Abstract
Microbial lipids called a sustainable alternative to traditional vegetable oils invariably capture the attention of researchers. In this study, the effect of limiting inorganic phosphorus (KH2PO4) and nitrogen ((NH4)2SO4) sources in lipid-rich culture medium on the efficiency of cellular lipid biosynthesis by Y. lipolytica yeast has been investigated. In batch cultures, the carbon source was rapeseed waste post-frying oil (50 g/dm3). A significant relationship between the concentration of KH2PO4 and the amount of lipids accumulated has been revealed. In the shake-flask cultures, storage lipid yield was correlated with lower doses of phosphorus source in the medium. In bioreactor culture in mineral medium with (g/dm3) 3.0 KH2PO4 and 3.0 (NH4)2SO4, the cellular lipid yield was 47.5% (w/w). Simultaneous limitation of both phosphorus and nitrogen sources promoted lipid accumulation in cells, but at the same time created unfavorable conditions for biomass growth (0.78 gd.m./dm3). Increased phosphorus availability with limited cellular access to nitrogen resulted in higher biomass yields (7.45 gd.m./dm3) than phosphorus limitation in a nitrogen-rich medium (4.56 gd.m./dm3), with comparable lipid yields (30% and 32%). Regardless of the medium composition, the yeast preferentially accumulated oleic and linoleic acids as well as linolenic acid up to 8.89%. Further, it is crucial to determine the correlation between N/P molar ratios, biomass growth and efficient lipid accumulation. In particular, considering the contribution of phosphorus as a component of coenzymes in many metabolic pathways, including lipid biosynthesis and respiration processes, its importance as a factor in the cultivation of the oleaginous microorganisms was highlighted.
1. Introduction
Among oleaginous microorganisms capable of accumulating lipids exceeding 20% of cell dry weight, the species Yarrowia lipolytica stands out as a model organism [1]. Moreover, the non-conventional yeast Y. lipolytica is a permissive species with wide-ranging biotechnological applications. Considering well-studied metabolism, fully sequenced genome and secretion capabilities that provide opportunities to obtain new microbial products previously obtained through other routes, they have become a model for oleaginous species in many basics and applications studies [2,3,4,5,6]. Single cell oil (SCO) extracted from cells of oleaginous microorganisms is promising for food technology and nutrition notably due to its content of polyunsaturated fatty acids. It is reported that SCOs in addition to polysaccharides or single cell proteins (SCPs) could be a valuable food additive. Microbial lipids may increase the nutritional value of the final product enriched with these components, being at the same time a potential substitute for vegetable lipids, e.g., palm oil, cocoa butter and other fatty acids of industrial importance [7,8].
Nowadays, PUFA-rich SCOs are mainly extracted from microalgae due to the innate ability to synthesize valuable fatty acids and lipid production efficiency, such as Schizochytrium sp. 50–77% of dry weight, Nitzschia sp. 45–47%, Nannochloropsis sp. 31–68% or Neochloris oleoabundans 35–54% oil content [9]. Oleaginous yeasts, compared to microalgae, have a rather lower ability to synthesize SCO. Remarkably, wild yeast strains of Y. lipolytica metabolizing glucose are able to store lipids up to 36% of cell dry weight, and even up to 50–60% in the case of feeding biomass with hydrophobic substrates [6,10]. In SCO production, lipid yield per unit dry weight is a critical factor; thus, solutions are still being sought to improve it [11]. Fields such as synthetic and system biology, along with metabolic engineering techniques, are used to improve lipid storage yield and obtain lipids rich in polyunsaturated fatty acids (PUFAs). By using various molecular biology methods, it has been possible to obtain Y. lipolytica yeast mutants capable of accumulating up to 25% eicosapentenoic acid in the total content of the fatty acids [12].
Oleaginous yeasts, depending on the type of carbon substrate in the culture medium, accumulate lipids through two different biochemical pathways: de novo for hydrophilic and ex novo for hydrophobic substrates. In recent years, research has been conducted to define and analyze genes associated with the activity of enzymes specific to both biosynthetic pathways [2,13]. There are reports that sometimes cellular lipid accumulation may occur through two pathways simultaneously [2].
The yeast Y. lipolytica is well known for its ability to metabolize complex lipid substrates, including industrial waste products [14]. Studies on SCO synthesis using including pork lard, stearin-an industrial derivative of animal fat, waste glycerol, molasses and other lignocellulosic wastes have been reported in the literature [11,15,16,17]. Nowadays, processed vegetable oils (waste cooking oils—WCO) have been considered a waste product with high potential for biotechnological application [18]. Y. lipolytica strains are able to produce many metabolites in media with waste cooking oils: citric acid [19], erythritol [20], lipases [21,22], and microbial lipids [23,24,25]. In this manner, environmentally burdensome wastes have found application in the cell culture of Y. lipolytica species. The enormous potential of Y. lipolytica in the utilization and management of hydrophobic industrial wastes was presented in a review by Wierzchowska et al. [26].
The oleaginous yeast accumulates significant amounts of SCO under stress conditions caused by limited access to nutrients. Biosynthesis of microbial oil is enhanced in media depleted of nutrients other than carbon. The key issue for efficient lipid accumulation is limiting access to nitrogen [27]. Unlike nitrogen, access of microorganism cells to carbon should be unrestricted, at a constantly high level [28]. There is a notion that a high carbon/nitrogen ratio and a high carbon/phosphorus ratio in the medium composition promotes lipid accumulation in oleaginous yeast cells [29]. Many studies have been carried out on the potential of oleaginous microorganisms to accumulate high lipid contents in medium with a limiting element (nitrogen, magnesium, phosphorus, iron, zinc, etc.) [29,30,31,32,33,34,35]. However, most of works was concerned the analysis of the effects of nutrient limitation on the synthesis of storage lipids via the de novo pathway. Therefore, there is still some need to explore how its limitation involves the ex novo biosynthesis route.
The dynamics of microbial processes is inextricably linked to the response of microorganisms to changes in the environmental conditions in which they grow. To ensure optimal production efficiency on an industrial scale, tolerance of microorganisms to environmental stress is highly desirable. In particular, this is due to the growing interest in microbial biotechnological processes. Understanding how physiological systems respond to changing environmental conditions resulting in a specific level of growth or production of desired metabolites is critical to finding new and optimizing already developed applications [36].
This paper attempts to evaluate the effect of limiting inorganic phosphorus and nitrogen sources as well as duration of culture on the efficiency of microbial oil production, fatty acid composition and growth of Y. lipolytica yeast with simultaneous waste valorization. The work assumed the use of oleaginous yeasts to manage waste from the food industry, specifically rapeseed post-frying oil, as an essential carbon source for cell growth.
2. Materials and Methods
2.1. Yeast strain and Culture Conditions
In the current work, the yeast strain Y. lipolytica KKP 379 from the Collection of Industrial Microorganisms Cultures belonging to Professor Wacław Dąbrowski Institute of Agricultural and Food Biotechnology—State Research Institute in Warsaw (Poland) was used. The yeast culture was stored at −20 °C using cryovials containing ceramic beads and cryoprotective agent (Protect Select, Technical Service Consultants Ltd., Heywood, UK).
Inoculation culture was conducted in YPG medium with the following composition: 10 g/dm3 yeast extract (Y), 20 g/dm3 peptone (P) and 20 g/dm3 glucose (G). The flasks were incubated at 28 °C for 24 h with a rotation amplitude of 140 rpm.
The yeast cells were incubated in 500 cm3 flasks at 28 °C with 140 rpm rotary shaker speed. Each flask contained 200 cm3 of sterile medium. Batch cultures were also conducted in a BIOFLO 3000 laboratory bioreactor from New Brunswick Scientific (USA) with a working volume of 4 dm3, fermentation temperature 28 °C and 0.025% (v/v) inoculum. Oxygenation control was applied using compressed air to maintain the relative degree of oxygenation in the culture medium at a level not lower than 30% of the initial oxygen concentration (variable agitation speed 300–600 rpm). The dissolved oxygen level was measured using an oxygen electrode. Changes in pH values were also monitored using a selective electrode. Parameters of cellular lipid biosynthesis in a batch culture of Y. lipolytica strain were calculated according to Fabiszewska et al. [2].
The yeast grew in a mineral medium containing 1.5 g/dm3 MgSO4, 0.16 g/dm3 FeSO4·H2O, 0.15 g/dm3 CaCl2, 0.08 g/dm3 MnCl2·4H2O, 0.02 g/dm3 ZnSO4 and KH2PO4, Na2HPO4, and (NH4)2SO4 at different levels. All inorganic chemicals were purchased from Avantor Performance Materials Poland S.A (Gliwice, Poland). In all batch cultures, the carbon source was waste rapeseed post-frying oil at 50 g/dm3. Waste oil, in which cod (Gadus morhua) fillets were fried at 170 °C in full immersion, came from a fish processing company in Podlaskie Voivodeship (Poland). The preliminary batch shaking flask cultures in duplicate have been conducted with statistical planning of experiments using a Latin square plan (4 × 4) (Table 1).

Table 1.
Latin Square plan 4 × 4-flask experiment design.
Bioreactor batch cultures have been conducted in media containing variable concentrations of KH2PO4, Na2HPO4 and (NH4)2SO4 (Table 2)

Table 2.
Composition of culture media used in yeast cultivation in a laboratory bioreactor.
2.2. General Analytical Techniques
Yeast biomass yield was determined by cell dry weight measured by thermogravimetric method. Cells were harvested by centrifugation at 8000 rpm, 4 °C for 10 min, washed using distilled water and dried at 105 °C to constant weight.
Determination of nitrogen content in the culture medium after biomass removal was carried out by the modified Kjeldahl method [37] with the sample mineralization step omitted. From 90 cm3 of the culture medium sample, ammonia was distilled (50 cm3 40% NaOH, distillation time—4 min) into 25 cm3 4% boric acid. The solution was titrated with 0.1 M HCl in the presence of Tashiro indicator. The nitrogen content of the sample was converted to the level of ammonium sulfate remaining in the medium.
Extraction of cellular lipids from dry material was performed in Soxhlet extractor using n-hexane as a solvent. The authors used modified Folch et al. [38] method extraction for batch shaking cultures by treating the dried and washed biomass four times with portions of a chloroform and methanol (2:1) mixture (1 cm3/1 gd.m). The solvents were separated by distillation under reduced pressure of 360 mbar. Distillation was carried out in a Buchi Rotavapor R-200 evaporator (Flawil, Switzerland). The lipid substrate consumption in the culture was evaluated using a simple extraction method with 10 cm3 portions of hexane. Magnesium sulfate was then added to the oil phase to remove water. After 10 min, the whole was filtered to remove the drying agent. The solvent was evaporated from the organic phase and the remaining oil was weighed.
2.3. Gas Chromatography
Microbial lipid samples extracted from yeast cells were derivatized with 14% solution of BF3 (boron trifluoride) in methanol added in equal volumes and heated for 2 h at 60 °C. Fatty acid composition of microbial lipids was determined by gas chromatography using a flame ionization detector (GC-FID) in an Agilent Technology 7820 (Santa Clara, CA, USA) with Zebron ZB-FFAP Capillary GC Column (30 m × 0.25 mm × 0.25 µm). Nitrogen has been used as a carrier gas at a flow rate of 35 mL/min. The temperature program was as follows: 80 °C (2 min) to 200 °C (10 min) (5 °C/min). Injector temperature: 250 °C; detector temperature: 290 °C; injection volume: 1 µL.
2.4. Statistical Analyses
Statistical elaboration of the results was performed using Statistica 13.0 set plus software (Statsoft, Cracow, Poland). A design of experiment was applied as a tool in investigating the effect of selected culture conditions on Y. lipolytica growth and lipid biosynthesis yield. A 4 × 4 latin square design method was used in the experiment provided in shaken flasks and p-value was then estimated at 0.01 (Table 1).
3. Results
3.1. Flask Scale Experiments
The preliminary experiment has been designed to evaluate the effect of culture time as well as limiting components such as phosphorus and nitrogen source on the growth of Y. lipolytica yeast biomass, cellular lipid yield, and the utilization of hydrophobic substrate as a carbon source. The cultures have been conducted in 16 medium variants, in which the concentrations of KH2PO4 (3.0, 7.0, 9.0, 11.0 g/dm3) and (NH4)2SO4 (4.0, 6.0, 8.0, 10.0 g/dm3) have been modified according to the experimental scheme (Table 1). The incubations were terminated after 3, 4, 5 and 6 days. As can be seen in Figure 1A, the value of biomass yield was closely related only to the time of culture (p-value 0.01). The effect of the duration of yeast cultivations was also significant for intracellular lipid content. The yeast cells accumulated higher amounts of lipids on the 5th and 6th days of culture. The level of KH2PO4 supplementation appeared to be a significant factor influencing lipid accumulation efficiency (p-value 0.01) (Figure 1B). Higher lipid biosynthesis yield was correlated with lower doses of inorganic phosphorus source in the medium. In the case of the level of the nitrogen source additive in the form of (NH4)2SO4, no significant relationship was found in the studied concentration range. It is noteworthy that none of the three analyzed factors was significantly associated with changes in the content of waste post-frying oil (carbon source) in the medium. Nevertheless, it decreased with time of culture (Figure 1C).
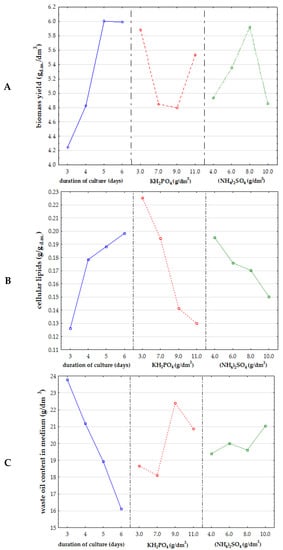
Figure 1.
Statistical analysis of correlation between (A) biomass yield (gd.m./dm3), (B) cellular lipid content (g/gd.m.), (C) waste post-frying oil content in medium (g/dm3) and concentration of KH2PO4 and (NH4)2SO4, and culture time of Y. lipolytica KKP 379 yeast in medium with 5% addition of post-frying rapeseed oil.
3.2. Bioreactor Scale Experiments
Four batch cultures of Y. lipolytica KKP 379 strain in a laboratory bioreactor have been conducted to evaluate the effect of the previously selected factors. In each culture, changes in the pH of the culture medium and the rate of oxygen consumption have been monitored (Figure 2). Generally, in all variants of the culture medium, the yeast completed the logarithmic growth phase after nearly 16 h, thus entering the stationary phase associated with the production of metabolites such as acids, which was reflected in a significant decrease in pH, but most importantly the cells began to accumulate storage lipids.
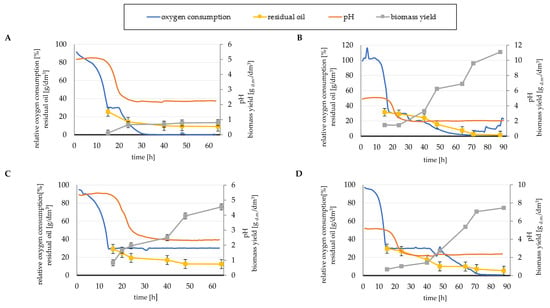
Figure 2.
Changes in relative oxygen consumption and pH of the strain Y. lipolytica KKP 379 grown in (A) M1, (B) M2, (C) M3 and (D) M4 medium with 5% rapeseed waste post-frying oil as a carbon source.
For M1 medium, in addition to the modification related to KH2PO4 (3.0 g/dm3) and (NH4)2SO4 (3.0 g/dm3) doses, the level of Na2HPO4 supplementation (1.1 g/dm3) was also adjusted due to the buffering properties of the medium. The molar ratios C/N and C/P were 71.8:1 and 114.9:1, respectively. A significant increase in the oxygen demand of the cells has been observed from the 20th hour of culture onwards, resulting in a decrease in the dissolved oxygen content of the medium (Figure 2A). During the entire cultivation process, the biomass yield value was marginal. Therefore, the high oxygen demand could be related to the increased lipid synthesis process due to the beginning of the stationary growth phase rather than to the oxygen demand caused by the intensive biomass growth, which did not occur. After 63 h of cultivation in mineral medium M1 with phosphorus and nitrogen source limitation, the efficiency of intracellular lipid accumulation by wild-type Y. lipolytica cells was 47.44%, which is considered an astonishing result. The yeast cells also efficiently utilized the waste carbon source, leaving only 9.11 g/dm3 unused. Despite the lipid yield result, considering the increase in biomass, the amount of SCO obtained per unit of substrate was very low (Table 3).

Table 3.
Parameters of Y. lipolytica KKP 379 yeasts strain culture in media with 5% rapeseed waste post-frying oil as a carbon source.
The residual oil content of the substrate was a reflection of the biomass yield in the M2 substrate (C/N = 26.7:1, C/P = 114.9:1). A significant increase in biomass yield after 2 days of culture combined with a decrease in the residual carbon source in the medium was noticeable (Figure 2B). After 88 h of culture, the cells had used almost all of the waste post-frying oil, reducing its initial content in the culture medium from 50 to 1.56 g/dm3, achieving at the same time the highest biomass yield (11.10 gd.m./dm3) among the other yeast culture variants (Table 3). In addition, there was a gradual decrease in oxygen demand at the end of the culture (71 h). Cells no longer multiplied intensively; moreover, lipids were not efficiently accumulated, gaining a final yield of 20.9%.
Oxygen turned out to be a factor worth observing also in the case of the next batch culture in M3 medium. The addition of (NH4)2SO4 was further increased to 10 g/dm3 (C/N = 21.5:1). Similarly, the level of the addition of phosphorus sources was slightly increased, KH2PO4 3.5 g/dm3 and Na2HPO4 2.5 g/dm3 (C/P = 81.6:1). In culture medium, the dissolved oxygen content remained constant from the 15th hour until the end of the cultivation process. The amplitude range of the agitation speed appeared to be sufficient to maintain the oxygenation level of the culture medium at a level close to 30%. The constantly low level of oxygen consumption by the cells indicated low oxygen demand, which could be associated with moderate growth of yeast biomass (Figure 2C). Although, the biomass yield was only 4.56 gd.m./dm3. The final waste post-frying oil content in the medium was relatively low at 12.44 g/dm3. Despite the low biomass yield, yeast consumption of the oily substrate was at a relatively high level, similar to the M1 medium. This is another example of the diversion of the available carbon source to the needs of storage lipid accumulation, not to the energy needs associated with growth. After 63 h, the efficiency of lipid storage was lower in this medium, but still at a satisfactory level of 32.67%.
When Y. lipolytica yeast was cultured in M4 medium, significant changes were observed after 2 days of yeast growth. After about 40 h, dissolved oxygen consumption increased due to the increase in biomass yield, which reached 7.45 gd.m./dm3 (Figure 2D). Thus, this resulted in a decrease in the lipid carbon source content of the culture medium; the final result after 88 h of culturing was a reduction to 5.33 g/dm3. Molar ratios of C/P = 49.6:1 and C/N = 53.1:1 yielded 2.24 g of microbial oil per dm3 of culture medium after 88 h (30.07% cell dry weight). As could be expected, the C/N ratio changed with the duration of the culture. After 24 h, when the most intensive period of cell growth was already over, the ratio decreased to C/N = 30.6:1, after the following hours it was already half (15.5:1), and at the end of the culture the molar C/N ratio was equal to 8.4:1.
The marked observation to emerge from the data comparison (Figure 3) was the dominance of oleic acid (C18:1) in the total fatty acids content of the cellular oils from each medium variant. When analyzing the composition of extracted oils in the context of their potential use as nutritionally valuable products, the content of linoleic (C18:2) and linolenic acids (C18:3) should be considered. The oleic acid content of the oils ranged from 53.89% (M2) to 60.44% (M3), while the linoleic acid content ranged from 17.39% (M4) to 22.58% (M2). For cellular lipids derived from culture in M2 medium, at the same time with the lowest proportion of oleic acid, the highest contents of linoleic acid and linolenic acid (8.89%) were noted. Importantly, considering all variants of the medium, the linolenic acid was at least, 5.82%. Saturated fatty acids were also determined in each of the cellular lipid samples. The highest percentage of stearic acid was found in samples from medium M1-8.40% and M4-10.93%. Palmitic acid (C16:0) from 1.40% for medium M4 to 4.76% for M2, palmitoleic acid (C16:1): 1.06% (M1)—2.62% (M2), arachidic acid (C20:0): 0.98% (M2)—1.55% (M4) and behenic acid (C22:0): 0.90% (M4)—2.42% (M3) were also identified. The content of myristic acid (C14:0) in the total pool of all fatty acids did not exceed 0.91%.
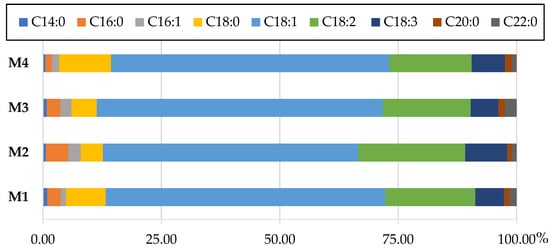
Figure 3.
Fatty acid composition [% w/w] of cellular lipids in Y. lipolytica KKP 379 grown in M1, M2, M3 and M4 medium with 5% rapeseed waste post-frying oil as a carbon source.
4. Discussion
4.1. Lipid Production in Phosphorus-Limited Media
Phosphorus is one of the basic elements for the cultivation of microorganisms, including oleaginous ones. Due to its incorporation into phospholipids, nucleic acids or coenzymes, the analyzed component has been considered crucial for the structure of yeast cells as well as their functioning. Phosphorus may also be stored as polymetaphosphate in biomass. Interestingly, the non-conventional yeast Y. lipolytica contains nearly half as much phosphorus compared to the common yeast species Sacharomyces cerevisiae [39].
According to Wu et al. [31], phosphorus limitation has the potential to be used as a regulator of lipid production by microorganisms, also in the presence of nitrogen-rich media. In other words, this approach provides new opportunities for microbial lipid biosynthesis in a more economical manner, due to the use of complex waste materials as a carbon source. Before the rapeseed oil after fish frying was used as substrate, in the present experiment, it was mechanically purified from the residue of the fried product suspended in it. In view of the above, the waste was not a source of nitrogen, but only a lipid carbon source. Taskin et al. [29] were the first to attempt to investigate the potential of cheese whey as a substrate for microbial oil production by Y. lipolytica B9. The analyzed by-product consisting of nearly 93% water and about 7% dry matter is particularly rich in phosphorus. One of the factors examined was the effect of supplementation of KH2PO4 as an additional phosphorus source on biomass growth and lipid accumulation levels. Similar to the current study, the authors found that phosphorus limitation increased the lipid content in yeast cells. The additional phosphorus source significantly decreased the efficiency of lipid substance accumulation in yeast cells compared to the medium without supplementation, when the efficiency was 44% on a dry weight basis. The addition of 1 and 2 g/dm3 KH2PO4 contributed to a cellular lipid content of 37 and 26%, respectively.
The effect of limiting access to mineral salts including potassium phosphate on SCO production by Y. lipolytica yeast was also studied by Hoarau et al. [35]. In the study, waste produced by ethanol distilleries named Distillery Spent Wash (DSW) was used. After all, the lipid accumulation efficiency was only 9.15% using a medium with 7.0 g/dm3 KH2PO4 and DSW. Moreover, phospholipids accounted for 34% of all lipids. However, this accumulation efficiency result was higher compared to the culture when raw DSW was used without additional phosphate supplementation (7.18%). The addition of 7 g/dm3 KH2PO4 and 1.5 g/dm3 MgSO4 to the culture medium resulted in the highest biomass yield of 7.34 gd.m./dm3. It is worth noting that the best growth of Y. lipolytica yeast was in DSW with the addition of KH2PO4 in the range of 7.5–8.0 g/dm3. The results of the current study appear to be consistent. Using M4 medium with 7.0 g/dm3 KH2PO4, 1.5 g/dm3 MgSO4 and 4 g/dm3 (NH4)2SO4, the biomass yield was 7.45 gd.m./dm3, but it was not the highest score. The yeast showed the best growth in medium with limiting additions of phosphorus source, 3.0 g/dm3 KH2PO4 and 1.1 g/dm3 Na2HPO4, with a relatively higher nitrogen source addition.
A valuable experiment was conducted by Wu et al. [31], whose results, similar to the current work, showed that better microbial lipid production efficiency correlated with low phosphorus source doses and higher C/P molar ratio. When yeast R. toruloides Y4 was cultivated in the medium with 3.6 g/L of KH2PO4 and 70 g/dm3 initial glucose content, which corresponded to a C/P molar ratio of 72:1, after 96 h of batch shaking the flask biomass yield was 18.6 gd.m./dm3 with a lipid content of 21.2%. Moreover, enhancing the limitation of phosphorus source to C/P= 9552:1 resulted in improved SCO production to 62.1% and better biomass growth of 19.4 gd.m./dm3. Surprisingly, when the nitrogen source was additionally restricted (C/N = 22.3:1), the accumulation efficiency was 63.3% with a biomass yield of 19.9 gd.m./dm3. Under phosphorus-limiting conditions, lipid efficiency was consistently close to 60%, even when C/N = 6.1:1 [31].
In the present experiments, the results of the flask-shaken cultures show that the level of KH2PO4 supplementation appeared to be a significant factor affecting the lipid accumulation efficiency, in contrast to the addition of nitrogen source in the form of (NH4)2SO4, for which no significant relationship was found over the concentration range studied. In a batch culture in a bioreactor, the highest efficiency of cellular lipid production by Y. lipolytica yeast has been observed for M1 medium, in which C/P was 114.9:1 and C/N = 71.8:1. However, in bioreactor culture mode, a comparable relationship was not found for the impact of the molar ratio C/P. At a C/P of 114.9, both the highest and lowest microbial lipid production among all substrates was obtained. Under the established conditions, this nitrogen addition was significant. In the experiment of Fabiszewska et al. [2], Y. lipolytica yeast cultured in YPG medium with high nitrogen content and therefore low C/N ratio did not produce SCO. This is in contrast to mineral media with glucose (MG7) and olive oil (MO7), where the conversion yield of storage lipids per biomass was 0.116 and 0.207 g/gd.m. These could be further examples that confirm the role of nitrogen limitation in culture medium.
4.2. Lipid Production in Nitrogen-Limited Media
Nitrogen is considered to be a special component, the limited amount of which in the medium has the greatest effect on the induction of lipogenesis in the cells of oil-producing species. In other words, when the culture medium is deficient in nitrogenous compounds or when its pool is depleted, the synthesis of nucleic acids and proteins is inhibited and the rate of biomass growth decreases [40]. According to Bellou et al. [32], in addition to magnesium limitation, the restriction of cellular access to nitrogen is the factor for efficient cellular lipid biosynthesis. Continuous culture of the wild yeast strain Y. lipolytica ACA-DC50109 resulted in an accumulation efficiency of 47.5% with a biomass yield of 12.2 g/L. Furthermore, lipid accumulation at high levels was provided by low nitrogen doses, but at levels that allowed Y. lipolytica yeast cells to grow. This correlation was related to the maintenance of the pentose-phosphate pathway properly function, which provides the coenzyme NADPH for the mechanism of lipogenesis. For this reason, understanding the physiology of oleaginous microorganisms is of great importance in efficient cellular lipids production.
Inorganic (NH4)2SO4 was the only source of nitrogen in the present study. The current experiment assumed the use of a medium containing only mineral components, excluding the carbon source. There are reports that the presence of organic nitrogen sources in the medium may stimulate microbial synthesis of lipid components [41,42]. Neither the preliminary study stage nor the bioreactor experiments proved a clear relationship between efficient cellular lipid accumulation via ex novo pathway and the level of nitrogen supplementation. In M3 medium (10 g/dm3 (NH4)2SO4), the lipid accumulation efficiency was 32.67%, while in M2 (8 g/dm3 (NH4)2SO4) it was only 20.90%. It is worth noting that in the medium with a lower dose of ammonium sulfate the biomass yield reached more than twice the value (Table 3). When the low addition of a nitrogen source in the M4 culture medium was compensated by a higher level of a phosphorus source, the accumulation efficiency was close to that for the phosphorus-limited M3 medium. This result further strengthened the hypothesis that the ratio of the two analyzed elements nitrogen and phosphorus can be considered an important factor for both yeast cell growth and lipid accumulation capacity.
The oil-producing species of the yeast Trichosporon oleaginosus, in the presence of xylose and limited access to nitrogen compounds, accumulated over 50% of lipids in the dry mass of cells. The type of limitation used (-N, -C and -CN) had a significant effect on the fatty acid profile of the oil extracted from yeast cells. The content of linoleic acid (C18:2 in the -cis configuration) showed the greatest variation depending on the conditions applied. When the researchers limited the access of the cells to nitrogen and carbon, the C18:2 content was 7.31% and 7.45% (w/w), respectively. When carbon source limitation was applied alone, the content of linoleic acid (as the main component of the cell membrane) increased threefold, accounting for 21.58% of all fatty acids [43]. In the current study, the linoleic acid content of the extracted oils was a maximum of 22.58% of total fatty acids. Notably, nutritionally valuable linolenic acid was present in each of the oil samples, with the highest percentage from M2 cultivation.
In the experiment, the highest percentage of SCO production by Y. lipolytica yeast was obtained for culture medium with 3.0 g/dm3 (NH4)2SO4 and 3.0 g/dm3 KH2PO4 and molar ratios of C/P = 114.9:1 and C/N = 71.8:1. A molar C/N ratio of 20:1 is considered to be the minimum for promoting lipid accumulation in oleaginous yeast cells [44], in the experiment, was not lower than 21.5. As found, simultaneous limited access to phosphorus and nitrogen promoted lipid accumulation, but inhibited biomass growth. When the nitrogen dose was increased, with the phosphorus dose unchanged (M2), yeast growth was improved, but the efficiency of the biosynthesis of lipids was significantly reduced. The decreased nitrogen-to-phosphorus ratio (N/P = 3,8:1) in M3 medium, compared to M2 medium (N/P = 4.3:1), resulted in half the biomass yield with 32% cellular lipid accumulation efficiency. Under different conditions, when the reduced nitrogen rate was accompanied by increased cell access to phosphorus sources in the culture medium (M4—N/P = 1:1), growth needs were met, resulting in one of the best biomass yields of 7.45 gd.m./dm3 with satisfactory SCO production efficiency. It can be summarized that phosphorus is needed by cells for growth, but in this context nitrogen is crucial.
Nevertheless, a greater addition of phosphorus source to a nitrogen-deficient medium may lead to the accumulation of higher storage lipid yields without negative effects on growth caused by reduced access to nitrogen. Both bioreactor experiments and preliminary studies emphasized the role of phosphorus as a substrate component in metabolic processes related to the basic metabolism of the yeast cell and SCO biosynthesis. Still, the role of phosphorus in combination with the issue of media supplementation with a nitrogen source needs further studies.
Using a C/N ratio of 30:1 led to an increase in saturated fatty acid production and a decrease in polyunsaturated fatty acids in Y. lipolytica yeast cells. The application of additional phosphorus limitation (C/P = 1043:1) in the medium did not alter the fatty acid group content of total lipid acids observed previously. Similar to the current experiment, total lipid content increased under limiting conditions, but did not exceed 30%. The highest biomass yield (over 7 gd.m./dm3) was achieved for culture in medium with C/N = 30:1, without limiting phosphorus source (C/P = 6:1) [33].
Microbial oil accumulated in lipid bodies consists mainly of TAG and steryl-esters in smaller amounts (neutral fractions), which are enclosed by polar fractions, e.g., phospholipids, glycolipids, and sphingolipids. Generally, the growth of oleaginous microorganisms may be divided into two phases. When cells grow in media rich in all the nutrients necessary for growth, biomass, also free of lipids, is produced. During this phase, lipids with a higher proportion of polar fractions, corresponding to cell membrane lipids, are accumulated. In a situation of limited access to sources such as nitrogen, phosphorus or sulfate, the lipid accumulation phase is stimulated, mainly of neutral fractions [40].
4.3. Cellular Oxygen Demand in Microbial Lipid Production
Based on the authors’ knowledge, there is no clear statement on the effect of the oxygenation degree on the efficiency of lipid accumulation. The link between dissolved oxygen in the medium and accumulation capacity by Y. lipolytica yeast is inconclusive. However, as a result of thought-provoking observations, the issue of oxygen demand by Y. lipolytica yeast appeared to be worth exploring. The concentration of oxygen available in the culture medium is a crucial variable in both the context of growth and efficient microbial lipid production by strictly aerobic yeast Y. lipolytica. It is predicted that an increase in substrate oxygenation may result in the stimulation of cells for growth [45].
The observations highlight the importance of the cellular oxygen demand in relation to growth, but also to the process of cellular lipid accumulation. Despite the negligible increase in biomass yield, the yeast still efficiently used oxygen from the substrate, which could be related to the intensively occurring process of lipid biosynthesis. Interestingly, it may be reasonably assumed that the cells should be exposed to a sufficiently high concentration of oxygen in the medium. Nevertheless, too high a concentration of dissolved oxygen may cause inhibition of cellular metabolism resulting from oxidative stress. Given the need to maintain an appropriate degree of oxygenation of the culture medium, agitation rate is an important parameter. Under conditions of increased cellular oxygen demand, high agitation rate is used. As is widely known, this kind of approach may have negative consequences. High shear forces and mechanical stress may make culture development impossible [46]. Therefore, despite the increase in cellular oxygen demand in bioreactor cultures, the set amplitude of the agitator rotation was not able to meet the demand of the yeast.
According to Bellou et al. [47], high dissolved oxygen (1.5 mg/dm3) in the culture media stimulates cellular lipid synthesis. Upregulation of ATP-citrate lyase (ATP-CL) and malic enzyme (ME), which are involved in microbial lipid synthesis, was observed under such conditions. The importance of malic enzyme for cellular metabolism of oleaginous microorganisms including Y. lipolytica yeast has been recognized as crucial. The product of reaction catalyzed by ME gives the product necessary for fatty acid synthesis, namely, coenzyme NADPH. Under conditions of limited access of cells to nitrogenous compounds, NADPH is produced only by enzymatic reactions involving ME. Conversely, increasing the dissolved oxygen concentration resulted in a decrease in NAD-dependent isocitrate dehydrogenase enzyme activity. When the Krebs cycle is inhibited due to the depletion of nitrogen sources by the cells and allosteric AMP (adenosinemonophosphate) activator concentrations being reduced, ICDH is deactivated. This phenomenon is considered to be characteristic of the de novo biosynthesis pathway [48]. When yeast cultures were conducted in glucose medium, it was observed that limited cellular access to phosphorus, as with nitrogen, results in a reduction in AMP levels to activate cellular phosphorus reserves.
There are two strategies to maximize cellular lipid accumulation. The first one, by deleting genes encoding for acyl-oxidase activity, assumes the prevention of the degradation of storage lipids [49]. The second one aims to support the production via the overexpression of genes encoding for ATP-CL and ME activity [50,51]. Wasylenko et al. [52] using 13C-Metabolic Flux Analysis singled the oxidative pentose-phosphate pathway out as the main source of lipogenic coenzyme NADPH rather than malic enzyme activity. In view of the above, the purpose for research on the role of phosphorus as a coenzyme component in processes related to cellular respiration and provision of energy necessary for SCO biosynthesis has been justified.
Many mechanisms and interactions accompanying the accumulation of lipid compounds via the de novo pathway have been recognized. Nevertheless, there is still a need to search for physiological and biochemical relationships that can explain, and thus support, efficient ex novo biosynthesis. The authors of this paper hypothesized a way in which the de novo and ex novo pathways could be related. The dissimilated fatty acids are degraded in the first step, which is mitochondrial β-oxidation. This process provides the energy necessary for cell growth and development by generating 1 mole of FADH and 1 mole of NADH per every 1 mole of acetyl-CoA produced before it is diverted to the Krebs cycle. Energy is also required for the production of intermediate metabolites [40]. Dissolved fatty acids after entering the cell are degraded in the first step, which is β-oxidation in peroxisomes (Figure 4). β-oxidation is a cyclic process that will be repeated until the lipid substrate is completely broken down. However, there is an anomaly to the cycle, leakage of certain fatty acids from the pathway could be observed, which may be stored in lipid bodies in the next step [48].
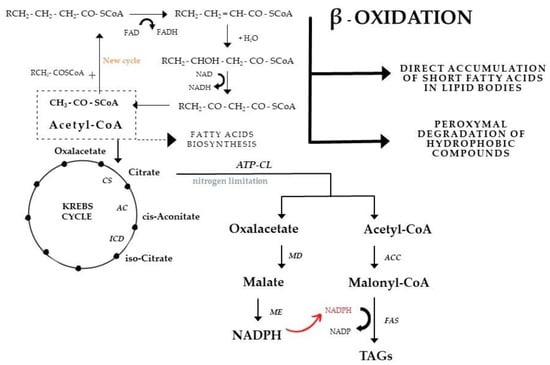
Figure 4.
A hypothetical combination of de novo and ex novo pathways of cellular lipid biosynthesis in media with hydrophobic carbon sources. CS—citrate synthase, AC—aconitase, ICD—iso-citrate dehydrogenase, ATP-CL—ATP-citrate lyase, MD—malate dehydrogenase, ME—malic enzyme, ACC—acetyl-CoA carboxylase, FAS—fatty acid synthetase. From [40], adapted.
5. Conclusions
To conclude, the results of the current research suggest that nitrogen limitation in culture media is crucial, but additional phosphorus limitation may further improve the efficiency of microbial lipid biosynthesis. To the authors’ current knowledge, phosphorus is a component of the culture medium, which still needs to be investigated in relation to the optimal level of its limitation in media stimulating SCO biosynthesis. In particular, evaluating the effect of the level of simultaneous substrate supplementation with phosphorus, nitrogen and the ratio of these two components seems to be one of the key issues in the context of efficient microbial lipid production combined with satisfactory biomass yield. Knowledge of the role of phosphorus as a component of coenzymes essential for a number of biochemical transformations occurring in yeast cells may prove crucial to improving microbial lipid biosynthesis and simultaneously supporting biomass growth, as well as considering respiratory processors. Further studies aimed at looking for strategies to stimulate microbial lipid biosynthesis via the ex novo pathway are worthy of consideration, as most of the available research work involves the de novo pathway. The approach is important for the basic research and from the practical points of view, especially if the possibility of oily waste applicability as a substrate in microbial culture is considered.
Author Contributions
Conceptualization, K.W.; Methodology, K.W., A.F., D.N. and B.Z.; Formal analysis: K.W. and A.F., Investigation, K.W., A.F., D.N. and B.Z.; Resources, A.F. and D.N.; Data Curation, K.W.; Writing—Original Draft Preparation, K.W.; Writing—Review & Editing, A.F. and B.Z.; Visualization, K.W.; Supervision, D.N. and A.F. All authors have read and agreed to the published version of the manuscript.
Funding
The study was financially supported by sources of the Ministry of Education and Science within funds of the Institute of Food Sciences of Warsaw University of Life Sciences (WULS), for scientific research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Beopoulos, A.; Cescut, J.; Haddouche, R.; Uribelarrea, J.L.; Molina-Jouve, C.; Nicaud, J.M. Yarrowia lipolytica as a model for bio-oil production. Prog. Lipid Res. 2009, 48, 375–387. [Google Scholar] [CrossRef]
- Fabiszewska, A.; Misiukiewicz-Stępień, P.; Paplińska-Goryca, M.; Zieniuk, B.; Białecka-Florjańczyk, E. An insight into storage lipid synthesis by Yarrowia lipolytica yeast relating to lipid and sugar substrates metabolism. Biomolecules 2019, 9, 685–697. [Google Scholar] [CrossRef] [PubMed]
- Groenewald, M.; Boekhout, T.; Neuvéglise, C.; Gaillardin, C.; van Dijck, P.W.; Wyss, M. Yarrowia lipolytica: Safety assessment of an oleaginous yeast with a great industrial potential. Crit. Rev. Microbiol. 2014, 40, 187–206. [Google Scholar] [CrossRef] [PubMed]
- Martínez, E.J.; Raghavan, V.; González-Andrés, F.; Gómez, X. New biofuel alternatives: Integrating waste management and single cell oil production. Int. J. Mol. Sci. 2015, 16, 9385–9405. [Google Scholar] [CrossRef]
- Pawar, P.P.; Odaneth, A.A.; Vadgama, R.N.; Lali, A.M. Simultaneous lipid biosynthesis and recovery for oleaginous yeast Yarrowia lipolytica. Biotechnol. Biofuels 2019, 12, 237. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, S.; Chevalot, I.; Galiotou-Panayotou, M.; Komaitis, M.; Marc, I.; Aggelis, G. Industrial derivative of tallow: A promising renewable substrate for microbial lipid, single-cell protein and lipase production by Yarrowia lipolytica. Electron. J. Biotechnol. 2007, 10, 425–435. [Google Scholar] [CrossRef]
- Cheirsilp, B.; Louhasakul, Y. Industrial wastes as a promising renewable source for production of microbial lipid and direct transesterification of the lipid into biodiesel. Bioresour. Technol. 2013, 142, 329–337. [Google Scholar] [CrossRef]
- Bharathiraja, B.; Sridharan, S.; Sowmya, V.; Yuvaraj, D.; Praveenkumar, R. Microbial oil—A plausible alternate resource for food and fuel application. Bioresour. Technol. 2017, 233, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, R.; Dufreche, S.; Zappi, M.; Bajpai, R. Microbial lipids from renewable resources: Production and characterization. J. Ind. Microbiol. Biotechnol. 2010, 37, 1271–1287. [Google Scholar] [CrossRef]
- Ratledge, C.; Wynn, J.P. The biochemistry and molecular biology of lipid accumulation in oleaginous microorganisms. Adv. Appl. Microbiol. 2002, 51, 1–52. [Google Scholar] [PubMed]
- Lopes, M.; Gomes, A.S.; Silva, C.M.; Belo, I. Microbial lipids and added value metabolites production by Yarrowia lipolytica from pork lard. J. Biotechnol. 2018, 265, 76–85. [Google Scholar] [CrossRef]
- Ji, X.J.; Ledesma-Amaro, R. Microbial lipid biotechnology to produce polyunsaturated fatty acids. Trends Biotechnol. 2020, 38, 832–834. [Google Scholar] [CrossRef]
- Patel, A.; Pruthi, V.; Pruthi, P.A. Synchronized nutrient stress conditions trigger the diversion of CDP-DG pathway of phospholipids synthesis towards de novo TAG synthesis in oleaginous yeast escalating biodiesel production. Energy 2017, 139, 962–974. [Google Scholar] [CrossRef]
- Fabiszewska, A.U.; Kotyrba, D.; Nowak, D. Assortment of carbon sources in medium for Yarrowia lipolytica lipase production: A statistical approach. Ann. Microbiol. 2015, 65, 1495–1503. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Papanikolaou, S.; Muniglia, L.; Chevalot, I.; Aggelis, G.; Marc, I. Accumulation of a cocoa-butter-like lipid by Yarrowia lipolytica cultivated on agro-industrial residues. Curr. Microbiol. 2003, 46, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Gajdos, P.; Nicaud, J.M.; Rossignol, T.; Čertík, M. Single cell oil production on molasses by Yarrowia lipolytica strains overexpressing DGA2 in multicopy. Appl. Microbiol. Biotechnol. 2015, 99, 8065–8074. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Liu, L.; Zeng, A.P.; Wei, D. From low-cost substrates to single cell oils synthesized by oleaginous yeasts. Bioresour. Technol. 2017, 245, 1507–1519. [Google Scholar] [CrossRef]
- Lopes, M.; Miranda, S.M.; Belo, I. Microbial valorization of waste cooking oils for valuable compounds production—A review. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2583–2616. [Google Scholar] [CrossRef]
- Liu, X.; Lv, J.; Xu, J.; Zhang, T.; Deng, Y.; He, J. Citric acid production in Yarrowia lipolytica SWJ-1b yeast when grown on waste cooking oil. Appl. Biochem. Biotechnol. 2015, 175, 2347–2356. [Google Scholar] [CrossRef]
- Xiaoyan, L.; Yu, X.; Lv, J.; Xu, J.; Xia, J.; Wu, Z.; Zhang, T.; Deng, Y. A cost-effective process for the coproduction of erythritol and lipase with Yarrowia lipolytica M53 from waste cooking oil. Food Bioprod. Process. 2017, 103, 86–94. [Google Scholar] [CrossRef]
- Nunes, P.M.B.; Martins, A.B.; Brigida, A.I.S.; Rocha Leao, M.H.M.; Amaral, P. Intracellular lipase production by Yarrowia lipolytica using different carbon sources. Chem. Eng. Trans. 2014, 38, 421–426. [Google Scholar]
- Lopes, M.; Miranda, S.M.; Alves, J.M.; Pereira, A.S.; Belo, I. Waste cooking oils as feedstock for lipase and lipid-rich biomass production. Eur. J. Lipid Sci. Technol. 2019, 121, 1800188–1800196. [Google Scholar] [CrossRef]
- El Bialy, H.; Gomaa, O.M.; Azab, K.S. Conversion of oil waste to valuable fatty acids using oleaginous yeast. World J. Microbiol. Biotechnol. 2011, 27, 2791–2798. [Google Scholar] [CrossRef]
- Katre, G.; Joshi, C.; Khot, M.; Zinjarde, S.; RaviKumar, A. Evaluation of single cell oil (SCO) from a tropical marine yeast Yarrowia lipolytica NCIM 3589 as a potential feedstock for biodiesel. AMB Express 2012, 2, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Tzirita, M.; Papanikolaou, S.; Chatzifragkou, A.; Quilty, B. Waste fat biodegradation and biomodification by Yarrowia lipolytica and a bacterial consortium composed of Bacillus spp. and Pseudomonas putida. Eng. Life Sci. 2018, 18, 932–942. [Google Scholar] [CrossRef]
- Wierzchowska, K.; Zieniuk, B.; Fabiszewska, A. Use of Non-Conventional Yeast Yarrowia lipolytica in Treatment or Upgradation of Hydrophobic Industry Wastes. Waste Biomass Valorization 2021, 1–23. Available online: https://link.springer.com/article/10.1007/s12649-021-01516-9 (accessed on 9 December 2021).
- Ratledge, C. Regulation of lipid accumulation in oleaginous micro-organisms. Biochem. Soc. Trans. 2002, 30, 1047–1050. [Google Scholar] [CrossRef]
- Ratledge, C.; Cohen, Z. Microbial and algal oils: Do they have a future for biodiesel or as commodity oils? Lipid Technol. 2008, 20, 155–160. [Google Scholar] [CrossRef]
- Taskin, M.; Saghafian, A.; Aydogan, M.N.; Arslan, N.P. Microbial lipid production by cold-adapted oleaginous yeast Yarrowia lipolytica B9 in non-sterile whey medium. Biofuels Bioprod. Biorefin. 2015, 9, 595–605. [Google Scholar] [CrossRef]
- Gill, C.O.; Hall, M.J.; Ratledge, C. Lipid accumulation in an oleaginous yeast (Candida 107) growing on glucose in single-stage continuous culture. Appl. Environ. Microbiol. 1977, 33, 231–239. [Google Scholar] [CrossRef]
- Wu, S.; Hu, C.; Jin, G.; Zhao, X.; Zhao, Z.K. Phosphate-limitation mediated lipid production by Rhodosporidium toruloides. Bioresour. Technol. 2010, 101, 6124–6129. [Google Scholar] [CrossRef]
- Bellou, S.; Triantaphyllidou, I.E.; Mizerakis, P.; Aggelis, G. High lipid accumulation in Yarrowia lipolytica cultivated under double limitation of nitrogen and magnesium. J. Biotechnol. 2016, 234, 116–126. [Google Scholar] [CrossRef]
- Kolouchová, I.; Maťátková, O.; Sigler, K.; Masák, J.; Řezanka, T. Lipid accumulation by oleaginous and non-oleaginous yeast strains in nitrogen and phosphate limitation. Folia Microbiol. 2016, 61, 431–438. [Google Scholar] [CrossRef]
- Huang, X.; Luo, H.; Mu, T.; Shen, Y.; Yuan, M.; Liu, J. Enhancement of lipid accumulation by oleaginous yeast through phosphorus limitation under high content of ammonia. Bioresour. Technol. 2018, 262, 9–14. [Google Scholar] [CrossRef]
- Hoarau, J.; Petit, T.; Grondin, I.; Marty, A.; Caro, Y. Phosphate as a limiting factor for the improvement of single cell oil production from Yarrowia lipolytica MUCL 30108 grown on pre-treated distillery spent wash. J. Water Process Eng. 2020, 37, 101392. [Google Scholar] [CrossRef]
- Timoumi, A.; Guillouet, S.E.; Molina-Jouve, C.; Fillaudeau, L.; Gorret, N. Impacts of environmental conditions on product formation and morphology of Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 2018, 102, 3831–3848. [Google Scholar] [CrossRef] [PubMed]
- AOAC. International Official Methods of Analysis, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2000; Volume 2. [Google Scholar]
- Folch, J.; Lees, M.; Stanley, G.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Michalik, B.; Biel, W.; Lubowicki, R.; Jacyno, E. Chemical composition and biological value of proteins of the yeast Yarrowia lipolytica growing on industrial glycerol. Can. J. Anim. Sci. 2014, 94, 99–104. [Google Scholar] [CrossRef][Green Version]
- Papanikolaou, S.; Aggelis, G. Lipids of oleaginous yeasts. Part I: Biochemistry of single cell oil production. Eur. J. Lipid Sci. Technol. 2011, 113, 1031–1051. [Google Scholar] [CrossRef]
- Beopoulos, A.; Mrozova, Z.; Thevenieau, F.; Le Dall, M.T.; Hapala, I.; Papanikolaou, S.; Chardot, T.; Nicaud, J.M. Control of lipid accumulation in the yeast Yarrowia lipolytica. Appl. Environ. Microbiol. 2008, 74, 7779–7789. [Google Scholar] [CrossRef]
- Tsigie, Y.A.; Wang, C.Y.; Truong, C.T.; Ju, Y.H. Lipid production from Yarrowia lipolytica Po1g grown in sugarcane bagasse hydrolysate. Bioresour. Technol. 2011, 102, 9216–9222. [Google Scholar] [CrossRef] [PubMed]
- Santek, M.I.; Miskulin, E.; Petrovic, M.; Beluhan, S.; Santek, B. Effect of carbon and nitrogen source concentrations on the growth and lipid accumulation of yeast Trichosporon oleaginosus in continuous and batch culture. J. Soc. Chem. Ind. 2017, 92, 1620–1629. [Google Scholar]
- Dobrowolski, A.; Mituła, P.; Rymowicz, W.; Mirończuk, A.M. Efficient conversion of crude glycerol from various industrial wastes into single cell oil by yeast Yarrowia lipolytica. Bioresour. Technol. 2016, 207, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Magdouli, S.; Brar, S.K.; Blais, J.F. Morphology and rheological behaviour of Yarrowia lipolytica: Impact of dissolved oxygen level on cell growth and lipid composition. Process Biochem. 2018, 65, 1–10. [Google Scholar] [CrossRef]
- Alonso, F.O.M.; Oliveira, E.B.L.; Dellamora-Ortiz, G.M.; Pereira-Meirelles, F.V. Improvement of lipase production at different stirring speeds and oxygen levels. Braz. J. Chem. Eng. 2005, 22, 9–18. [Google Scholar] [CrossRef]
- Bellou, S.; Makri, A.; Triantaphyllidou, I.E.; Papanikolaou, S.; Aggelis, G. Morphological and metabolic shifts of Yarrowia lipolytica induced by alteration of the dissolved oxygen concentration in the growth environment. Microbiology 2014, 160, 807–817. [Google Scholar] [CrossRef]
- Beopoulos, A.; Nicaud, J.M. Yeast: A new oil producer? Oléagineux Corps Gras Lipides 2012, 19, 22–28. [Google Scholar] [CrossRef]
- Dulermo, T.; Nicaud, J.M. Involvement of the G3P shuttle and oxidation pathway in the control of TAG synthesis and lipid accumulation in Yarrowia lipolytica. Metab. Eng. 2011, 13, 482–491. [Google Scholar] [CrossRef]
- Blazeck, J.; Hill, A.; Liu, L.; Knight, R.; Miller, J.; Pan, A.; Otoupal, P.; Alper, H.S. Harnessing Yarrowia lipolytica lipogenesis to create a platform for lipid and biofuel production. Nat. Commun. 2014, 5, 3131. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, L.; Chen, H.; Chen, Y.Q.; Chen, W.; Song, Y.; Ratledge, C. Enhanced lipid accumulation in the yeast Yarrowia lipolytica by over-expression of ATP: Citrate lyase from Mus musculus. J. Biotechnol. 2014, 192, 78–84. [Google Scholar] [CrossRef]
- Wasylenko, T.M.; Ahn, W.S.; Stephanopoulos, G. The oxidative pentose phosphate pathway is the primary source of NADPH for lipid overproduction from glucose in Yarrowia lipolytica. Metab. Eng. 2015, 30, 27–39. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).