Comparative Analysis of Collagen-Containing Waste Biodegradation, Amino Acid, Peptide and Carbohydrate Composition of Hydrolysis Products
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Objects of Research
4.2. Biodegradation of Collagen-Containing Waste by Enzymes
4.3. Analysis of Peptide and Protein Profile
4.4. Analysis of the Amino Acid Composition of Proteins
4.5. Analysis of the Carbohydrate Composition of Hydrolysis Products
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arslan, Y.E.; Derkus, T.S.; Emregul, E.; Emregul, K.C. Fabrication of human hair keratin/jellyfish collagen/eggshell-derived hydroxyapatite osteoinductivebiocomposite scaffolds for bone tissue engineering: From waste to regenerative medicine products. Colloids Surf. B 2017, 154, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Bhagwat, P.K.; Dandge, P.B. Collagen and collagenolytic proteases: A review. Biocatal. Agric. Biotechnol. 2018, 15, 43–55. [Google Scholar] [CrossRef]
- Bilek, S.E.; Bayram, S.K. Fruit juice drink production containing hydrolyzed collagen. J. Funct. Foods 2015, 14, 562–569. [Google Scholar] [CrossRef]
- Da Costa Reichenbach, B.R.S.; Keller, N.; Nartey, L.; Wandel, S.; Jüni, P.; Trelle, S. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: A network meta-analysis. Lancet 2017, 390, e21–e33. [Google Scholar] [CrossRef]
- De Luca, P.; Colombini, A.; Carimati, G.; Beggio, M.; de Girolamo, L.; Volpi, P. Intra-Articular Injection of Hydrolyzed Collagen to Treat Symptoms of Knee Osteoarthritis. A Functional In Vitro Investigation and a Pilot Retrospective Clinical Study. J. Clin. Med. 2019, 8, 975. [Google Scholar] [CrossRef] [PubMed]
- Felician, F.F.; Xia, C.; Qi, W.; Xu, H. Collagen from marine biological sources and medical applications. Chem. Biodivers. 2018, 15, e1700557. [Google Scholar] [CrossRef] [PubMed]
- Felician, F.F.; Yu, R.H.; Li, M.Z.; Li, C.J.; Chen, H.Q.; Jiang, Y.; Xu, H.M. The wound healing potential of collagen peptides derived from the jellyfish Rhopilemaesculentum. Chin. J. Traumatol. 2019, 22, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Ferrario, C.; Rusconi, F.; Pulaj, A.; Macchi, R.; Landini, P.; Paroni, M.; Candia Carnevali, M.D. From food waste to innovative biomaterial: Sea urchin-derived collagen for applications in skin regenerative medicine. Mar. Drugs 2020, 18, 414. [Google Scholar] [CrossRef]
- Freire, V.; Bureau, N.J. Injectable corticosteroids: Take precautions and use caution Seminars in musculoskeletal radiology. Thieme 2016, 20, 401–408. [Google Scholar]
- Fu, Y.; Young, J.F.; Rasmussen, M.K.; Dalsgaard, T.K.; Lametsch, R.; Aluko, R.E.; Therkildsen, M. Angiotensin I–converting enzyme–inhibitory peptides from bovine collagen: Insights into inhibitory mechanism and transepithelial transport. Food Res. Int. 2016, 89, 373–381. [Google Scholar] [CrossRef]
- Goh, K.L.; Hiller, J.; Haston, J.L.; Holmes, D.F.; Kadler, K.E.; Murdoch, A.; Wess, T.J. Analysis of collagen fibril diameter distribution in connective tissues using small-angle X-ray scattering. Biochim. Biophys. BBA-Gen. Subj. 2005, 1722, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Hashim, P.; Hashim, M.D. Collagen in food and beverage industries. J. Food Sci. Technol. 2015, 22, 1–8. [Google Scholar]
- Jeevithan, E.; Bao, B.; Zhang, J.; Hong, S.; Wu, W. Purification, characterization and antioxidant properties of low molecular weight collagenous polypeptide (37 kDa) prepared from whale shark cartilage (Rhincodontypus). J. Food Sci. Technol. 2015, 52, 6312–6322. [Google Scholar] [CrossRef] [PubMed]
- Jhample, S.B.; Bhagwat, P.K.; Dandge, P.B. Statistical media optimization for enhanced production of fibrinolytic enzyme from newly isolated Proteus penneri SP-20. Biocatal. Agric. Biotechnol. 2015, 4, 370–379. [Google Scholar] [CrossRef]
- Sukhikh, S.; Noskova, S.; Ivanova, S.; Ulrikh, E.; Izgaryshev, A.; Babich, O. Chondroprotection and molecular mechanism of action of phytonutraceuticals on osteoarthritis. Molecules 2021, 26, 2391. [Google Scholar] [CrossRef]
- Asyakina, L.K.; Fotina, N.V.; Izgarysheva, N.V.; Slavyanskiy, A.A.; Neverova, O.A. Geroprotective potential of in vitro bioactive compounds isolated from yarrow (Achilleae millefolii L.) cell cultures. Foods Raw Mater. 2021, 9, 126–134. [Google Scholar] [CrossRef]
- Babich, O.; Sukhikh, S.; Prosekov, A.; Asyakina, L.; Ivanova, S. Medicinal Plants to Strengthen Immunity during a Pandemic. Pharmaceuticals 2020, 13, 313. [Google Scholar] [CrossRef]
- Kozlowska, J.; Sionkowska, A.; Skopinska-Wisniewska, J.; Piechowicz, K. Northern pike (Esoxlucius) collagen: Extraction, characterization and potential application. Int. J. Biol. Macromol. 2015, 81, 220–227. [Google Scholar] [CrossRef]
- Sukhikh, S.; Babich, O.; Prosekov, A.; Patyukov, N.; Ivanova, S. Future of Chondroprotectors in the Treatment of Degenerative Processes of Connective Tissue. Pharmaceuticals 2020, 13, 220. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R.F.; Collins, J.A.; Diekman, B.O. Ageing and the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 412–420. [Google Scholar] [CrossRef]
- Mashiko, T.; Takada, H.; Wu, S.H.; Kanayama, K.; Feng, J.; Tashiro, K.; Takato, T. Therapeutic effects of a recombinant human collagen peptide bioscaffold with human adipose-derived stem cells on impaired wound healing after radiotherapy. Tissue Eng. Regen. Med. 2018, 12, 1186–1194. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, A.; Bay-Jensen, A.C.; Van Spil, W.E.; Larkin, J.; Levesque, M.C. Osteoarthritis Year in Review 2016: Biomarkers (biochemical markers). Osteoarthr. Cartil. 2017, 25, 199–208. [Google Scholar] [CrossRef]
- Nishikimi, A.; Koyama, Y.I.; Ishihara, S.; Kobayashi, S.; Tometsuka, C.; Kusubata, M.; Kuwaba, K.; Hayashida, O.; Hattori, S.; Katagiri, K. Collagen-derived peptides modulate CD4+ T-cell differentiation and suppress allergic responses in mice. Immun. Inflamm. Dis. 2018, 6, 245–255. [Google Scholar] [CrossRef]
- Pal, G.K.; Suresh, P.V. Sustainable valorisation of seafood by-products: Recovery of collagen and development of collagen-based novel functional food ingredients. Innov. Food Sci. Emerg. Technol. 2016, 37, 201–215. [Google Scholar] [CrossRef]
- Paré, F.; Tardif, G.; Fahmi, H.; Ouhaddi, Y.; Pelletier, J.P.; Martel-Pelletier, J. In vivo protective effect of adipsin-deficiency on spontaneous knee osteoarthritis in aging mice. Aging 2020, 12, 2880. [Google Scholar] [CrossRef]
- Richette, P.; Latourte, A.; Frazier, A. Safety and efficacy of paracetamol and NSAIDs in osteoarthritis: Which drug to recommend? Expert Opin. Drug Saf. 2015, 14, 1259–1268. [Google Scholar] [CrossRef]
- Sader, M.S.; Martins, V.C.; Gomez, S.; LeGeros, R.Z.; Soares, G.A. Production and in vitro characterization of 3D porous scaffolds made of magnesium carbonate apatite (MCA)/anionic collagen using a biomimetic approach. Mater. Sci. Eng. C 2013, 33, 4188–4196. [Google Scholar] [CrossRef]
- Schmidt, M.M.; Dornelles, R.C.P.; Mello, R.O.; Kubota, E.H.; Mazutti, M.A.; Kempka, A.P.; Demiate, I.M. Collagen extraction process. J. Food Sci. Technol. 2016, 23, 913–922. [Google Scholar]
- Sionkowska, A. Biopolymeric nanocomposites for potential biomedical applications. Polym. Int 2016, 65, 1123–1131. [Google Scholar] [CrossRef]
- Sun, C.; Che, Y.; Lu, S. Preparation and application of collagen scaffold-encapsulated silver nanoparticles and bone morphogenetic protein 2 for enhancing the repair of infected bone. Biotechnol. Lett. 2015, 37, 467–473. [Google Scholar] [CrossRef]
- Taylor, N. Nonsurgical management of osteoarthritis knee pain in the older adult. Clin. Geriatr. Med. 2017, 33, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Trojian, T.H.; Concoff, A.L.; Joy, S.M.; Hatzenbuehler, J.R.; Saulsberry, W.J.; Coleman, C.I. AMSSM scientific statement concerning viscosupplementation injections for knee osteoarthritis: Importance for individual patient outcomes. Br. J. Sports Med. 2016, 50, 84–92. [Google Scholar] [CrossRef]
- Wahyudi, H.; Reynolds, A.A.; Li, Y.; Owen, S.C.; Yu, S.M. Targeting collagen for diagnostic imaging and therapeutic delivery. J. Control. Release 2016, 240, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Chen, B.; Wang, Y.; Wang, X.; Han, D.; Ding, D.; Zheng, Y.; Cao, Y.; Zhan, H.; Zhou, Y. The effectiveness of manual therapy for relieving pain, stiffness, and dysfunction in knee osteoarthritis: A systematic review and metaanalysis. Pain Physician 2017, 20, 229–243. [Google Scholar] [CrossRef][Green Version]
- Xie, F.; Kovic, B.; Jin, X.; He, X.; Wang, M.; Silvestre, C. Economic and humanistic burden of osteoarthritis: A systematic review of large sample studies. Pharmacoeconomics 2016, 34, 1087–1100. [Google Scholar] [CrossRef]
- Toledano, M.; Asady, S.; Toledano-Osorio, M.; García-Godoy, F.; Serrera-Figallo, M.-A.; Benítez-García, J.A.; Osorio, R. Differential Biodegradation Kinetics of Collagen Membranes for Bone Regeneration. Polymers 2020, 12, 1290. [Google Scholar] [CrossRef]
- Hon, W.N.; Yi, Z.; Rafea, N.; Sujay, P. Monitoring the Degradation of Collagen Hydrogels by Collagenase Clostridium histolyticum. Gels 2020, 6, 46. [Google Scholar]
- Dey, S.; Dora, K. Optimization of the production of shrimp waste protein hydrolysate using microbial proteases adopting response surface methodology. J. Food Sci. Technol. 2014, 51, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M. Processing of collagen based biomaterials and the resulting materials properties. Biomed. Eng. Online 2019, 18, 24. [Google Scholar] [CrossRef]
- Avery, N.C.; Bailey, A.J. Enzymic and non-enzymic cross-linking mechanisms in relation to turnover of collagen: Relevance to aging and exercise. Scand. J. Med. Sci. Sports 2005, 15, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Bailey, A.J.; Paul, R.G.; Knott, L. Mechanisms of maturation and ageing of collagen. Mech. Ageing Dev. 1998, 106, 1–56. [Google Scholar] [CrossRef]
- Avery, N.C.; Bailey, A.J. The effects of the Maillard reaction on the physical properties and cell interactions of collagen. Pathol. Biol. 2006, 54, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, Y.; Zhao, H.; Wang, H. Effect of heat treatment on the enzymatic stability of grass carp skin collagen and its ability to form fibrils in vitro. J. Sci. Food Agric. 2015, 95, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, C.; Atamaz, F.; Hepguler, S.; Argin, M.; Arkun, R. The safety and efficacy of intraarticular hyaluronan with/without corticosteroid in knee osteoarthritis: 1-year, single-blind, randomized study. Rheumatol. Int. 2006, 26, 314–319. [Google Scholar] [CrossRef]
- Atamaz, F.; Kirazli, Y.; Akkoc, Y. A comparison of two different intraarticular hyaluronan drugs and physical therapy in the management of knee osteoarthritis. Rheumatol. Int. 2006, 26, 873–878. [Google Scholar] [CrossRef]
- Aubry, L.; De-Oliveira-Ferreira, C.; Santé-Lhoutellier, V.; Ferraro, V. Redox Potential and Antioxidant Capacity of Bovine Bone Collagen Peptides towards Stable Free Radicals, and Bovine Meat Lipids and Proteins. Effect of Animal Age, Bone Anatomy and Proteases—A Step Forward towards Collagen-Rich Tissue Valorisation. Molecules 2020, 25, 5422. [Google Scholar] [CrossRef] [PubMed]
- Mishra, K.; Ojha, H.; Chandhury, N.K. Estimation of antiradical properties of antioxidants using DPPH• assay: A critical review and results. Food Chem. 2012, 130, 1036–1043. [Google Scholar] [CrossRef]
- Ji, D.; Roman, M.; Zhou, J.; Hildreth, J. Determination of chondroitin sulfate content in raw materials and dietary supplements by high-performance liquid chromatography with ultraviolet detection after enzymatic hydrolysis: Single-laboratory validation. J. AOXA Int. 2007, 90, 659–669. [Google Scholar] [CrossRef]
- Baburina, M.I.; Ivankin, A.N.; Stanovova, I.A. Chemical and biotechnological processing of collagen-containing raw materials into functional components of feed suitable for production of high-quality meat from farm animals. Earth Environ. Sci. 2017, 85, 012037. [Google Scholar] [CrossRef]
- Mezenova, T.Y.; Agafonova, S.V.; Mezenova, O.Y.; Baidalinova, L.S.; Grimm, T. Obtaining and estimating the potential of protein nutraceuticals from highly mineralized collagen-containing beef raw materials. Theory Pract. Meat Process. 2021, 6, 10–22. [Google Scholar] [CrossRef]
- Mao, J.S.; Cui, Y.L.; Wang, X.H.; Sun, Y.; Yin, Y.J.; Zhao, H.M.; De Yao, K. A preliminary study on chitosan and gelatin polyelectrolyte complex cytocompatibility by cell cycle and apoptosis analysis. Biomaterials 2004, 25, 3973–3981. [Google Scholar] [CrossRef] [PubMed]
- Angela, S. Optimisation of a Hydrolysed Collagen Production Process from Heat-Treated Bovine Bone Based on Acid and Enzymatic Proteolysis. Master’s Thesis, Queensland University of Technology, Brisbane City, Australia, 2020; pp. 32–38. [Google Scholar]
- Enzymes Market Size 2021. Global Comprehensive Research Study: Business Strategy, Industry Share, Supply-Demand, Growth Statistics, Growing Trends, Top Manufactures, Regional Forecast Analysis 2027. 2021. Available online: https://www.precisionreports.co/enquiry/request-sample/17207228 (accessed on 1 October 2021).
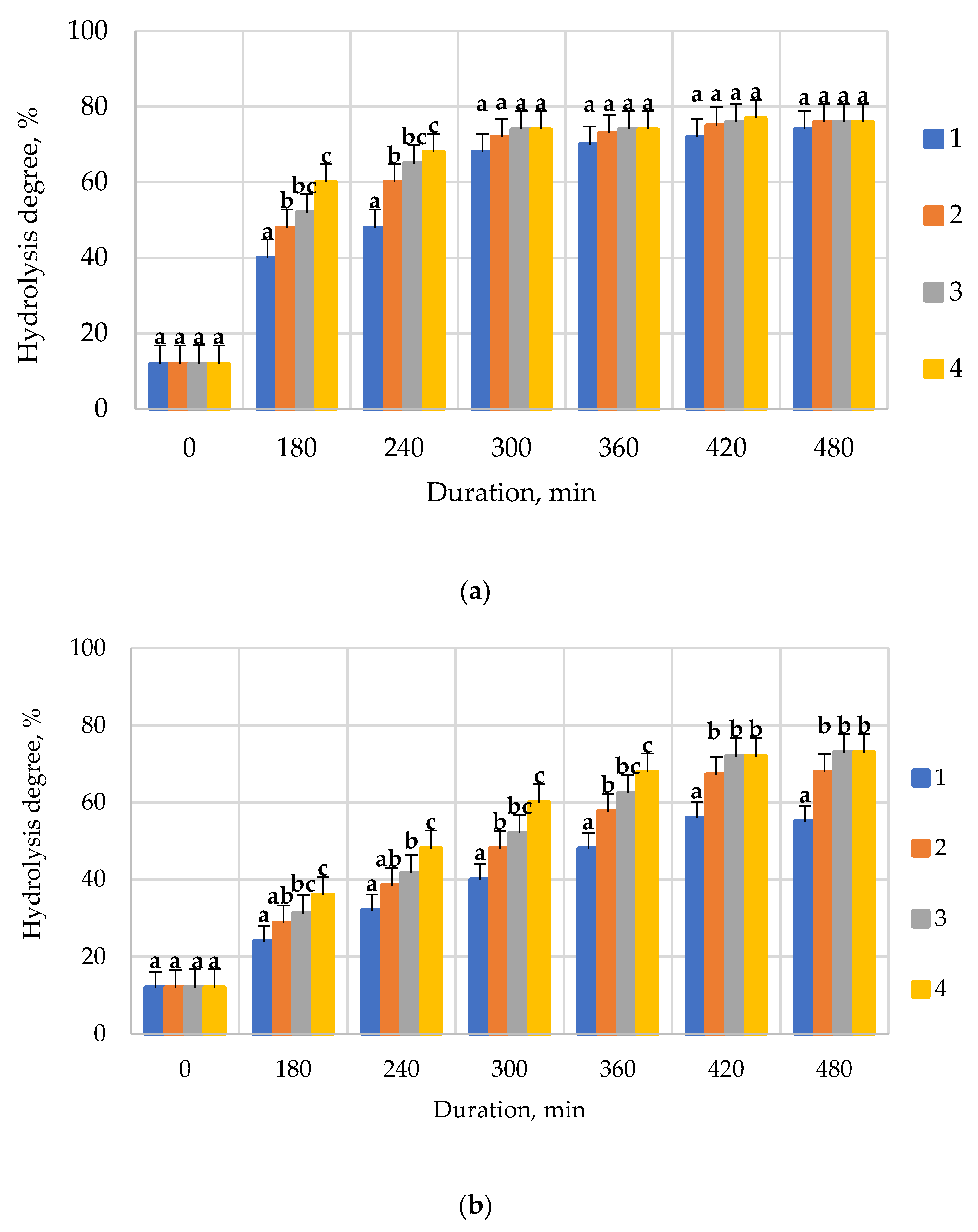
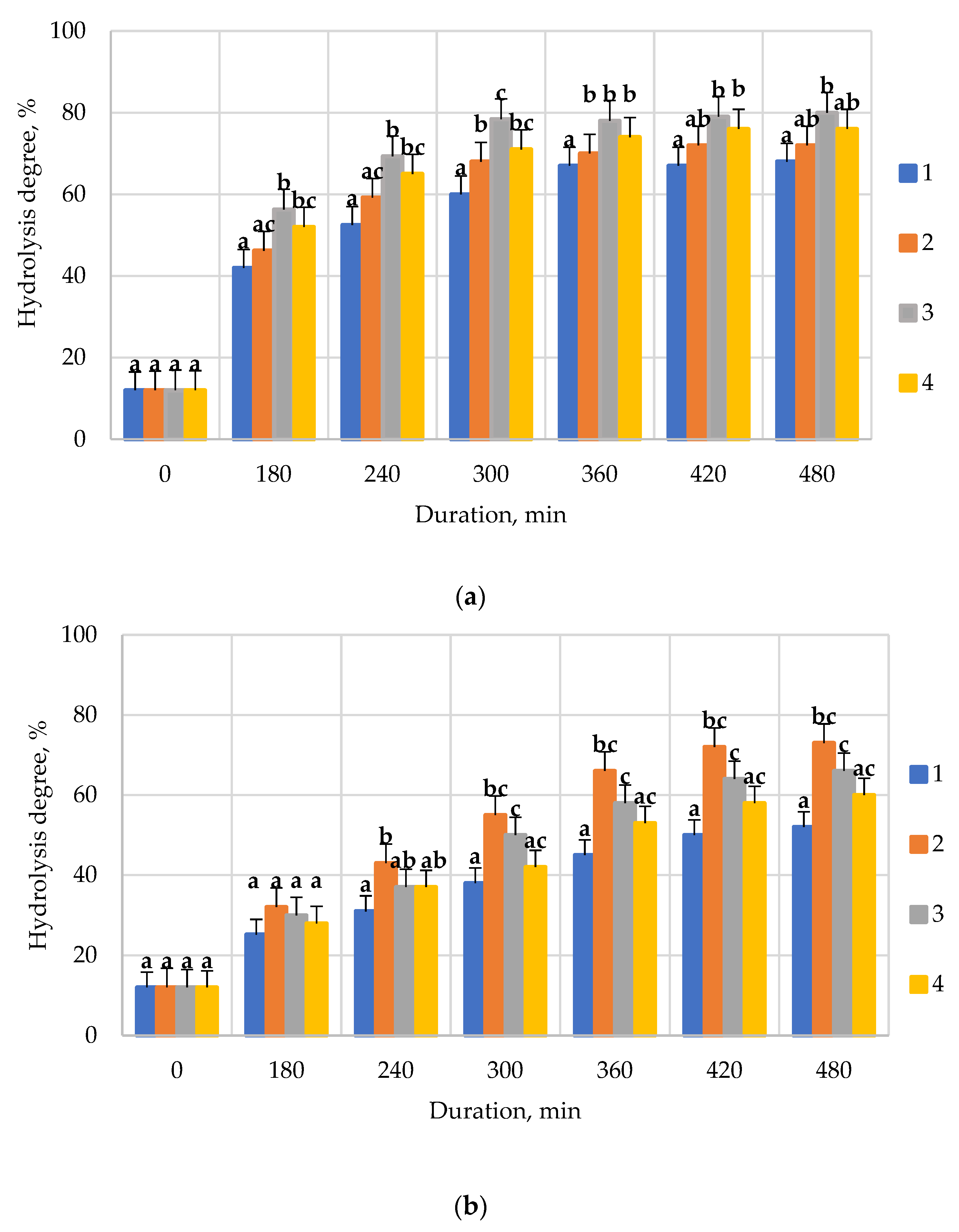
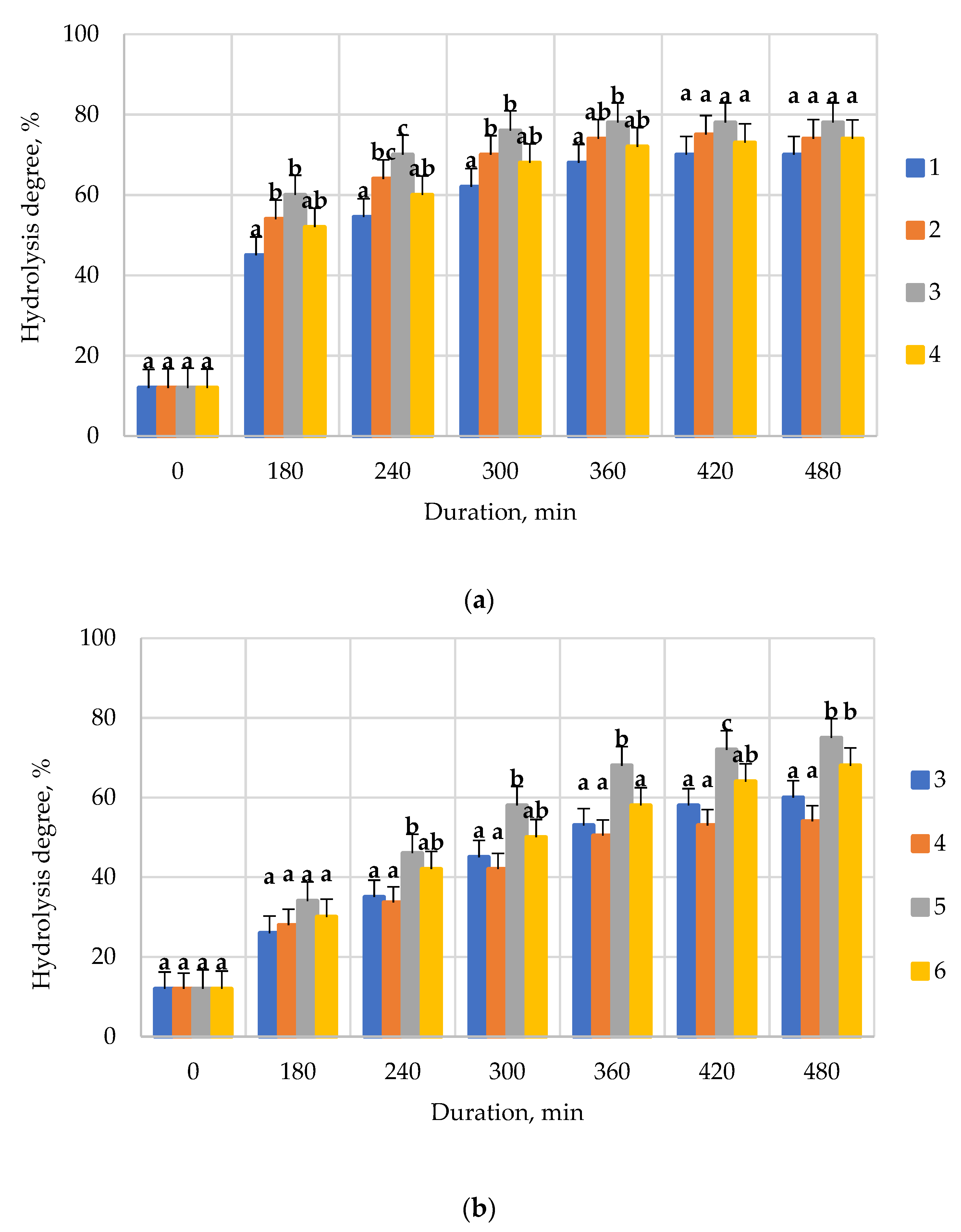

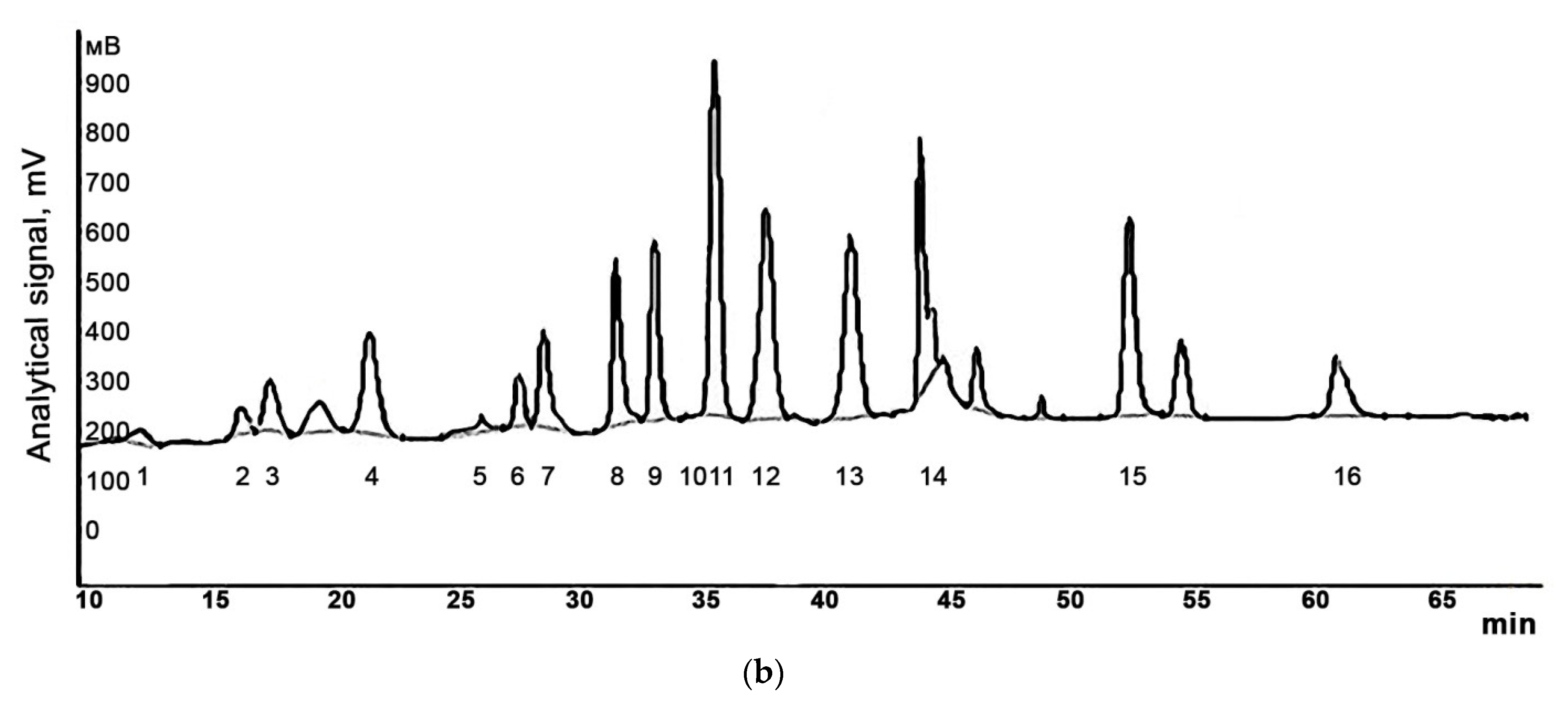
| Conditions | Enzyme Preparations | |
|---|---|---|
| Collagenase | Neutrase 1.5 MG | |
| Enzyme concentration, U/g | 250 | 250 |
| Duration, h | 6 | 7 |
| pH | 7.0 | 6.0 |
| Temperature, °C | 40 | 50 |
| Hydrolysis degree, % | 77–78 | 72–74 |
| The Molecular Weight of the Protein Profile, kDa | The Proportion of Protein and Peptide Fraction in the Hydrolyzate, % | |
|---|---|---|
| I | II | |
| 66.0–89.0 | 0.1 ± 0.003 | 0.3 ± 0.001 |
| 57.0–65.0 | 0.8 ± 0.02 | 0.6 ± 0.02 |
| 45.0–57.0 | 3.6 ± 0.11 | 3.8 ± 0.11 |
| 36.0–45.0 | 2.1 ± 0.06 | 1.9 ± 0.05 |
| 29.0–36.0 | 23.8 ± 0.71 | 22.4 ± 0.72 |
| 14.0–29.0 | 15.7 ± 0.47 | 14.5 ± 0.46 |
| 6.5–14.0 | 13.4 ± 0.40 | 12.8 ± 0.38 |
| 3.5–6.5 | 37.6 ± 0.45 | 39.2 ± 1.17 |
| 3.0–3.5 | 48.3 ± 1.44 | 46.7 ± 1.40 |
| Indicator | The Proportion of Carbohydrate Components in the Hydrolyzate, % | |
|---|---|---|
| I | II | |
| Hexose | 0.33 ± 0.01 | 0.28 ± 0.01 |
| Hexosamines | 2.14 ± 0.06 | 2.12 ± 0.06 |
| Free hexosamines | 6.87 ± 0.20 | 6.64 ± 0.20 |
| Hyaluronic acid | 1.61 ± 0.04 | 1.58 ± 0.04 |
| Chondroitin sulfates | 6.32 ± 0.18 | 6.31 ± 0.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sukhikh, S.; Noskova, S.; Ivanova, S.; Ulrikh, E.; Izgaryshev, A.; Larichev, T.; Kozlova, O.; Prosekov, A.; Babich, O. Comparative Analysis of Collagen-Containing Waste Biodegradation, Amino Acid, Peptide and Carbohydrate Composition of Hydrolysis Products. Appl. Sci. 2021, 11, 11511. https://doi.org/10.3390/app112311511
Sukhikh S, Noskova S, Ivanova S, Ulrikh E, Izgaryshev A, Larichev T, Kozlova O, Prosekov A, Babich O. Comparative Analysis of Collagen-Containing Waste Biodegradation, Amino Acid, Peptide and Carbohydrate Composition of Hydrolysis Products. Applied Sciences. 2021; 11(23):11511. https://doi.org/10.3390/app112311511
Chicago/Turabian StyleSukhikh, Stanislav, Svetlana Noskova, Svetlana Ivanova, Elena Ulrikh, Alexsander Izgaryshev, Timothy Larichev, Oksana Kozlova, Alexander Prosekov, and Olga Babich. 2021. "Comparative Analysis of Collagen-Containing Waste Biodegradation, Amino Acid, Peptide and Carbohydrate Composition of Hydrolysis Products" Applied Sciences 11, no. 23: 11511. https://doi.org/10.3390/app112311511
APA StyleSukhikh, S., Noskova, S., Ivanova, S., Ulrikh, E., Izgaryshev, A., Larichev, T., Kozlova, O., Prosekov, A., & Babich, O. (2021). Comparative Analysis of Collagen-Containing Waste Biodegradation, Amino Acid, Peptide and Carbohydrate Composition of Hydrolysis Products. Applied Sciences, 11(23), 11511. https://doi.org/10.3390/app112311511









