Anti-Aging Effects of Polyoxometalates on Skin
Abstract
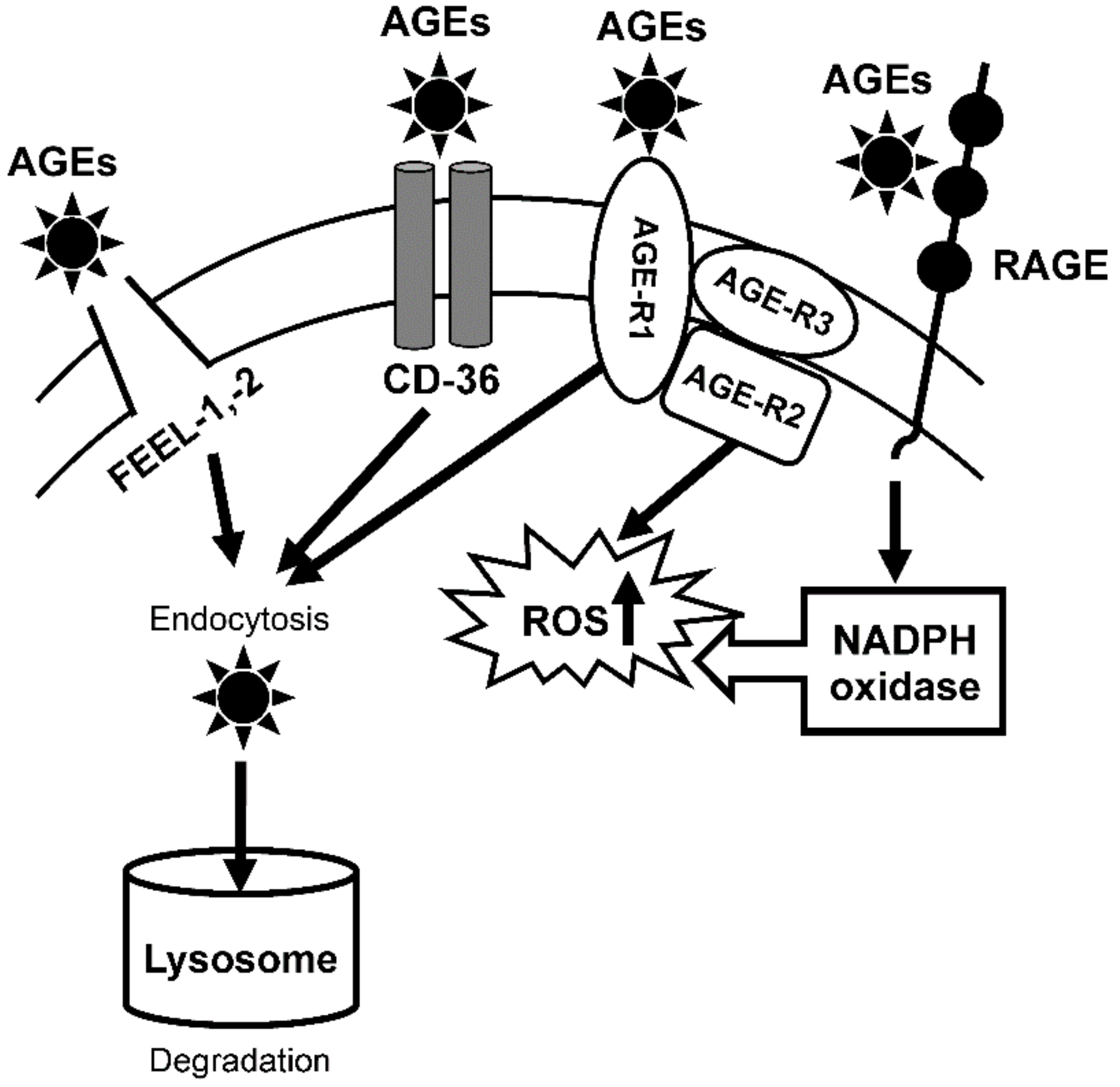
1. Introduction
2. Materials and Methods
2.1. PMs
2.2. Materials
2.3. Glycation of BSA
2.4. Cell Cultures
2.5. Effects of VBs on the mRNA Expression of AGE Receptors and Heat Shock Proteins (Hsps) in Cells Treated with AGE
2.6. Effects of VBs on the mRNA Expression of AQP-1 and AQP-3 in Cells Treated with H2O2
2.7. qRT-PCR
2.8. Effects of VBs on Cell Viability under Oxidative Stress Conditions
2.9. Measurement of Intracellular ROS Production
2.10. Measurement of SOD Production
2.11. Evaluation of the Production of Hyaluronan and Elastin
2.12. Statistical Analysis
3. Results
3.1. Anti-Aging Effects of VBs in an AGE-Induced Stress Environment
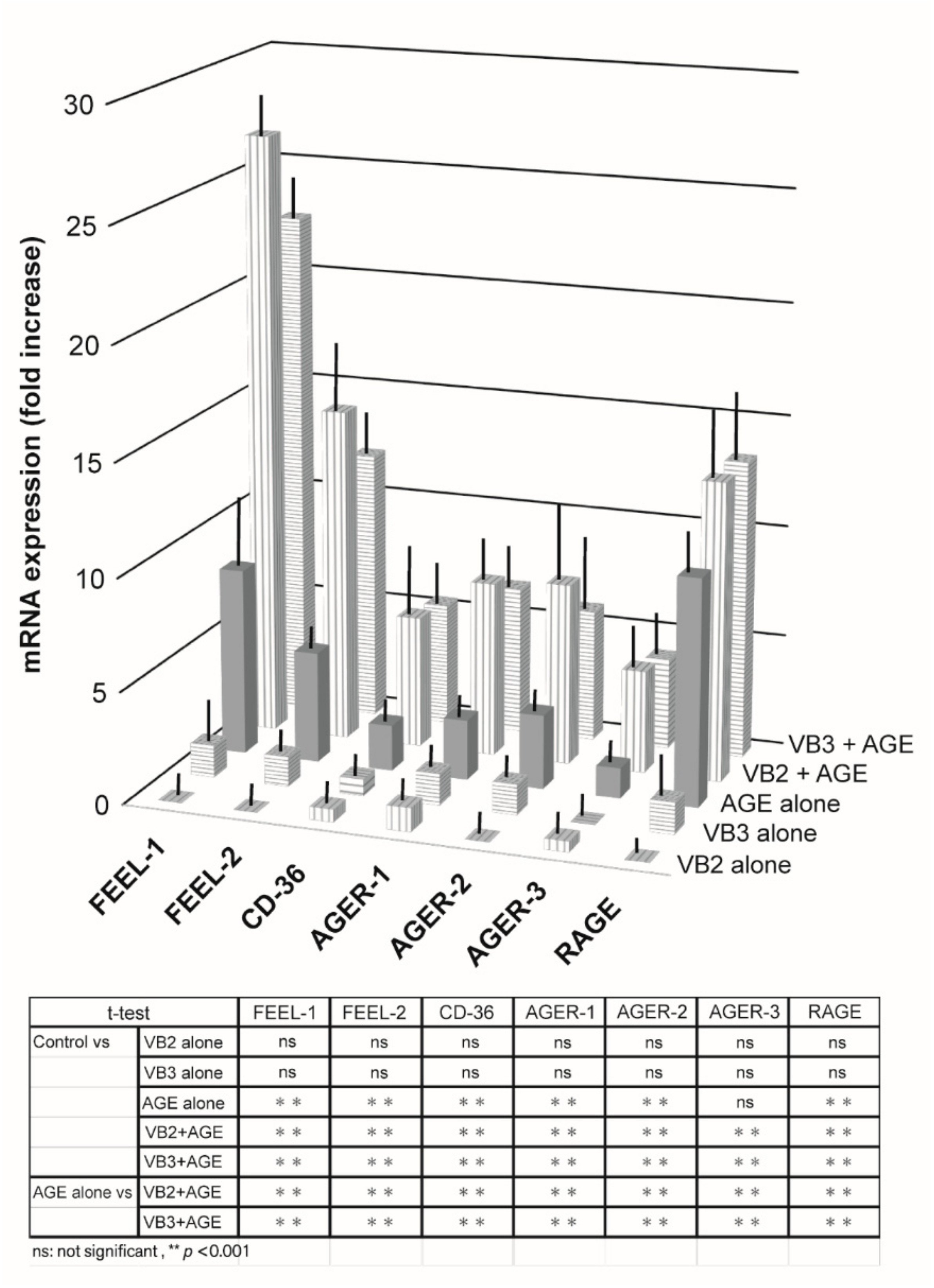
3.1.1. Expression of AGE Receptor mRNAs
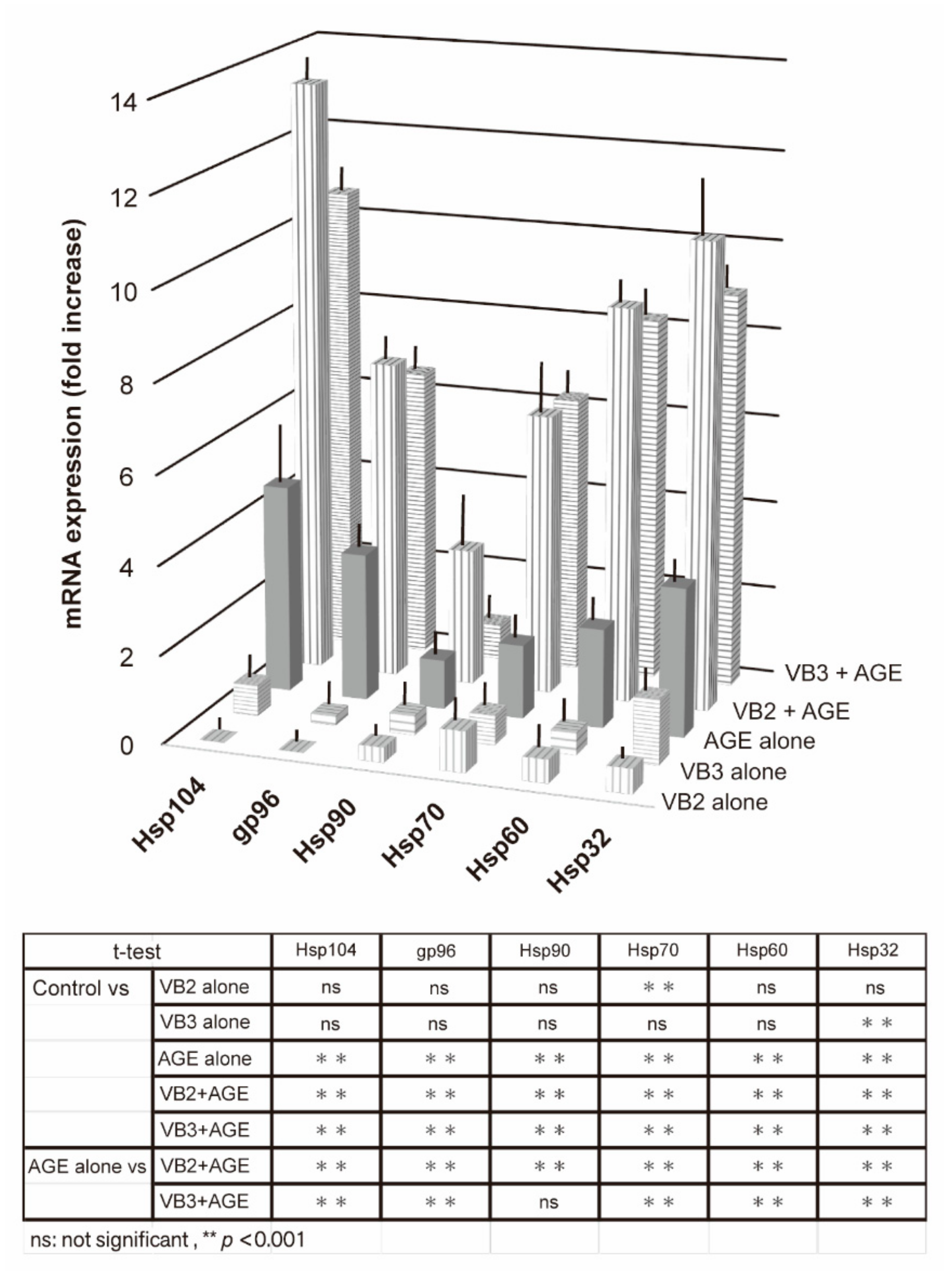
3.1.2. Expression of Hsp mRNAs
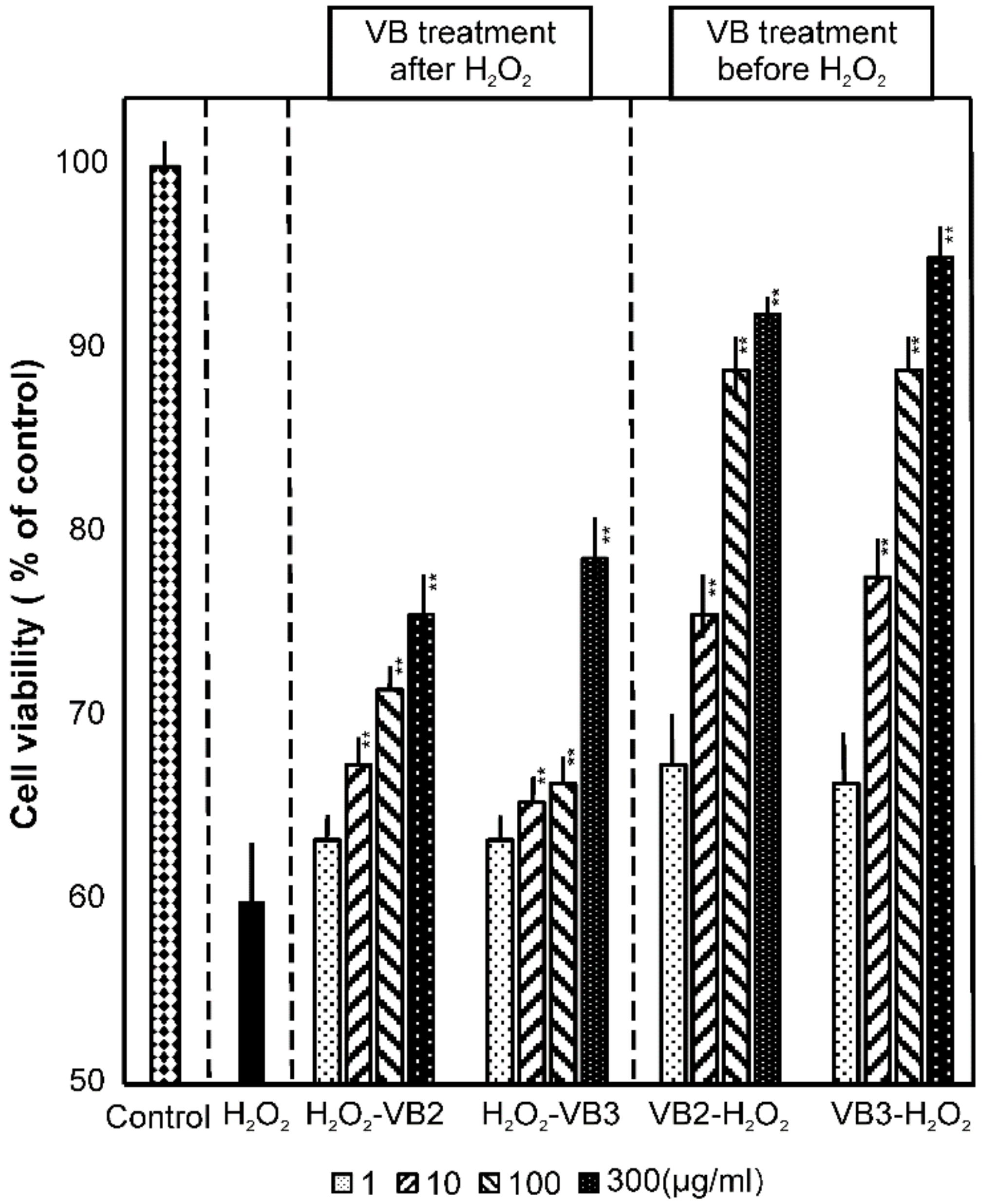
3.2. Effects of VBs on Cell Viability under Oxidative Stress Conditions
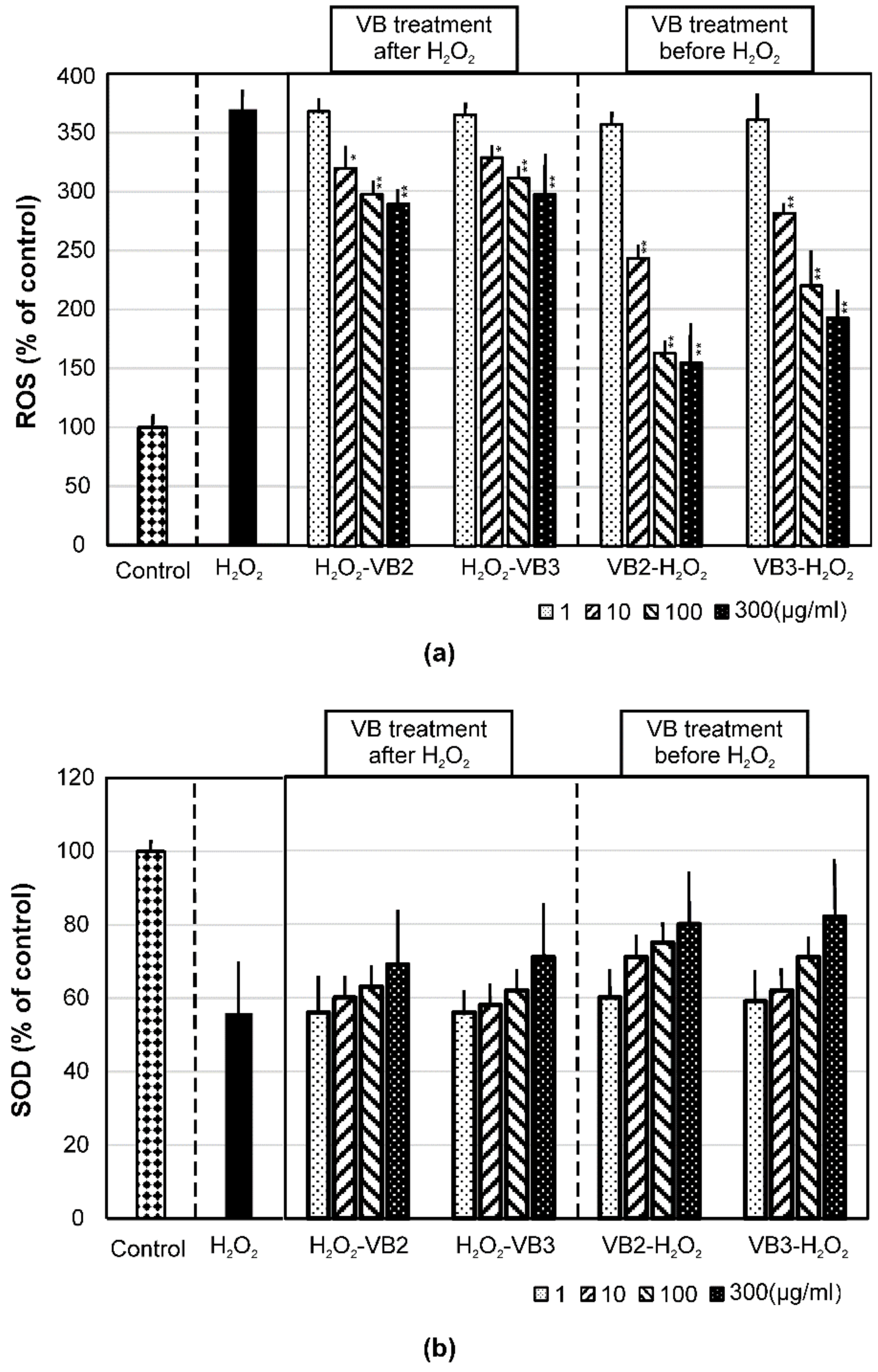
3.3. Effects of VBs on the Production of Intracellular ROS and SOD
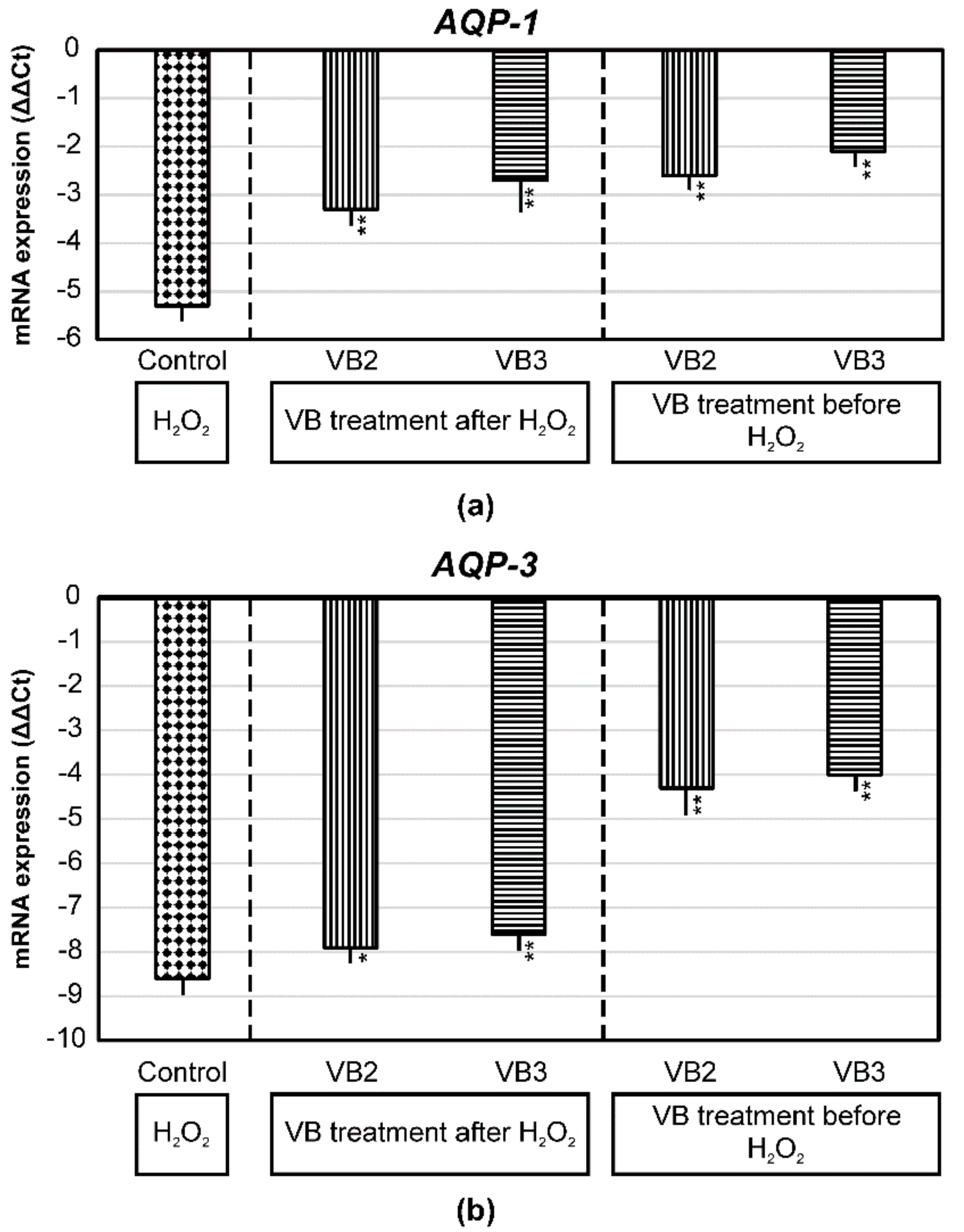
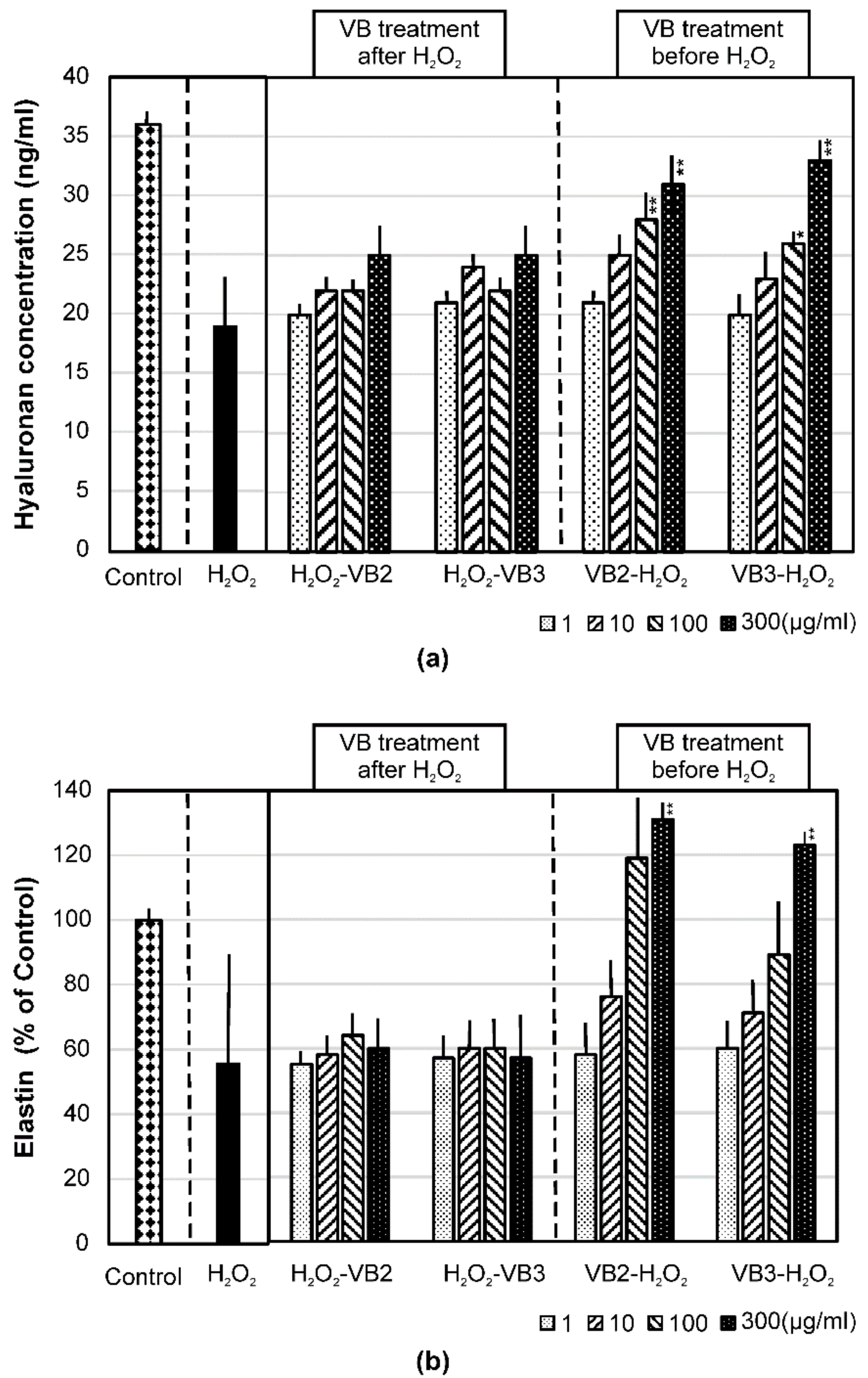
3.4. Effects of VBs on the mRNA Expression of AQP-1 and AQP-3 and on the Production of Hyaluronan and Elastin under Oxidative Stress Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Babior, B.M. Phagocytes and oxidative stress. Am. J. Med. 2000, 109, 33–44. [Google Scholar] [CrossRef]
- Ben-Porath, I.; Weinberg, R. A The signals and pathways activating cellular senescence. Int. J. Biochem. Cell Biol. 2005, 37, 961–976. [Google Scholar] [CrossRef] [PubMed]
- Richter, T.; von Zglinicki, T. A continuous correlation between oxidative stress and telomere shortening in fibroblasts. Exp. Gerontol. 2007, 42, 1039–1042. [Google Scholar] [CrossRef]
- Mizutani, H.; Tada-Oikawa, S.; Hiraku, Y.; Oikawa, S.; Kojima, M.; Kawanishi, S. Mechanism of apoptosis induced by a new topoisomerase inhibitor through the generation of hydrogen peroxide. J. Biol. Chem. 2002, 277, 30684–30689. [Google Scholar] [CrossRef]
- Brunet, A.; Sweeney, L.B.; Sturgill, J.F.; Chua, K.F.; Greer, P.L.; Lin, Y.; Tran, H.; Ross, S.E.; Mostoslavsky, R.; Cohen, H.Y.; et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 2004, 303, 2011–2015. [Google Scholar] [CrossRef] [PubMed]
- Yagi, M.; Yonei, Y. Glycative Stress and Anti-Aging: 7. Glycative stress and skin aging. Glycative Stress Res. 2018, 5, 50–54. [Google Scholar]
- Saxena, S.; Vekaria, H.; Sullivan, P.G.; Seifert, A.W. Connective tissue fibroblasts from highly regenerative mammals are refractory to ROS-induced cellular senescence. Nat. Commun. 2019, 10, 4400. [Google Scholar] [CrossRef]
- Reiser, K.M. Nonenzymatic glycation of collagen in aging and diabetes. Proc. Soc. Exp. Biol. Med. 1998, 218, 23–37. [Google Scholar] [CrossRef]
- Reddy, V.P.; Obrenovich, M.E.; Atwood, C.S.; Perry, G.; Smith, M.A. Involvement of Maillard reactions in Alzheimer disease. Neurotox. Res. 2002, 4, 191–209. [Google Scholar] [CrossRef]
- Schram, M.T.; Schalkwijk, C.G.; Bootsma, A.H.; Fuller, J.H.; Chaturvedi, N.; Stehouwer, C.D.; EURODIAB Prospective Complications Study Group. Advanced glycation end products are associated with pulse pressure in type 1 diabetes: The EURODIAB Prospective Complications Study. Hypertension 2005, 46, 232–237. [Google Scholar] [CrossRef]
- Brownlee, M.; Vlassara, H.; Kooney, A.; Ulrich, P.; Cerami, A. Aminoguanidine prevents diabetes-induced arterial wall protein cross-linking. Science 1986, 232, 1629–1632. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Fujii, K.; Soshi, S.; Tanaka, T. Reductions in degree of mineralization and enzymatic collagen cross-links and increases in glycation-induced pentosidine in the femoral neck cortex in cases of femoral neck fracture. Osteoporos. Int. 2006, 17, 986–995. [Google Scholar] [CrossRef]
- Kawanishi, S.; Hiraku, Y.; Oikawa, S. Mechanism of guanine-specific DNA damage by oxidative stress and its role in carcinogenesis and aging. Mutat. Res. 2001, 488, 65–76. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef]
- Xu, Y.; Yao, H.; Wang, Q.; Xu, W.; Liu, K.; Zhang, J.; Zhao, H.; Hou, G. Aquaporin-3 Attenuates oxidative stress-induced nucleus pulposus cell apoptosis through regulating the P38 MAPK pathway. Cell. Physiol. Biochem. 2018, 50, 1687–1697. [Google Scholar] [CrossRef]
- Quan, T.; Qin, Z.; Xia, W.; Shao, Y.; Voorhees, J.J.; Fisher, G.J. Matrix-degrading metalloproteinases in photoaging. J. Investig. Dermatol. Symp. Proc. 2009, 14, 20–24. [Google Scholar] [CrossRef]
- Xu, Q.; Hou, W.; Zheng, Y.; Liu, C.; Gong, Z.; Lu, C.; Lai, W.; Maibach, H.I. Ultraviolet A-induced cathepsin K expression is mediated via MAPK/AP-1 pathway in human dermal fibroblasts. PLoS ONE 2014, 9, e102732. [Google Scholar] [CrossRef]
- Yamase, T. Polyoxometalates active against tumors, viruses, and bacteria Review. Prog. Mol. Subcell. Biol. 2013, 54, 65–116. [Google Scholar] [CrossRef] [PubMed]
- Dan, K.; Miyashita, K.; Seto, Y.; Fujita, H.; Yamase, T. Mechanism of the protective effect of heteropolyoxotungstate against herpes simplex virus type 2. Pharmacology 2003, 67, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Dan, K.; Fujinami, K.; Sumitomo, H.; Ogiwara, Y.; Suhara, S.; Konno, Y.; Sawada, M.; Soga, Y.; Takada, A.; Takanashi, K.; et al. Application of antiviral polyoxometalates to living environments-Antiviral moist hand towels and stationery items. Appl. Sci. 2020, 10, 8246. [Google Scholar] [CrossRef]
- Maeda, S.; Matsui, T.; Ojima, A.; Takeuchi, M.; Yamagishi, S. Sulforaphane inhibits advanced glycation end product-induced pericyte damage by reducing expression of receptor for advanced glycation end products. Nutr. Res. 2014, 34, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Kartal, B.; Akçay, A.; Palabiyik, B. Oxidative stress upregulates the transcription of genes involved in thiamine metabolism. Turk. J. Biol. 2018, 42, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Chen, Z.W.; Li, H.M.; Yan, X.F.; Feng, B. AGE/RAGE-induced EMP release via the NOX-derived ROS pathway. J. Diabetes Res. 2018, 2018, 6823058. [Google Scholar] [CrossRef]
- Stoll, S.J.; Bartsch, S.; Kroll, J. HOXC9 regulates formation of parachordal lymphangioplasts and the thoracic duct in zebrafish via stabilin 2. PLoS ONE 2013, 8, e58311. [Google Scholar] [CrossRef][Green Version]
- Draude, G.; Lorenz, R.L. TGF-beta1 downregulates CD36 and scavenger receptor A but upregulates LOX-1 in human macrophages. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H1042–H1048. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Ishibashi, K.; Ishibashi, K.; Bhutto, I.A.; Tian, J.; Lutty, G.A.; Handa, J.T. The expression of advanced glycation endproduct receptors in rpe cells associated with basal deposits in human maculas. Exp. Eye Res. 2006, 82, 840–848. [Google Scholar] [CrossRef]
- Casselmann, C.; Reimann, A.; Friedrich, I.; Schubert, A.; Silber, R.E.; Simm, A. Age-dependent expression of advanced glycation end product receptor genes in the human heart. Gerontology 2004, 50, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Bründl, J.; Wallinger, S.; Breyer, J.; Weber, F.; Evert, M.; Georgopoulos, N.T.; Rosenhammer, B.; Burger, M.; Otto, W.; Rubenwolf, P. Expression, Localization and potential significance of aquaporins in benign and malignant human prostate tissue. BMC Urol. 2018, 18, 75. [Google Scholar] [CrossRef]
- Tamura, Y.; Adachi, H.; Osuga, J.; Ohashi, K.; Yahagi, N.; Sekiya, M.; Okazaki, H.; Tomita, S.; Iizuka, Y.; Shimano, H.; et al. FEEL-1 and FEEL-2 are endocytic receptors for advanced glycation end products. J. Biol. Chem. 2003, 278, 12613–12617. [Google Scholar] [CrossRef] [PubMed]
- Koyama, H.; Yamamoto, H.; Nishizawa, Y. RAGE and soluble RAGE: Potential therapeutic targets for cardiovascular diseases. Mol. Med. 2007, 13, 625–635. [Google Scholar] [CrossRef]
- Lee, A.C.; Lam, J.K.; Shiu, S.W.; Wong, Y.; Betteridge, D.J.; Tan, K.C. Serum level of soluble receptor for advanced glycation end products is associated with a disintegrin and metalloproteinase 10 in type 1 diabetes. PLoS ONE 2015, 10, e0137330. [Google Scholar] [CrossRef]
- Inoue, Y.; Hanazono, Y.; Noi, K.; Kawamoto, A.; Kimatsuka, M.; Harada, R.; Takeda, K.; Kita, R.; Iwamasa, N.; Shibata, K.; et al. Split conformation of Chaetomium thermophilum Hsp104 disaggregase. Structure 2021, 29, 721–730.e6. [Google Scholar] [CrossRef] [PubMed]
- Udono, H.; Levey, D.L.; Srivastava, P.K. Cellular Requirements for tumor-specific immunity elicited by heat shock proteins: Tumor rejection antigen gp96 primes CD8+T cells in vivo. Proc. Natl. Acad. Sci. USA 1994, 91, 3077–3081. [Google Scholar] [CrossRef] [PubMed]
- Pozo, F.M.; Oda, T.; Sekimoto, T.; Murakumo, Y.; Masutani, C.; Hanaoka, F.; Yamashita, T. Molecular chaperone Hsp90 regulates REV1-mediated mutagenesis. Mol. Cell. Biol. 2011, 31, 3396–3409. [Google Scholar] [CrossRef]
- Rapoport, I.; Boll, W.; Yu, A.; Böcking, T.; Kirchhausen, T. A motif in the clathrin heavy chain required for the Hsc70/auxilin uncoating reaction. Mol. Biol. Cell 2008, 19, 405–413. [Google Scholar] [CrossRef]
- Cuervo, A.M. Chaperone-mediated autophagy: Dice’s ‘wild’ idea about lysosomal selectivity. Nat. Rev. Mol. Cell Biol. 2011, 12, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Horwich, A.L. Chaperonin-mediated protein folding. J. Biol. Chem. 2013, 288, 23622–23632. [Google Scholar] [CrossRef] [PubMed]
- Barakat, B.M.; Ahmed, H.I.; Bahr, H.I.; Elbahaie, A.M. Protective Effect of Boswellic Acids Against Doxorubicin-induced hepatotoxicity: Impact on Nrf2/HO-1 defense pathway. Oxid. Med. Cell. Longev. 2018, 2018, 8296451. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, H.; Cegielska, A.; Wojciechowska, A.; Delobel, P. Prospective, randomized, investigator-blinded, split-face evaluation of a topical crosslinked hyaluronic acid serum for post-procedural improvement of skin quality and biomechanical attributes. J. Drugs Dermatol. 2018, 17, 442–450. [Google Scholar]
- Kirkpatrick, C.E.; Megella, C. Use of ivermectin in treatment of Aelurostrongylus abstrusus and Toxocara cati infection in a cat. J. Am. Vet. Med. Assoc. 1987, 190, 1309–1310. [Google Scholar] [PubMed]
- Song, I.B.; Gu, H.; Han, H.J.; Lee, N.Y.; Cha, J.Y.; Son, Y.K.; Kwon, J. Effects of 7-MEGATM 500 on oxidative stress, inflammation, and skin regeneration in H2O2-treated skin cells. Toxicol. Res. 2018, 34, 103–110. [Google Scholar] [CrossRef] [PubMed]

| Target | Primer | References or NCBI Reference Sequence |
|---|---|---|
| FEEL-1 (Stabilin-1) | Forward: AGG ACT GCC GCT ACG AAG TA | 18 |
| Reverse: CAC TGC CCT GCT GTG TGT AG | ||
| FEEL-2 (Stabilin-2) | Forward: TCT GAA GGC AGG TCT CAC CTA | 18 |
| Reverse: CTG GGG AGC AGA AAT TTT GTA | ||
| CD-36 | Forward: GAG AAC TGT TAT GGG GCT AT | 19 |
| Reverse: TTC AAC TGG AGA GGC AAA GG | ||
| AGE receptor-1 | Forward: GTG GGA AAA TGG CAC AAC TT | 20 |
| Reverse: CTG GCC ACG TCC CTA TTT TA | ||
| AGE receptor-2 | Forward: AGG GCC GTA AGG AGA GAG AG | 21 |
| Reverse: GTG GCG TCT GTC TGT GTG TC | ||
| AGE receptor-3 | Forward: GAT AAC AAT TCT GGG CAC GG | 21 |
| Reverse: TGG AGC ACT GGT GAG GTC TAT G | ||
| RAGE | Forward: GAA ACT GAA CAC AGG CCG GA | 22 |
| Reverse: CAC GGA CTC GGT AGT TGG AC | ||
| Hsp-104 | Forward: TCA TCG ACA AGG ACA GCA AG | NM0012583 |
| Reverse: GGC TGT GAG GAG GTA AGC AG | ||
| gp96 | Forward: TGG GAA GAG GTT CCA GAA TG | AJ890084.1 |
| Reverse: GTT GCC AGA CCA TCC GTA CT | ||
| Hsp-90 | Forward: TGG ACA GCA AAC ATG GAG AG | BC121062 |
| Reverse: AGA CAG GAG CGC AGT TTC AT | ||
| Hsp-70 | Forward: AGT GGT GGC CAC TAA TGG AG | BC112963 |
| Reverse: CAA TCC TTG CTT GAT GCT GA | ||
| Hsp-60 | Forward: GCA ATG TGT CCA GAG CAA GA | AF143723.1 |
| Reverse: AAG CTC AAC AGC TGG GAA AA | ||
| Hsp-32 | Forward: TCC GAT GGG TCC TTA CAC TC | NM002133.2 |
| Reverse: TAA GGA AGC CAG CCA AGA GA | ||
| AQP-1 | Forward: TCT GTA GCC CTT GGA CAC CT | 23 |
| Reverse: CAA AGG ACC GAG CAG GGT TA | ||
| AQP-3 | Forward: TGC TAC CTA CCC CTC TGG AC | 23 |
| Reverse: GCC AGC ACA CAC ACG ATA AG | ||
| GAPDH | Forward: AGG GCT GCT TTT AAC TCT GGT | 22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujinami, K.; Dan, K.; Tanaka-Kagawa, T.; Kawamura, I. Anti-Aging Effects of Polyoxometalates on Skin. Appl. Sci. 2021, 11, 11948. https://doi.org/10.3390/app112411948
Fujinami K, Dan K, Tanaka-Kagawa T, Kawamura I. Anti-Aging Effects of Polyoxometalates on Skin. Applied Sciences. 2021; 11(24):11948. https://doi.org/10.3390/app112411948
Chicago/Turabian StyleFujinami, Katsuyuki, Katsuaki Dan, Toshiko Tanaka-Kagawa, and Ikuo Kawamura. 2021. "Anti-Aging Effects of Polyoxometalates on Skin" Applied Sciences 11, no. 24: 11948. https://doi.org/10.3390/app112411948
APA StyleFujinami, K., Dan, K., Tanaka-Kagawa, T., & Kawamura, I. (2021). Anti-Aging Effects of Polyoxometalates on Skin. Applied Sciences, 11(24), 11948. https://doi.org/10.3390/app112411948







