PPG | 35.4 ± 3.6 | 86.1 ± 1.5 | 172.5 ± 0.5 | 21.8 | 47.4 |
| PLA/3MWCNT_s | 63.7 ± 0.4 | 82.0 ± 0.9 | 176.9 ± 0.6 | 19.5 | 47.2 |
| PLA/3MWCNT/20PPG_s | 54.2 ± 2.2 | 86.9 ± 5.3 | 170.3 ± 0.5 | 2.9 | 48.2 |
| PLA/3MWCNT/20PPG | 50.4 ± 10.7 | 85.6 ± 0.8 | 172.7 ± 0.3 | 20.7 | 50.9 |
| Sample | At 0 °C | Temperature of tan δ Peak (°C) | Temperature of E″ Maximum (°C) | |
|---|---|---|---|---|
| E′ (GPa) | E″ (MPa) | |||
| PLA | 4.0 | 24.7 | 66.4 | 60.9 |
| PLA/20PPG | 1.7 | 92.9 | 52.5 | 33.8 |
| PLA/2MWCNT_s | 5.1 | 33.2 | 71.5 | 61.4 |
| PLA/2MWCNT/20PPG_s | 3.4 | 121.3 | 57.7 | 38.8 |
| PLA/3MWCNT_s | 6.7 | 41.4 | 73.7 | 63.0 |
| PLA/3MWCNT/20PPG_s | 2.7 | 110.6 | 57.1 | 33.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, M.M.; Lopes, P.E.; Li, Y.; Pötschke, P.; Ferreira, F.N.; Paiva, M.C. Polylactic Acid/Carbon Nanoparticle Composite Filaments for Sensing. Appl. Sci. 2021, 11, 2580. https://doi.org/10.3390/app11062580
Silva MM, Lopes PE, Li Y, Pötschke P, Ferreira FN, Paiva MC. Polylactic Acid/Carbon Nanoparticle Composite Filaments for Sensing. Applied Sciences. 2021; 11(6):2580. https://doi.org/10.3390/app11062580
Chicago/Turabian StyleSilva, Mariana M., Paulo E. Lopes, Yilong Li, Petra Pötschke, Fernando N. Ferreira, and Maria C. Paiva. 2021. "Polylactic Acid/Carbon Nanoparticle Composite Filaments for Sensing" Applied Sciences 11, no. 6: 2580. https://doi.org/10.3390/app11062580
APA StyleSilva, M. M., Lopes, P. E., Li, Y., Pötschke, P., Ferreira, F. N., & Paiva, M. C. (2021). Polylactic Acid/Carbon Nanoparticle Composite Filaments for Sensing. Applied Sciences, 11(6), 2580. https://doi.org/10.3390/app11062580








