Evaluation of Pig Manure for Environmental or Agricultural Applications through Gasification and Soil Leaching Experiments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials Characterization
2.2. Biochar Production and Activation
2.3. Leaching of Manure and Biochars through the Soil
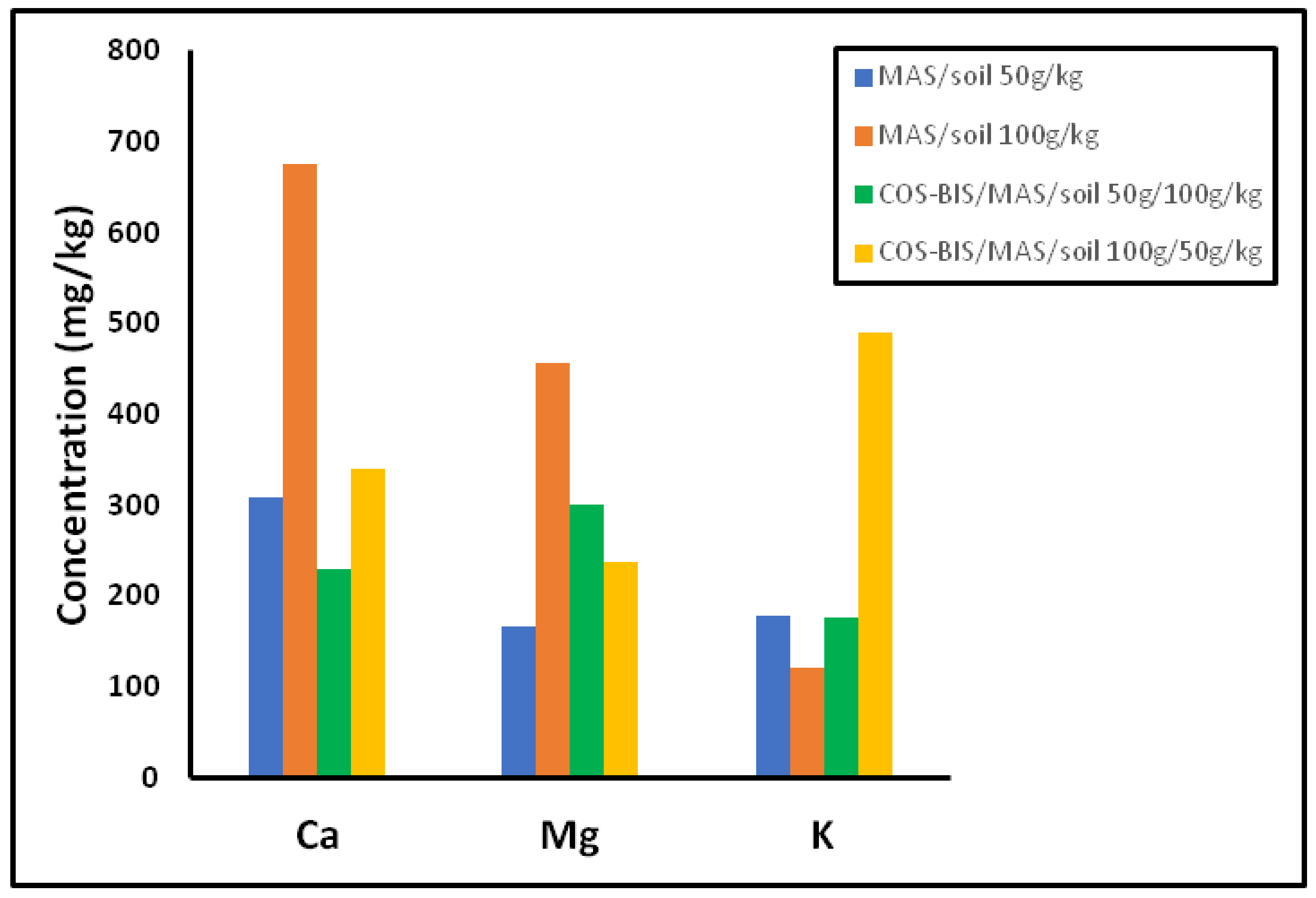
3. Results and Discussion
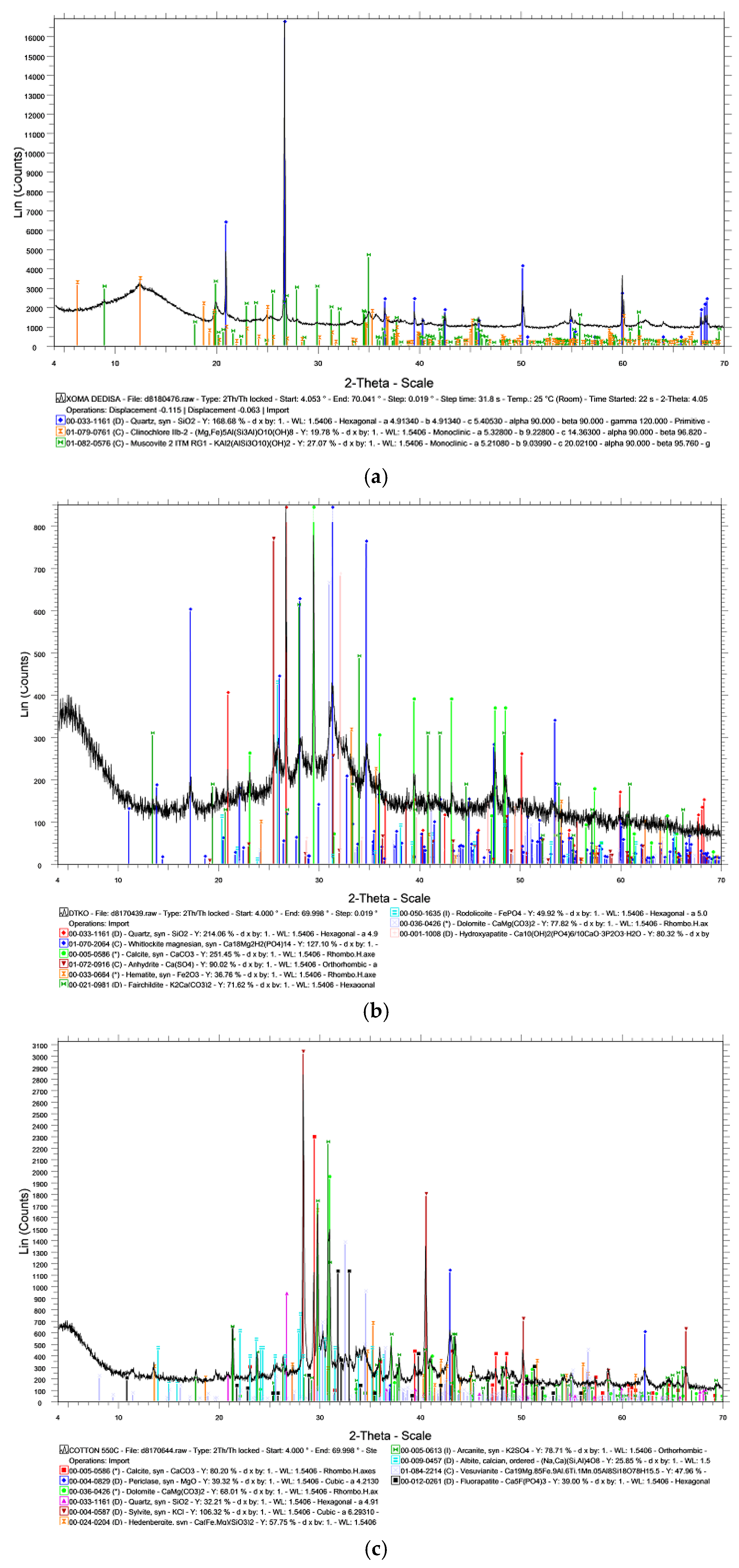
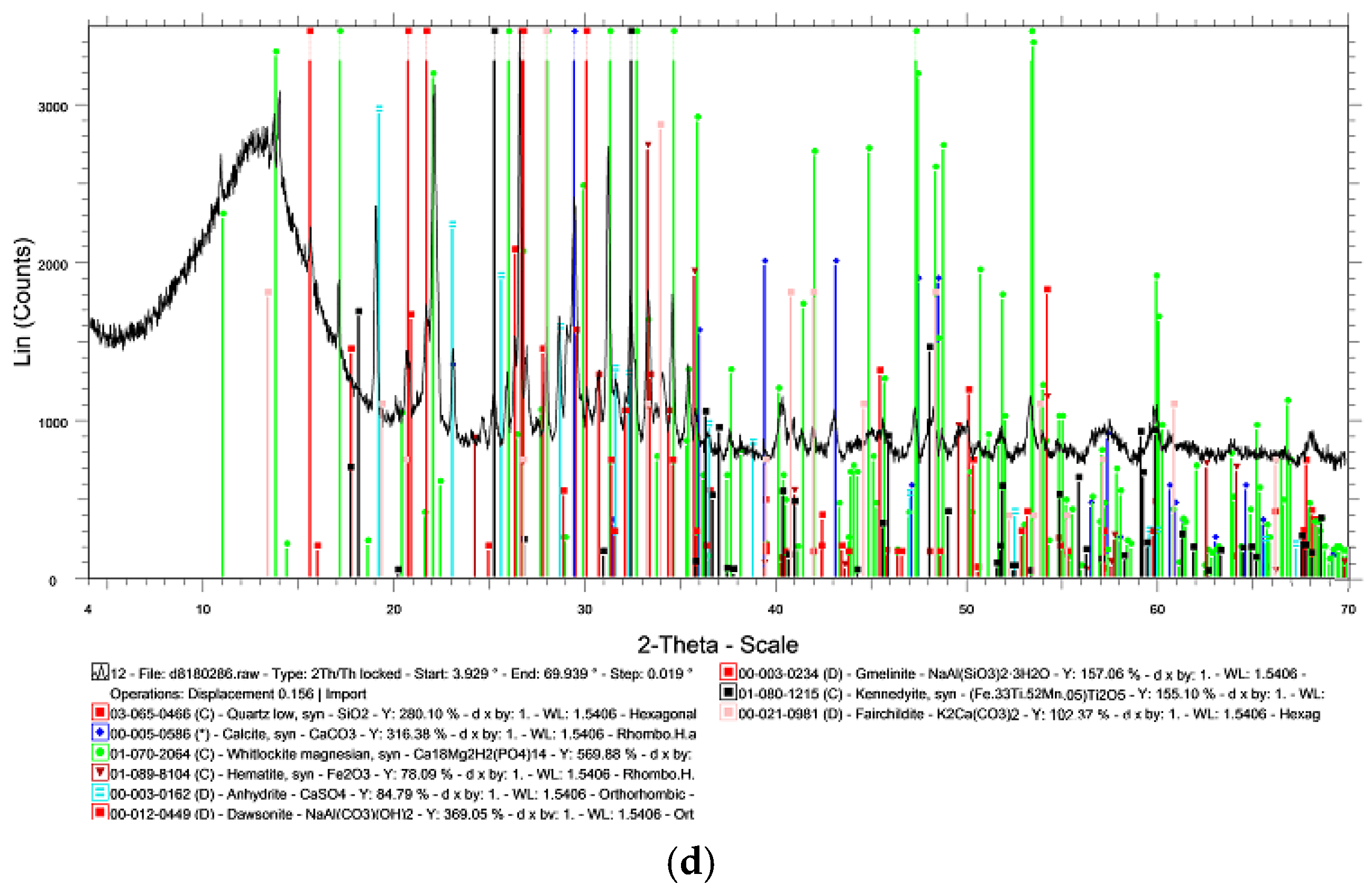
3.1. Chemical and Mineralogical Properties of Raw Materials
3.2. Physical Activation of Manure
3.2.1. Yield of Products under Different Atmosphere
3.2.2. Structural Characteristics and Chemical Functional Groups under Different Atmosphere
3.3. Leachability of Manure Blends through the Soil
3.3.1. Physicochemical Properties before and after Incubation
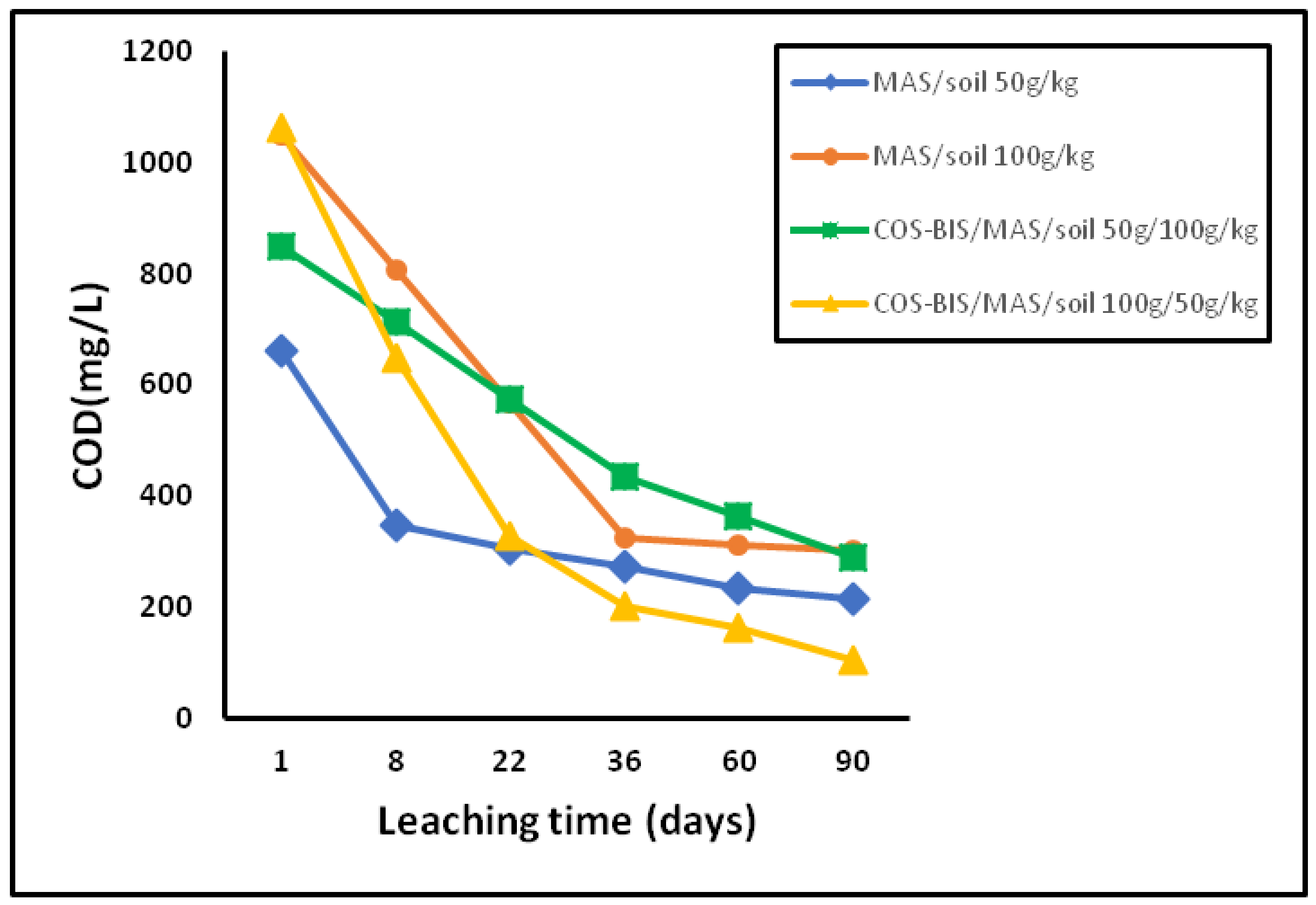
3.3.2. Leaching of Nitrogen, Phosphorous Anions and Phenols
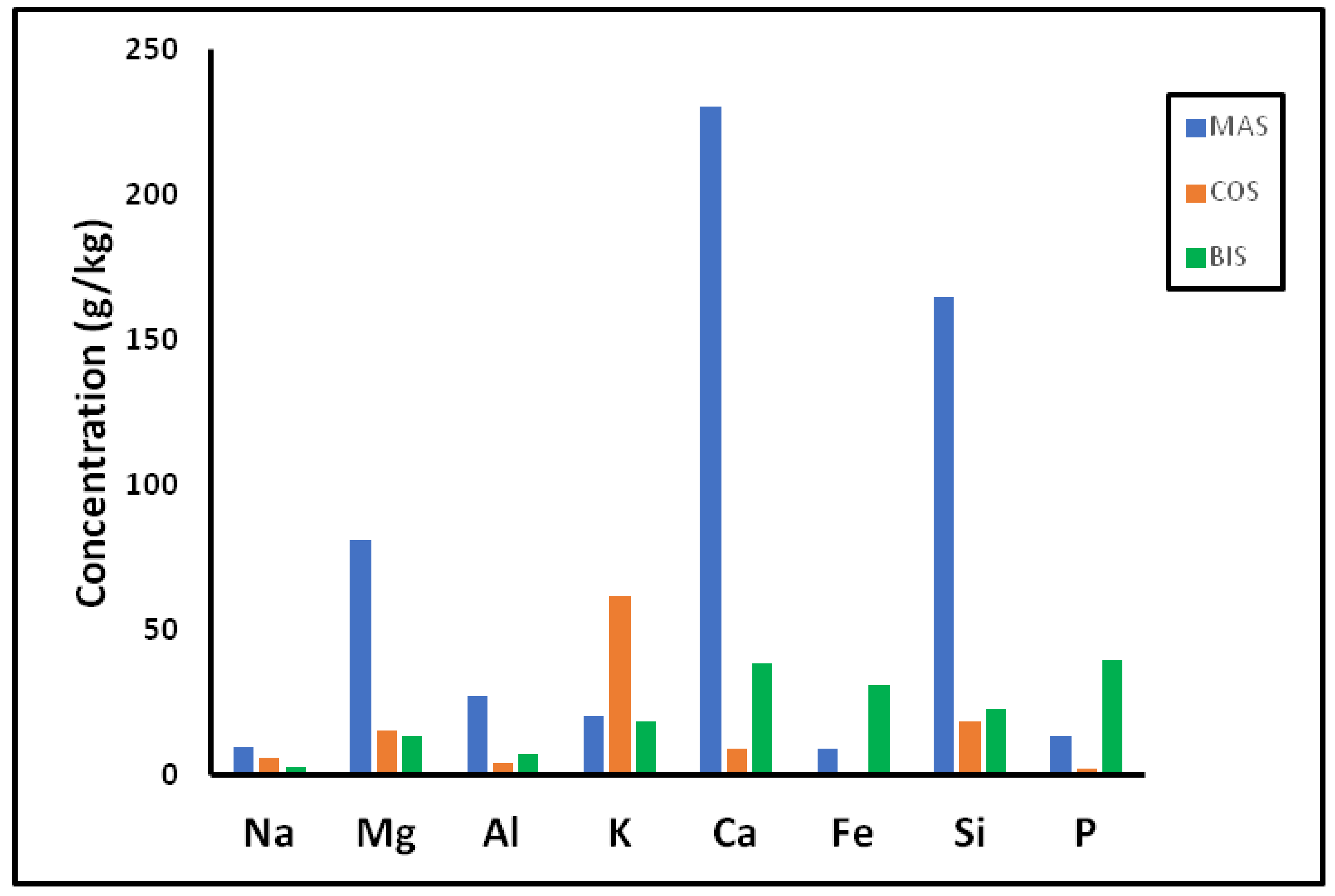
3.3.3. Leaching of Metals
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoogwijk, M.; Faaj, A.; Van den Broek, R.; Berndes, G.; Gielen, D.; Turkenburg, W. Exploration of the ranges of the global potential of biomass for energy. Biomass Bioenergy 2003, 25, 119. [Google Scholar] [CrossRef]
- Liang, J.; Yang, Z.; Tang, L.; Zeng, G.; Yu, M.; Li, X.; Luo, Y. Changes in heavy metal mobility and availability from contaminated wetland soil remediated with combined biochar-compost. Chemosphere 2017, 181, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Cambier, P.; Michaud, A.; Paradelo, R.; Germain, M.; Mercier, V.; Guerin-Lebourg, A.; Houot, S. Trace metal availability in soil horizons amended with various urban waste composts during 17 years-Monitoring and modeling. Sci. Total Environ. 2019, 651, 2961–2974. [Google Scholar] [CrossRef] [PubMed]
- Gondek, K.; Mierzwa-Hersztek, M.; Kopec, M. Mobility of heavy metals in sandy soil after application of composts produced from maize straw, sewage sludge and biochar. J. Environ. Manag. 2018, 210, 87–95. [Google Scholar] [CrossRef]
- Vamvuka, D.; Sfakiotakis, S.; Pantelaki, O. Evaluation of gaseous and solid products from the pyrolysis of waste biomass blends for energetic and environmental applications. Fuel 2019, 236, 574–582. [Google Scholar] [CrossRef]
- Cao, Y.; Gao, Y.; Qi, Y.; Li, J. Biochar-enhanced composts reduce the potential leaching of nutrients and heavy metals and suppress plant-parasitic nematodes in excessively fertilized cucumber soils. Environ. Sci. Pollut. Res. 2018, 25, 7589–7599. [Google Scholar] [CrossRef]
- Li, F.; Wu, X.; Ji, W.; Gui, X.; Chen, Y.; Zhao, J.; Zhou, C.; Ren, T. Effects of pyrolysis temperature on properties of swine manure biochar and its environmental risks of heavy metals. J. Anal. Appl. Pyrolysis 2020, 152, 104945. [Google Scholar] [CrossRef]
- Qin, X.; Guo, S.; Zhai, L.; Pan, J.; Khoshnevisan, B.; Wu, S.; Wang, H.; Yang, B.; Ji, J.; Liu, H. How long-term excessive manure application affects soil phosphorous species and risk of phosphotous loss in fluvo-aquic soil. Environ. Pollut. 2020, 266, 115304. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, X.; Li, Y.; Han, L. Influence of pyrolysis temperature on chemical speciation, leaching ability and environmental risk of heavy metals in biochar derived from cow manure. Biores. Technol. 2020, 302, 122850. [Google Scholar] [CrossRef]
- Xu, Y.; Qu, W.; Sun, B.; Peng, K.; Zhang, X.; Xu, J.; Gao, F.; Yan, Y.; Bai, T. Effects of added calcium-based additives on swine manure derived biochar characteristics and heavy metals immobilization. Waste Manag. 2021, 123, 69–79. [Google Scholar] [CrossRef]
- Vamvuka, D.; Papaioannou, G.; Alexandrakis, S.; Stratakis, A. Control of the mobility of heavy metals in soil from disposal of bio-solid and olive by-roduct ashes using waste additives. Environ. Pollut. 2020, 266, 115136. [Google Scholar] [CrossRef]
- Vamvuka, D. Biomass, Bioenergy and the Environment, 1st ed.; Tziolas Publications: Salonica, Greece, 2009. [Google Scholar]
- Manolikaki, I.; Mangolis, A.; Diamantopoulos, E. The impact of biochars prepared from agricultural residues on phosphorous release and availability in two fertile soils. J. Environ. Manag. 2016, 181, 536–543. [Google Scholar] [CrossRef]
- Vamvuka, D.; Sfakiotakis, S. Thermal behavior and reactivity of swine sludge and olive by-products during co-pyrolysis and co-combustion. Waste Biomass Valoriz. 2019, 10, 1433–1442. [Google Scholar] [CrossRef]
- Sadaf, J.; Shah, G.; Shahzad, K.; Ali, N.; Shahid, M.; Ali, S.; Hussain, R.; Ahmed, Z.; Traore, B.; Ismail, I.; et al. Improvements in wheat productivity and soil quality can accomplish by co-application of biochars and chemical fertilizers. Sci. Total Environ. 2017, 608, 715–724. [Google Scholar] [CrossRef]
- Varma, A.; Mondal, P. Pyrolysis of sugarcane bagasse in semi batch reactor: Effects of process parameters on product yields and characterization of products. Ind. Crops Prod. 2017, 95, 704–717. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D. Morphology of modified biochar and its potential for phenol removal from aqueous solutions. Front. Environ. Sci. 2016, 4, 26. [Google Scholar] [CrossRef] [Green Version]
- Vamvuka, D.; Dermitzakis, S.; Pentari, D. Valorization of Meat and Bone Meal through pyrolysis for soil amendment or lead adsorption from wastewaters. Food Bioprod. Process. 2018, 109, 148–157. [Google Scholar] [CrossRef]
- Idrees, M.; Batool, S.; Ullah, H.; Hussain, Q.; Al-Wabel, M.; Ahmad, M.; Hussain, A.; Riaz, M.; Ok, Y.; Kong, J. Adsorption and thermodynamic mechanisms of manganese removal from aqueous media bybiowaste-derived biochars. J. Mol. Liq. 2018, 266, 373–380. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, X.; Yuan, X.; Xie, R.; Han, L. Characteristics, adsorption behaviors, Cu(II) adsorption mechanisms by cow manure biochar derived at various pyrolysis temperatures. Biores. Technol. 2021, 331, 125013. [Google Scholar] [CrossRef]
- Wang, K.; Peng, N.; Zhao, P.; Chen, M.; Deng, F.; Yu, X.; Zhang, D.; Chen, J.; Sun, J. Effect of a low-cost and highly efficient passivator synthesized by alkali-fused fly ash and swine manure on the leachability of heavy metals in a multi-metal contaminated soil. Chemosphere 2021, 279, 130558. [Google Scholar] [CrossRef]
- Jiang, B.; Lin, Y.; Mbog, J. Biochar derived from swine manure digestate and applied on the removals of heavy metals and antibiotics. Biores. Technol. 2018, 270, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Sheng, H.; Gu, C.; Marc, R.; Wang, Y.; Bian, Y.; Jiang, X.; Wang, F. Biochar combined with compost to reduce the mobility, bioavailability and plant uptake of 2,2’,4,4’-tetrabrominated diphenyl ether in soil. J. Hazard. Mater. 2019, 374, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Mao, X.; Pi, L.; Wu, T.; Hu, Y. Adsorptive and capacitive properties of the activated carbons derived from pig manure residues. J. Environ. Chem. Eng. 2019, 7, 103066. [Google Scholar] [CrossRef]
- Vamvuka, D.; Sfakiotakis, S. Gasification reactivity and mass spectrometric analysis of gases of energy crop chars under a CO2 atmosphere. Energy Fuels 2015, 29, 3215–3223. [Google Scholar] [CrossRef]
- Prodana, M.; Bastos, A.; Amaro, A.; Cardoso, D.; Morgado, R.; Machado, A.; Verheijen, F.; Keizer, J.; Loureiro, S. Biomonitoring tools for biochar and biochar-compost amended soil under viticulture: Looking at expose and effects. Appl. Soil Ecol. 2019, 137, 120–128. [Google Scholar] [CrossRef]
- Badescu, I.; Bulgariu, D.; Bulgariu, L. Alternative utilization of algal biomass (Ulva sp.) loaded with Zn (II) ions for improving of soil quality. J. Appl. Phycol. 2017, 29, 1069–1079. [Google Scholar] [CrossRef]
- Limwikran, T.; Kheoruenromne, I.; Suddhiprakarn, A.; Prakongkep, N.; Gilkes, R. Dissolution of K, Ca and P from biochar grains in tropical soils. Geoderma 2018, 312, 139–150. [Google Scholar] [CrossRef]
- Ye, S.; Zeng, G.; Wu, H.; Liang, J.; Zhang, C.; Dai, J.; Xiong, W.; Song, B.; Wu, S.; Yu, J. The effects of activated biochar addition on remediation efficiency of co-composting with contaminated wetland soil. Resour. Conserv. Recycl. 2019, 140, 278–285. [Google Scholar] [CrossRef]
- Forjan, R.; Rodriguez-Vila, A.; Cerqueira, B.; Covelo, E. Comparison of the effects of compost versus compost and biochar on the recovery of a mine soil by improving the nutrient content. J. Geochem. Explor. 2017, 183, 46–57. [Google Scholar] [CrossRef]
- Ippolito, J.; Stromberger, M.; Lentz, R.; Dungan, R. Hardwood biochar and manure co-application to a calcareous soil. Chemosphere 2016, 142, 84–91. [Google Scholar] [CrossRef]
- Wei, X.; Liu, D.; Li, W.; Lia, L.; Wang, Z.; Huang, W.; Huang, W. Biochar addition for accelerating bioleaching of heavy metals from swine manure and reserving the nutrients. Sci. Total Environ. 2018, 632, 1553–1559. [Google Scholar] [CrossRef]
- Bhushan, B.; Nayak, A.; Kotnala, S. Green synthesis of highly porous activated carbon from jackfruit peel: Effect of operating factors on its physic-chemical characteristics. Mater. Today Proc. 2020, 44, 187–191. [Google Scholar] [CrossRef]
- Bubba, M.; Anichini, B.; Bakari, Z.; Bruzzoniti, M.; Camisa, R.; Caprini, C.; Checchini, L.; Fibbi, D.; El Ghadraouia, A.; Liguori, F.; et al. Physicochemical properties and sorption capacities of sawdust-based biochars and commercial activated carbons towards ethoxylated alkylphenols and their phenolic metabolites in effluent wastewater from a textile district. Sci. Total Environ. 2020, 708, 135217. [Google Scholar] [CrossRef]
- Vamvuka, D.; Machairas, E.; Sfakiotakis, S.; Pantelaki, O. Physically activated agricultural waste biochars for production of pollutant adsorbents. J. Chem. Eng. Res. Updates 2020, 7, 6–15. [Google Scholar] [CrossRef]
- Gong, H.; Tan, Z.; Zhang, L.; Huang, Q. Preparation of biochar with high absorbability and its nutrient adsorption-desorption behavior. Sci. Total Environ. 2019, 694, 133728. [Google Scholar] [CrossRef]
- Jeffery, S.; Verheijen, F.; van der Velde, M.; Bastos, A. A quantitative review on the effects of biochar application to soils on crop productivity using meta-analysis. Agric. Ecosyst. Environ. 2011, 144, 175–187. [Google Scholar] [CrossRef]
- ASTM. Refractories, activated carbon, advanced ceramics. In ASTM Book of Standards; American Society for Testing Materials: West Conshohocken, PA, USA, 2007; Volume 15.01. [Google Scholar]
- Ye, L.; Zhang, J.; Zhao, J.; Luo, Z.; Tu, S.; Yin, Y. Properties of biochar obtained from pyrolysis of bamboo shoot shell. J. Anal. Appl. Pyrolysis 2015, 114, 172–178. [Google Scholar] [CrossRef]
- Sfakiotakis, S. Study on the Exploitation of Agricultural, Urban and Industrial Wastes of Crete for Power Production-Thermal and Kinetic Analyses. Ph.D. Thesis, Technical University of Crete, Chania, Greece, 2016. [Google Scholar]
- Stylianou, M.; Christou, A.; Dalias, P.; Polycarpou, P.; Michael, C.; Agapiou, A.; Papanastasiou, P.; Fatta-Kassinos, D. Ohysicochemical and structural characterization of biochar derived from the pyrolysis of biosolids, cattle manure and spent coffee grounds. J. Energy Inst. 2020, 93, 2063–2073. [Google Scholar] [CrossRef]
- Shen, X.; Zeng, J.; Zhang, D.; Wang, F.; Li, Y.; Yi, W. Effect of pyrolysis temperature on characteristics, chemical speciation and environmental risk of Cr, Mn, Cu and Zn in biochars derived from pig manure. Sci. Total Environ. 2020, 704, 135283. [Google Scholar] [CrossRef]
- IR Spectrum Table by Frequency Range; Merck kGaA: Darustadt, Germany, 2021.
- Yuan, H.; Lu, T.; Wang, Y.; Chen, Y.; Lei, T. Sewage sludge biochar: Nutrient composition and its effect on the leaching of soil nutrients. Geoderma 2016, 267, 17–23. [Google Scholar] [CrossRef]
- Yang, X.; Chang, K.; Kim, Y.; Zhang, J.; Yoo, G. Effects of different biochar amendments on carbon loss and leachate characterization from an agricultural soil. Chemosphere 2019, 226, 625–635. [Google Scholar] [CrossRef]
- EBC. European Biochar Certificate, Guidelines for a Sustainable Production of Biochar, Version 4.8; European Biochar Foundation: Arbaz, Switzerland, 2014. [Google Scholar]
- Boostani, H.; Najafi-Ghiri, M.; Hardie, A.; Khalili, D. Comparison of Pb stabilization in a contaminated calcareous soil by application of vermicompost and sheep manure and their biochars produced at two temperatures. Appl. Geochem. 2019, 102, 121–128. [Google Scholar] [CrossRef]


| Sample | Volatiles | Fixed Carbon | Ash | C | H | N | O | S |
|---|---|---|---|---|---|---|---|---|
| Manure (MAS) | 51.9 | 16.5 | 31.6 | 35.8 | 5.3 | 3.6 | 22.2 | 1.5 |
| Cotton stems (COS) | 73.4 | 18.3 | 8.3 | 46.8 | 6.5 | 1.6 | 36.7 | 0.2 |
| Bio-solid (BIS) | 65.6 | 16.6 | 17.8 | 43.0 | 6.6 | 7.9 | 22.9 | 1.8 |
| Temperature (°C) | Activation Gas | Specific Surface Area (m2/g) | Micropore Volume × 102 (cm3/g) | Average Pore Size (Å) |
|---|---|---|---|---|
| 700 | N2 | 47.6 | 4.4 | 37.0 |
| H2Ov | 231.4 | 15.9 | 33.0 | |
| 800 | N2 | 68.9 | 5.9 | 49.3 |
| H2Ov | 228.7 | 21.5 | 45.1 | |
| CO2 | 233.3 | 16.2 | 33.3 |
| Wavenumber (cm−1)/Functional Groups | N2 | H2O | CO2 |
|---|---|---|---|
| 500–600/C-X halo compounds | 556 | 564 | |
| 592 | 592 | ||
| 650–1000/C=C alkenes | 666 | ||
| 1000–1400/C-O alcohols, ethers | 1046 | 1030 | 1036 |
| 1114 | 1090 | ||
| 1146 | |||
| 1156 | |||
| 1234 | |||
| 1300–1600/C-H alkanes, C=C aromatic compounds | 1454 | 1550 | 1440 |
| 1600–1670/C = C alkenes | 1650 | 1602 | |
| 1650–1750/C = O carboxylic acids | 1714 | ||
| 2000–2400/O = C = O carbon dioxide | 2318 | ||
| 2350 | |||
| 2500–3000/C-H aldehydes, alkanes | 2848 | ||
| 2918 | |||
| 3000–4000/-OH alcohols, carboxylic acids | 3292 | 3436 |
| Sample | Before Incubation | After Incubation | ||||||
|---|---|---|---|---|---|---|---|---|
| d (g/cm3) | pH | EC (mS/cm) | WHC (g/g) | d (g/cm3) | pH | EC (mS/cm) | WHC (g/g) | |
| Soil | 1.06 | 7.80 | 1.05 | 0.92 | ||||
| MAS/soil 50 g/kg | 1.02 | 7.70 | 1.10 | 0.94 | 1.0 | 6.35 | 0.20 | 1.0 |
| MAS/soil 100 g/kg | 1.01 | 7.60 | 1.10 | 0.94 | 0.63 | 6.30 | 0.40 | 1.0 |
| COS-BIS/MAS/soil 50 g/100 g/kg | 1.01 | 7.68 | 1.06 | 0.94 | 0.64 | 6.38 | 0.45 | 1.18 |
| COS-BIS/MAS/soil 100 g/50 g/kg | 1.0 | 7.79 | 1.05 | 0.94 | 0.61 | 6.41 | 0.20 | 1.20 |
| Sample | Leaching Time (days) | pH | EC (mS/cm) | NO3− (mg/L) | PO43− (mg/L) | Phenols (mg/L) |
|---|---|---|---|---|---|---|
| MAS/soil 50 g/kg | 1 | 8.1 | 2.8 | 26 | 6 | 1.7 |
| 8 | 8.1 | 0.8 | 45 | 31 | ||
| 22 | 7.5 | 0.9 | 38 | 33 | ||
| 36 | 7.4 | 1.0 | 32 | 34 | ||
| 60 | 7.2 | 0.6 | 25 | 36 | ||
| 90 | 7.1 | 0.4 | 20 | 40 | ||
| MAS/soil 100 g/kg | 1 | 6.2 | 4.9 | 43 | 62 | 2.4 |
| 8 | 6.4 | 3.6 | 42 | 69 | ||
| 22 | 6.5 | 2.3 | 41 | 75 | ||
| 36 | 6.7 | 1.1 | 40 | 82 | ||
| 60 | 7.0 | 0.7 | 30 | 85 | ||
| 90 | 7.2 | 0.4 | 21 | 89 | ||
| COS-BIS/MAS/soil 50 g/100 g/kg | 1 | 6.8 | 2.6 | 27 | 21 | 2.9 |
| 8 | 7.1 | 2.1 | 26 | - | ||
| 22 | 7.4 | 1.5 | 25 | - | ||
| 36 | 7.7 | 1.0 | 24 | - | ||
| 60 | 7.8 | 1.0 | 20 | - | ||
| 90 | 7.8 | 1.0 | 17 | - | ||
| COS-BIS/MAS/soil 100 g/50 g/kg | 1 | 8.5 | 3.8 | 22 | 5 | 1.7 |
| 8 | 7.9 | 1.4 | 20 | - | ||
| 22 | 7.8 | 0.7 | 17 | - | ||
| 36 | 7.6 | 0.6 | 13 | - | ||
| 60 | 7.5 | 0.5 | 10 | - | ||
| 90 | 7.5 | 0.4 | 7 | - |
| Sample | MAS/Soil 50 g/kg | MAS/Soil 100 g/kg | COS-BIS/MAS/Soil 50 g/100 g/kg | COS-BIS/MAS/Soil 100 g/50 g/kg |
|---|---|---|---|---|
| Mn | - | 461.8 | 214.6 | - |
| Ni | - | 89.3 | 136.0 | - |
| Cu | 92.0 | 111.1 | 119.8 | 137.8 |
| Zn | - | 487.0 | 144.8 | - |
| Sr | 504.5 | 1144.9 | 413.3 | 703.4 |
| Pb | 7.3 | - | - | 4.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vamvuka, D.; Raftogianni, A. Evaluation of Pig Manure for Environmental or Agricultural Applications through Gasification and Soil Leaching Experiments. Appl. Sci. 2021, 11, 12011. https://doi.org/10.3390/app112412011
Vamvuka D, Raftogianni A. Evaluation of Pig Manure for Environmental or Agricultural Applications through Gasification and Soil Leaching Experiments. Applied Sciences. 2021; 11(24):12011. https://doi.org/10.3390/app112412011
Chicago/Turabian StyleVamvuka, Despina, and Adamantia Raftogianni. 2021. "Evaluation of Pig Manure for Environmental or Agricultural Applications through Gasification and Soil Leaching Experiments" Applied Sciences 11, no. 24: 12011. https://doi.org/10.3390/app112412011
APA StyleVamvuka, D., & Raftogianni, A. (2021). Evaluation of Pig Manure for Environmental or Agricultural Applications through Gasification and Soil Leaching Experiments. Applied Sciences, 11(24), 12011. https://doi.org/10.3390/app112412011






