Alginate Modification and Lectin-Conjugation Approach to Synthesize the Mucoadhesive Matrix
Abstract
:1. Introduction
2. Alginate Structure and Properties
2.1. Chemical Structure of Alginates
2.2. Biocompatibility and Degradability of Alginates
2.3. Mucoadhesive Properties of Alginates
3. Alginate Application in Drug Delivery
3.1. Alginate Hydrogel
3.2. Alginate Ester
3.3. Alginate Dialdehyde
4. Alginate Modification and Coupling Strategies
4.1. Carboxylic Groups
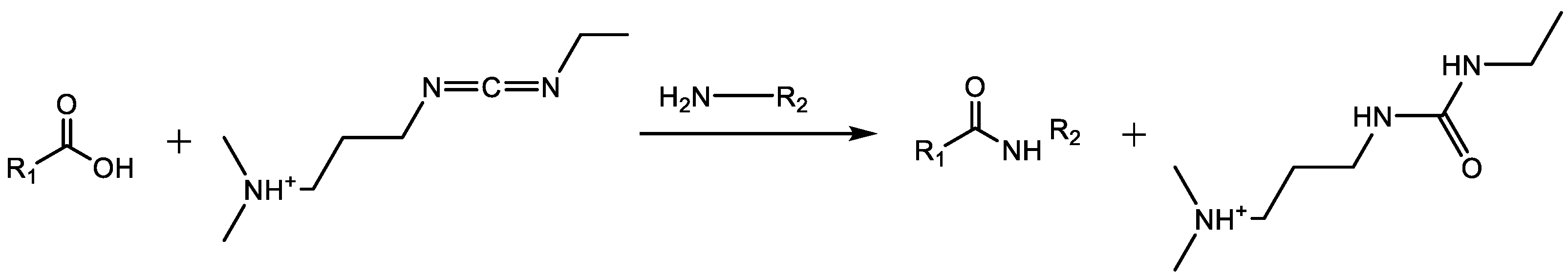
4.1.1. Carbodiimide
4.1.2. Ugi Multicomponent Reaction
4.2. Hydroxyl Groups
4.2.1. Acetylation and Esterification of Hydroxyl Groups
4.2.2. Phosphorylation
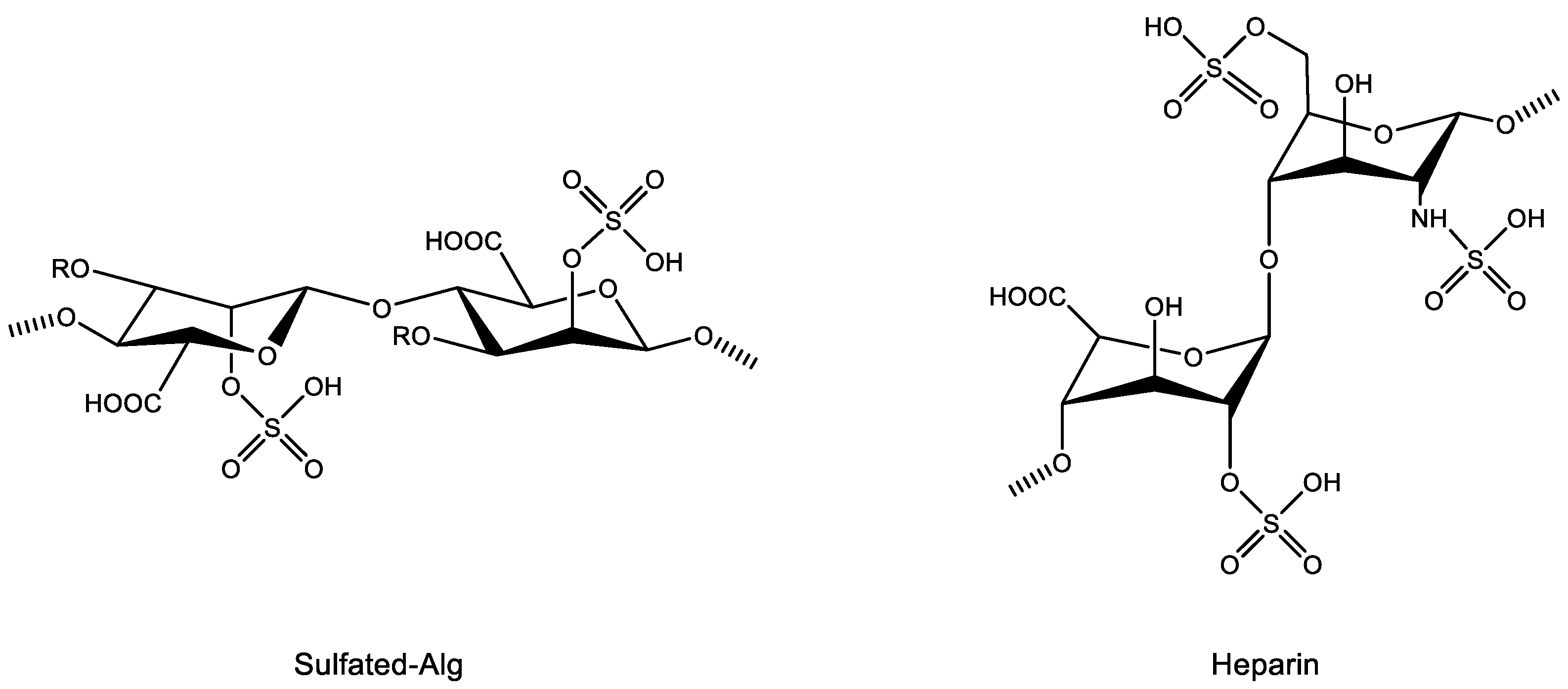
4.2.3. Sulfation
4.2.4. Oxidation and Reduction
4.2.5. Graft Copolymerization
5. Lectin-ADA Conjugation
5.1. Lectin

5.2. Lectin Biomedical Applications
5.3. Lectin Mediated Drug Delivery
5.4. Lectin-Alginate Dialdehyde Conjugate
6. Conclusions and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Pawar, S.N.; Edgar, K.J. Alginate derivatization: A review of chemistry, properties and applications. Biomaterials 2012, 33, 3279–3305. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [Green Version]
- Szabo, L.; Gerber-Lemaire, S.; Wandrey, C. Strategies to Functionalize the Anionic Biopolymer Na-Alginate without Restricting Its Polyelectrolyte Properties. Polymers 2020, 12, 919. [Google Scholar] [CrossRef] [Green Version]
- Lavín de Juan, L.; García Recio, V.; Jiménez López, P.; Girbés Juan, T.; Cordoba-Diaz, M.; Cordoba-Diaz, D. Pharmaceutical applications of lectins. J. Drug Deliv. Sci. Technol. 2017, 42, 126–133. [Google Scholar] [CrossRef]
- Chettri, D.; Boro, M.; Sarkar, L.; Verma, A.K. Lectins: Biological significance to biotechnological application. Carbohydr. Res. 2021, 506, 108367. [Google Scholar] [CrossRef]
- Haltner, E.; Easson, J.H.; Lehr, C.-M. Lectins and bacterial invasion factors for controlling endo- and transcytosis of bioadhesive drug carrier systems. Eur. J. Pharm. Biopharm. 1997, 44, 3–13. [Google Scholar] [CrossRef]
- Gabor, F.; Schwarzbauer, A.; Wirth, M. Lectin-mediated drug delivery: Binding and uptake of BSA-WGA conjugates using the Caco-2 model. Int. J. Pharm. 2002, 237, 227–239. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Koller, R.; Wirth, M.; Gabor, F. Lectin-Grafted PLGA Microcarriers Loaded with Fluorescent Model Drugs: Characteristics, Release Profiles, and Cytoadhesion Studies. Sci. Pharm. 2014, 82, 193–205. [Google Scholar] [CrossRef] [Green Version]
- Kesavan, K.; Nath, G.; Pandit, J.K. Sodium alginate based mucoadhesive system for gatifloxacin and its in vitro antibacterial activity. Sci. Pharm. 2010, 78, 941–957. [Google Scholar] [CrossRef] [Green Version]
- Bies, C.; Lehr, C.-M.; Woodley, J.F. Lectin-mediated drug targeting: History and applications. Adv. Drug. Deliv. Rev. 2004, 56, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Salomonsen, T.; Jensen, H.M.; Stenbæk, D.; Engelsen, S.B. Rapid Determination of Alginate Monomer Compostion using Raman Spectroscopy and Chemometrics. In Gums and Stabilisers for the Food Industry 14; The Royal Society of Chemistry: London, UK, 2008; pp. 543–551. [Google Scholar]
- Dodero, A.; Vicini, S.; Alloisio, M.; Castellano, M. Rheological properties of sodium alginate solutions in the presence of added salt: An application of Kulicke equation. Rheol. Acta 2020, 59, 365–374. [Google Scholar] [CrossRef]
- Teng, K.; An, Q.; Chen, Y.; Zhang, Y.; Zhao, Y. Recent Development of Alginate-Based Materials and Their Versatile Functions in Biomedicine, Flexible Electronics, and Environmental Uses. ACS Biomater. Sci. Eng.. 2021, 7, 1302–1337. [Google Scholar] [CrossRef]
- Orive, G.; Tam, S.K.; Pedraz, J.L.; Hallé, J.-P. Biocompatibility of alginate–poly-l-lysine microcapsules for cell therapy. Biomaterials 2006, 27, 3691–3700. [Google Scholar] [CrossRef] [PubMed]
- Orive, G.; Ponce, S.; Hernández, R.M.; Gascón, A.R.; Igartua, M.; Pedraz, J.L. Biocompatibility of microcapsules for cell immobilization elaborated with different type of alginates. Biomaterials 2002, 23, 3825–3831. [Google Scholar] [CrossRef]
- Feng, L.; Cao, Y.; Xu, D.; You, S.; Han, F. Influence of sodium alginate pretreated by ultrasound on papain properties: Activity, structure, conformation and molecular weight and distribution. Ultrason. Sonochem. 2016, 32, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Cao, Y.; Xu, D.; Wang, S.; Zhang, J. Molecular weight distribution, rheological property and structural changes of sodium alginate induced by ultrasound. Ultrason. Sonochem. 2017, 34, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Burana-osot, J.; Hosoyama, S.; Nagamoto, Y.; Suzuki, S.; Linhardt, R.J.; Toida, T. Photolytic depolymerization of alginate. Carbohydr. Res. 2009, 344, 2023–2027. [Google Scholar] [CrossRef]
- Kurowiak, J.; Kaczmarek-Pawelska, A.; Mackiewicz, A.G.; Bedzinski, R. Analysis of the Degradation Process of Alginate-Based Hydrogels in Artificial Urine for Use as a Bioresorbable Material in the Treatment of Urethral Injuries. Processes 2020, 8, 304. [Google Scholar] [CrossRef] [Green Version]
- Esposito, P.; Colombo, I.; Lovrecich, M. Investigation of surface properties of some polymers by a thermodynamic and mechanical approach: Possibility of predicting mucoadhesion and biocompatibility. Biomaterials 1994, 15, 177–182. [Google Scholar] [CrossRef]
- Jindal, A.B.; Wasnik, M.N.; Nair, H.A. Synthesis of thiolated alginate and evaluation of mucoadhesiveness, cytotoxicity and release retardant properties. Indian J. Pharm. Sci. 2010, 72, 766–774. [Google Scholar] [CrossRef] [Green Version]
- Mackaya-Navarro, L.; Campos-Requena, V.H. Mucoadhesive alginate synthesis: A multivariate calibration approach. New J. Chem. 2020, 44, 20267–20274. [Google Scholar] [CrossRef]
- Bernkop-Schnürch, A.; Kast, C.E.; Richter, M.F. Improvement in the mucoadhesive properties of alginate by the covalent attachment of cysteine. J. Control Release 2001, 71, 277–285. [Google Scholar] [CrossRef]
- Liu, Z.; Li, J.; Nie, S.; Liu, H.; Ding, P.; Pan, W. Study of an alginate/HPMC-based in situ gelling ophthalmic delivery system for gatifloxacin. Int. J. Pharm. 2006, 315, 12–17. [Google Scholar] [CrossRef]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for Biomedical Applications: Their Characteristics and the Mechanisms behind Them. Gels 2017, 3, 6. [Google Scholar] [CrossRef] [Green Version]
- Augst, A.D.; Kong, H.J.; Mooney, D.J. Alginate hydrogels as biomaterials. Macromol. Biosci. 2006, 6, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Abasalizadeh, F.; Moghaddam, S.V.; Alizadeh, E.; Akbari, E.; Kashani, E.; Fazljou, S.M.B.; Torbati, M.; Akbarzadeh, A. Alginate-based hydrogels as drug delivery vehicles in cancer treatment and their applications in wound dressing and 3D bioprinting. J. Biol. Eng. 2020, 14, 8. [Google Scholar] [CrossRef]
- Dianawati, D.; Mishra, V.; Shah, N.P. Role of calcium alginate and mannitol in protecting Bifidobacterium. Appl. Environ. Microbiol. 2012, 78, 6914–6921. [Google Scholar] [CrossRef] [Green Version]
- Kuo, C.K.; Ma, P.X. Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: Part 1. Structure, gelation rate and mechanical properties. Biomaterials 2001, 22, 511–521. [Google Scholar] [CrossRef]
- Aguero, L.; Zaldivar-Silva, D.; Pena, L.; Dias, M.L. Alginate microparticles as oral colon drug delivery device: A review. Carbohydr. Polym. 2017, 168, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Drury, J.L.; Dennis, R.G.; Mooney, D.J. The tensile properties of alginate hydrogels. Biomaterials 2004, 25, 3187–3199. [Google Scholar] [CrossRef]
- Fernández Farrés, I.; Norton, I.T. Formation kinetics and rheology of alginate fluid gels produced by in-situ calcium release. Food Hydrocoll. 2014, 40, 76–84. [Google Scholar] [CrossRef] [Green Version]
- Mørch, Ý.A.; Donati, I.; Strand, B.L.; Skjåk-Bræk, G. Effect of Ca2+, Ba2+, and Sr2+ on Alginate Microbeads. Biomacromolecules 2006, 7, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Eiselt, P.; Lee, K.Y.; Mooney, D.J. Rigidity of Two-Component Hydrogels Prepared from Alginate and Poly(ethylene glycol)−Diamines. Macromolecules 1999, 32, 5561–5566. [Google Scholar] [CrossRef]
- Araiza-Verduzco, F.; Rodríguez-Velázquez, E.; Cruz, H.; Rivero, I.A.; Acosta-Martínez, D.R.; Pina-Luis, G.; Alatorre-Meda, M. Photocrosslinked Alginate-Methacrylate Hydrogels with Modulable Mechanical Properties: Effect of the Molecular Conformation and Electron Density of the Methacrylate Reactive Group. Materials 2020, 13, 534. [Google Scholar] [CrossRef] [Green Version]
- Drury, J.L.; Boontheekul, T.; Mooney, D.J. Cellular Cross-linking of Peptide Modified Hydrogels. J. Biomech. Eng. 2004, 127, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Yallapu, M.M.; Jaggi, M.; Chauhan, S.C. Design and engineering of nanogels for cancer treatment. Drug Discov. 2011, 16, 457–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirrahimi, M.; Abed, Z.; Beik, J.; Shiri, I.; Shiralizadeh Dezfuli, A.; Mahabadi, V.P.; Kamran Kamrava, S.; Ghaznavi, H.; Shakeri-Zadeh, A. A thermo-responsive alginate nanogel platform co-loaded with gold nanoparticles and cisplatin for combined cancer chemo-photothermal therapy. Pharmacol. Res. 2019, 143, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Hosseinifar, T.; Sheybani, S.; Abdouss, M.; Hassani Najafabadi, S.A.; Shafiee Ardestani, M. Pressure responsive nanogel base on Alginate-Cyclodextrin with enhanced apoptosis mechanism for colon cancer delivery. J. Biomed. Mater. Res. A 2018, 106, 349–359. [Google Scholar] [CrossRef]
- Sun, Z.; Yi, Z.; Zhang, H.; Ma, X.; Su, W.; Sun, X.; Li, X. Bio-responsive alginate-keratin composite nanogels with enhanced drug loading efficiency for cancer therapy. Carbohydr. Polym. 2017, 175, 159–169. [Google Scholar] [CrossRef]
- Suzuki, S.; Kurachi, S.; Wada, N.; Takahashi, K. Selective Modification of Aliphatic Hydroxy Groups in Lignin Using Ionic Liquid. Catalysts 2021, 11, 120. [Google Scholar] [CrossRef]
- Pawar, S.N.; Edgar, K.J. Alginate esters via chemoselective carboxyl group modification. Carbohydr. Polym. 2013, 98, 1288–1296. [Google Scholar] [CrossRef] [PubMed]
- Broderick, E.; Lyons, H.; Pembroke, T.; Byrne, H.; Murray, B.; Hall, M. The characterisation of a novel, covalently modified, amphiphilic alginate derivative, which retains gelling and non-toxic properties. J. Colloid. Interface Sci. 2006, 298, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Ballester, N.M.; Bataille, B.; Benabbas, R.; Alonso, B.; Soulairol, I. Development of alginate esters as novel multifunctional excipients for direct compression. Carbohydr. Polym. 2020, 240, 116280. [Google Scholar] [CrossRef]
- Raja, M.; Liu, C.; Huang, Z. Nanoparticles Based on Oleate Alginate Ester as Curcumin Delivery Aystem. Curr. Drug Deliv. 2015, 12, 613–627. [Google Scholar] [CrossRef]
- Zhang, N.; Wardwell, P.R.; Bader, R.A. Polysaccharide-based micelles for drug delivery. Pharmaceutics 2013, 5, 329–352. [Google Scholar] [CrossRef] [Green Version]
- Gomez, C.G.; Rinaudo, M.; Villar, M.A. Oxidation of sodium alginate and characterization of the oxidized derivatives. Carbohydr. Polym. 2007, 67, 296–304. [Google Scholar] [CrossRef]
- Jejurikar, A.; Seow, X.T.; Lawrie, G.; Martin, D.; Jayakrishnan, A.; Grøndahl, L. Degradable alginate hydrogels crosslinked by the macromolecular crosslinker alginate dialdehyde. J. Mater. Chem. 2012, 22, 9751–9758. [Google Scholar] [CrossRef]
- Bouhadir, K.H.; Hausman, D.S.; Mooney, D.J. Synthesis of cross-linked poly(aldehyde guluronate) hydrogels. Polymer 1999, 40, 3575–3584. [Google Scholar] [CrossRef]
- Omtvedt, L.A.; Dalheim, M.O.; Nielsen, T.T.; Larsen, K.L.; Strand, B.L.; Aachmann, F.L. Efficient Grafting of Cyclodextrin to Alginate and Performance of the Hydrogel for Release of Model Drug. Sci. Rep. 2019, 9, 9325. [Google Scholar] [CrossRef]
- Fan, L.-H.; Pan, X.-R.; Zhou, Y.; Chen, L.-Y.; Xie, W.-G.; Long, Z.-H.; Zheng, H. Preparation and characterization of crosslinked carboxymethyl chitosan–oxidized sodium alginate hydrogels. J. Appl. Polym. Sci. 2011, 122, 2331–2337. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Joshi, N.; Jayakrishnan, A.; Banerjee, R. Self-crosslinked oxidized alginate/gelatin hydrogel as injectable, adhesive biomimetic scaffolds for cartilage regeneration. Acta Biomater. 2014, 10, 3650–3663. [Google Scholar] [CrossRef] [PubMed]
- Naghizadeh, Z.; Karkhaneh, A.; Khojasteh, A. Self-crosslinking effect of chitosan and gelatin on alginate based hydrogels: Injectable in situ forming scaffolds. Mater. Sci. Eng. C 2018, 89, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Applications of the Ninhydrin Reaction for Analysis of Amino Acids, Peptides, and Proteins to Agricultural and Biomedical Sciences. J. Agric. Food Chem. 2004, 52, 385–406. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Jayakrishnan, A. Self-cross-linking biopolymers as injectable in situ forming biodegradable scaffolds. Biomaterials 2005, 26, 3941–3951. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Mohanty, M.; Umashankar, P.R.; Jayakrishnan, A. Evaluation of an in situ forming hydrogel wound dressing based on oxidized alginate and gelatin. Biomaterials 2005, 26, 6335–6342. [Google Scholar] [CrossRef] [PubMed]
- Aderibigbe, B.A.; Buyana, B. Alginate in Wound Dressings. Pharmaceutics 2018, 10, 42. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, L.; Priddy, L.B.; Esancy, C.; Li, M.-T.A.; Stevens, H.Y.; Jiang, X.; Tran, L.; Rowe, D.W.; Guldberg, R.E. Hydrogel-based Delivery of rhBMP-2 Improves Healing of Large Bone Defects Compared with Autograft. Clin. Orthop. Relat. Res. 2015, 473, 2885–2897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Resmi, R.; Parvathy, J.; John, A.; Joseph, R. Injectable self-crosslinking hydrogels for meniscal repair: A study with oxidized alginate and gelatin. Carbohydr. Polym. 2020, 234, 115902. [Google Scholar] [CrossRef]
- Sandvig, I.; Karstensen, K.; Rokstad, A.M.; Aachmann, F.L.; Formo, K.; Sandvig, A.; Skjak-Braek, G.; Strand, B.L. RGD-peptide modified alginate by a chemoenzymatic strategy for tissue engineering applications. J. Biomed. Mater. Res. A 2015, 103, 896–906. [Google Scholar] [CrossRef]
- Cohen, B.; Pinkas, O.; Foox, M.; Zilberman, M. Gelatin–alginate novel tissue adhesives and their formulation–strength effects. Acta Biomater. 2013, 9, 9004–9011. [Google Scholar] [CrossRef]
- Foox, M.; Raz-Pasteur, A.; Berdicevsky, I.; Krivoy, N.; Zilberman, M. In vitro microbial inhibition, bonding strength, and cellular response to novel gelatin–alginate antibiotic-releasing soft tissue adhesives. Polym. Adv. Technol. 2014, 25, 516–524. [Google Scholar] [CrossRef]
- Yang, J.S.; Jiang, B.; He, W.; Xia, Y.M. Hydrophobically modified alginate for emulsion of oil in water. Carbohydr. Polym. 2012, 87, 1503–1506. [Google Scholar] [CrossRef]
- Schulz, A.; Gepp, M.M.; Stracke, F.; von Briesen, H.; Neubauer, J.C.; Zimmermann, H. Tyramine-conjugated alginate hydrogels as a platform for bioactive scaffolds. J. Biomed. Mater. Res. 2019, 107, 114–121. [Google Scholar] [CrossRef] [Green Version]
- Ooi, H.W.; Mota, C.; ten Cate, A.T.; Calore, A.; Moroni, L.; Baker, M.B. Thiol–Ene Alginate Hydrogels as Versatile Bioinks for Bioprinting. Biomacromolecules 2018, 19, 3390–3400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galant, C.; Kjøniksen, A.-L.; Nguyen, G.T.M.; Knudsen, K.D.; Nyström, B. Altering Associations in Aqueous Solutions of a Hydrophobically Modified Alginate in the Presence of β-Cyclodextrin Monomers. J. Phys. Chem. B 2006, 110, 190–195. [Google Scholar] [CrossRef]
- Dalheim, M.O.; Vanacker, J.; Najmi, M.A.; Aachmann, F.L.; Strand, B.L.; Christensen, B.E. Efficient functionalization of alginate biomaterials. Biomaterials 2016, 80, 146–156. [Google Scholar] [CrossRef]
- Xu, J.B.; Bartley, J.P.; Johnson, R.A. Preparation and characterization of alginate hydrogel membranes crosslinked using a water-soluble carbodiimide. J. Appl. Polym. Sci. 2003, 90, 747–753. [Google Scholar] [CrossRef]
- Lee, K.Y.; Bouhadir, K.H.; Mooney, D.J. Degradation behavior of covalently cross-linked poly(aldehyde guluronate) hydrogels. Macromolecules 2000, 33, 97–101. [Google Scholar] [CrossRef]
- Maiti, S.; Singha, K.; Ray, S.; Dey, P.; Sa, B. Adipic acid dihydrazide treated partially oxidized alginate beads for sustained oral delivery of flurbiprofen. Pharm. Dev. Technol. 2009, 14, 461–470. [Google Scholar] [CrossRef]
- Bu, H.; Kjøniksen, A.-L.; Knudsen, K.D.; Nyström, B. Rheological and Structural Properties of Aqueous Alginate during Gelation via the Ugi Multicomponent Condensation Reaction. Biomacromolecules 2004, 5, 1470–1479. [Google Scholar] [CrossRef]
- Bu, H.; Kjøniksen, A.-L.; Knudsen, K.D.; Nyström, B. Effects of Surfactant and Temperature on Rheological and Structural Properties of Semidilute Aqueous Solutions of Unmodified and Hydrophobically Modified Alginate. Langmuir 2005, 21, 10923–10930. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Ishii, D.; Iwata, T. Synthesis and characterization of alginic acid ester derivatives. Carbohydr. Polym. 2017, 171, 229–235. [Google Scholar] [CrossRef]
- Chamberlain, N.H.; Cunningham, G.E.; Speakman, J.B. Alginic Acid Diacetate. Nature 1946, 158, 553. [Google Scholar] [CrossRef]
- Wassermann, A. Alginic Acid-acetate. Nature 1946, 158, 271. [Google Scholar] [CrossRef] [PubMed]
- Skjd k-Bræk, G.; Paoletti, S.; Gianferrara, T. Selective acetylation of mannuronic acid residues in calcium alginate gels. Carbohydr. Res. 1989, 185, 119–129. [Google Scholar] [CrossRef]
- Skjd k-Bræk, G.; Zanetti, F.; Paoletti, S. Effect of acetylation on some solution and gelling properties of alginates. Carbohydr. Res. 1989, 185, 131–138. [Google Scholar] [CrossRef]
- Pawar, S.N.; Edgar, K.J. Chemical modification of alginates in organic solvent systems. Biomacromolecules 2011, 12, 4095–4103. [Google Scholar] [CrossRef] [PubMed]
- Babak, V.G.; Skotnikova, E.A.; Lukina, I.G.; Pelletier, S.; Hubert, P.; Dellacherie, E. Hydrophobically Associating Alginate Derivatives: Surface Tension Properties of Their Mixed Aqueous Solutions with Oppositely Charged Surfactants. J. Colloid. Interface Sci. 2000, 225, 505–510. [Google Scholar] [CrossRef]
- Yu, Y.; Leng, C.; Liu, Z.; Jia, F.; Zheng, Y.; Yuan, K.; Yan, S. Preparation and characterization of biosurfactant based on hydrophobically modified alginate. Colloid. J. 2014, 76, 622–627. [Google Scholar] [CrossRef]
- Meng, Y.; Wu, C.; Zhang, J.; Cao, Q.; Liu, Q.; Yu, Y. Amphiphilic alginate as a drug release vehicle for water-insoluble drugs. Colloid. J. 2015, 77, 754–760. [Google Scholar] [CrossRef]
- Wu, J.; Wu, Z.; Zhang, R.; Yuan, S.; Lu, Q.; Yu, Y. Synthesis and micelle properties of the hydrophobic modified alginate. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 742–747. [Google Scholar] [CrossRef]
- Coleman, R.J.; Lawrie, G.; Lambert, L.K.; Whittaker, M.; Jack, K.S.; Grondahl, L. Phosphorylation of alginate: Synthesis, characterization, and evaluation of in vitro mineralization capacity. Biomacromolecules 2011, 12, 889–897. [Google Scholar] [CrossRef]
- Kim, H.S.; Song, M.; Lee, E.J.; Shin, U.S. Injectable hydrogels derived from phosphorylated alginic acid calcium complexes. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 51, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Munarin, F.; Kabelac, C.; Coulombe, K.L.K. Heparin-modified alginate microspheres enhance neovessel formation in hiPSC-derived endothelial cells and heterocellular in vitro models by controlled release of vascular endothelial growth factor. J. Biomed. Mater. Res. A 2021, 109, 1726–1736. [Google Scholar] [CrossRef]
- Mhanna, R.; Kashyap, A.; Palazzolo, G.; Vallmajo-Martin, Q.; Becher, J.; Möller, S.; Schnabelrauch, M.; Zenobi-Wong, M. Chondrocyte culture in three dimensional alginate sulfate hydrogels promotes proliferation while maintaining expression of chondrogenic markers. Tissue Eng. Part A 2014, 20, 1454–1464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, L.; Cheng, C.; Nie, C.; He, C.; Deng, J.; Wang, L.; Xia, Y.; Zhao, C. Anticoagulant sodium alginate sulfates and their mussel-inspired heparin-mimetic coatings. J. Mater. Chem. B 2016, 4, 3203–3215. [Google Scholar] [CrossRef]
- Freeman, I.; Kedem, A.; Cohen, S. The effect of sulfation of alginate hydrogels on the specific binding and controlled release of heparin-binding proteins. Biomaterials 2008, 29, 3260–3268. [Google Scholar] [CrossRef]
- Arlov, Ø.; Skjåk-Bræk, G. Sulfated Alginates as Heparin Analogues: A Review of Chemical and Functional Properties. Molecules 2017, 22, 778. [Google Scholar] [CrossRef] [Green Version]
- Emami, Z.; Ehsani, M.; Zandi, M.; Foudazi, R. Controlling alginate oxidation conditions for making alginate-gelatin hydrogels. Carbohydr. Polym. 2018, 198, 509–517. [Google Scholar] [CrossRef]
- Sarker, B.; Papageorgiou, D.G.; Silva, R.; Zehnder, T.; Gul, E.N.F.; Bertmer, M.; Kaschta, J.; Chrissafis, K.; Detsch, R.; Boccaccini, A.R. Fabrication of alginate-gelatin crosslinked hydrogel microcapsules and evaluation of the microstructure and physico-chemical properties. J. Mater. Chem. B 2014, 2, 1470–1482. [Google Scholar] [CrossRef] [Green Version]
- Balakrishnan, B.; Lesieur, S.; Labarre, D.; Jayakrishnan, A. Periodate oxidation of sodium alginate in water and in ethanol–water mixture: A comparative study. Carbohydr. Res. 2005, 340, 1425–1429. [Google Scholar] [CrossRef]
- Zhao, H.; Heindel, N.D. Determination of Degree of Substitution of Formyl Groups in Polyaldehyde Dextran by the Hydroxylamine Hydrochloride Method. Pharm. Res. 1991, 8, 400–402. [Google Scholar] [CrossRef]
- Tomić, S.L.; Nikodinović-Runić, J.; Vukomanović, M.; Babić, M.M.; Vuković, J.S. Novel Hydrogel Scaffolds Based on Alginate, Gelatin, 2-Hydroxyethyl Methacrylate, and Hydroxyapatite. Polymers 2021, 13, 932. [Google Scholar] [CrossRef] [PubMed]
- Bednarzig, V.; Karakaya, E.; Egaña, A.L.; Teßmar, J.; Boccaccini, A.R.; Detsch, R. Advanced ADA-GEL bioink for bioprinted artificial cancer models. Bioprinting 2021, 23, e00145. [Google Scholar] [CrossRef]
- Sato, S.; Sakamoto, T.; Miyazawa, E.; Kikugawa, Y. One-pot reductive amination of aldehydes and ketones with α-picoline-borane in methanol, in water, and in neat conditions. Tetrahedron 2004, 60, 7899–7906. [Google Scholar] [CrossRef]
- Cosenza, V.A.; Navarro, D.A.; Stortz, C.A. Usage of α-picoline borane for the reductive amination of carbohydrates. ARKIVOC 2011, 2011, 182–194. [Google Scholar] [CrossRef] [Green Version]
- Kapishon, V.; Whitney, R.A.; Champagne, P.; Cunningham, M.F.; Neufeld, R.J. Polymerization Induced Self-Assembly of Alginate Based Amphiphilic Graft Copolymers Synthesized by Single Electron Transfer Living Radical Polymerization. Biomacromolecules 2015, 16, 2040–2048. [Google Scholar] [CrossRef]
- Praphakar, R.A.; Munusamy, M.A.; Alarfaj, A.A.; Kumar, S.S.; Rajan, M. Zn2+ cross-linked sodium alginate-g-allylamine-mannose polymeric carrier of rifampicin for macrophage targeting tuberculosis nanotherapy. New J. Chem. 2017, 41, 11324–11334. [Google Scholar] [CrossRef]
- Sharon, N.; Lis, H. Lectins, 2nd ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003. [Google Scholar]
- El-Maradny, Y.A.; El-Fakharany, E.M.; Abu-Serie, M.M.; Hashish, M.H.; Selim, H.S. Lectins purified from medicinal and edible mushrooms: Insights into their antiviral activity against pathogenic viruses. Int. J. Biol. Macromol. 2021, 179, 239–258. [Google Scholar] [CrossRef]
- Sharon, N.; Lis, H. History of lectins: From hemagglutinins to biological recognition molecules. Glycobiology 2004, 14, 53R–62R. [Google Scholar] [CrossRef] [Green Version]
- Espinosa, J.F.; Asensio, J.L.; García, J.L.; Laynez, J.; Bruix, M.; Wright, C.; Siebert, H.-C.; Gabius, H.-J.; Cañada, F.J.; Jiménez-Barbero, J. NMR investigations of protein–carbohydrate interactions. Eur. J. Biochem. 2000, 267, 3965–3978. [Google Scholar] [CrossRef]
- Jiménez-Barbero, J.; Javier Cañada, F.; Asensio, J.L.; Aboitiz, N.; Vidal, P.; Canales, A.; Groves, P.; Gabius, H.-J.; Siebert, H.-C. Hevein Domains: An Attractive Model to Study Carbohydrate–Protein Interactions at Atomic Resolution. Adv. Carbohydr. Chem. Biochem. 2006, 60, 303–354. [Google Scholar]
- Biari, K.E.; Gaudioso, Á.; Fernández-Alonso, M.C.; Jiménez-Barbero, J.; Cañada, F.J. Peptidoglycan Recognition by Wheat Germ Agglutinin. A View by NMR. Nat. Prod. Commun. 2019, 14, 1934578X19849240. [Google Scholar] [CrossRef] [Green Version]
- Tateno, H.; Nakamura-Tsuruta, S.; Hirabayashi, J. Comparative analysis of core-fucose-binding lectins from Lens culinaris and Pisum sativum using frontal affinity chromatography. Glycobiology 2009, 19, 527–536. [Google Scholar] [CrossRef] [Green Version]
- Coulibaly, F.S.; Youan, B.B.C. Current status of lectin-based cancer diagnosis and therapy. AIMS Mol. Sci. 2017, 4, 1–27. [Google Scholar] [CrossRef]
- Li, C.; Simeone, D.M.; Brenner, D.E.; Anderson, M.A.; Shedden, K.A.; Ruffin, M.T.; Lubman, D.M. Pancreatic Cancer Serum Detection Using a Lectin/Glyco-Antibody Array Method. J. Proteome Res. 2009, 8, 483–492. [Google Scholar] [CrossRef] [Green Version]
- Minko, T. Drug targeting to the colon with lectins and neoglycoconjugates. Adv. Drug Deliv. Rev. 2004, 56, 491–509. [Google Scholar] [CrossRef] [PubMed]
- Gabor, F.; Bogner, E.; Weissenboeck, A.; Wirth, M. The lectin-cell interaction and its implications to intestinal lectin-mediated drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 459–480. [Google Scholar] [CrossRef] [PubMed]
- Wijetunge, S.S.; Wen, J.; Yeh, C.-K.; Sun, Y. Lectin-Conjugated Liposomes as Biocompatible, Bioadhesive Drug Carriers for the Management of Oral Ulcerative Lesions. ACS Appl. Bio Mater. 2018, 1, 1487–1495. [Google Scholar] [CrossRef]
- Wang, J.; Duan, T.; Sun, L.; Liu, D.; Wang, Z. Functional gold nanoparticles for studying the interaction of lectin with glycosyl complex on living cellular surfaces. Anal. Biochem. 2009, 392, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Mo, Y.; Lim, L.-Y. Mechanistic study of the uptake of wheat germ agglutinin-conjugated PLGA nanoparticles by A549 cells. J. Pharm. Sci. 2004, 93, 20–28. [Google Scholar] [CrossRef]
- Wang, C.; Ho, P.C.; Lim, L.Y. Wheat germ agglutinin-conjugated PLGA nanoparticles for enhanced intracellular delivery of paclitaxel to colon cancer cells. Int. J. Pharm. 2010, 400, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Chen, D.; Qiao, M.; Lu, Z.; Hu, H. Preparation and evaluation of lectin-conjugated PLGA nanoparticles for oral delivery of thymopentin. J. Control. Release 2006, 116, 337–345. [Google Scholar] [CrossRef]
- Mo, Y.; Lim, L.-Y. Preparation and in vitro anticancer activity of wheat germ agglutinin (WGA)-conjugated PLGA nanoparticles loaded with paclitaxel and isopropyl myristate. J. Control. Release 2005, 107, 30–42. [Google Scholar] [CrossRef]
- Leong, K.H.; Chung, L.Y.; Noordin, M.I.; Onuki, Y.; Morishita, M.; Takayama, K. Lectin-functionalized carboxymethylated kappa-carrageenan microparticles for oral insulin delivery. Carbohydr. Polym. 2011, 86, 555–565. [Google Scholar] [CrossRef]
- Apfelthaler, C.; Anzengruber, M.; Gabor, F.; Wirth, M. Poly–(l)–glutamic acid drug delivery system for the intravesical therapy of bladder cancer using WGA as targeting moiety. Eur. J. Pharm. Biopharm. 2017, 115, 131–139. [Google Scholar] [CrossRef]
- Apfelthaler, C.; Skoll, K.; Ciola, R.; Gabor, F.; Wirth, M. A doxorubicin loaded colloidal delivery system for the intravesical therapy of non-muscle invasive bladder cancer using wheat germ agglutinin as targeter. Eur. J. Pharm. Biopharm. 2018, 130, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.-C.; Chang, Y.-H.; Rajesh, R. Targeted delivery of etoposide, carmustine and doxorubicin to human glioblastoma cells using methoxy poly(ethylene glycol) poly(Õ caprolactone) nanoparticles conjugated with wheat germ agglutinin and folic acid. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 96, 114–128. [Google Scholar] [CrossRef]
- Chiu, H.I.; Ayub, A.D.; Mat Yusuf, S.N.A.; Yahaya, N.; Abd Kadir, E.; Lim, V. Docetaxel-Loaded Disulfide Cross-Linked Nanoparticles Derived from Thiolated Sodium Alginate for Colon Cancer Drug Delivery. Pharmaceutics 2020, 12, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glavas-Dodov, M.; Steffansen, B.; Crcarevska, M.S.; Geskovski, N.; Dimchevska, S.; Kuzmanovska, S.; Goracinova, K. Wheat germ agglutinin-functionalised crosslinked polyelectrolyte microparticles for local colon delivery of 5-FU: In vitro efficacy and in vivo gastrointestinal distribution. J. Microencapsul. 2013, 30, 643–656. [Google Scholar] [CrossRef]
- Murata, M.; Yonamine, T.; Tanaka, S.; Tahara, K.; Tozuka, Y.; Takeuchi, H. Surface Modification of Liposomes Using Polymer-Wheat Germ Agglutinin Conjugates to Improve the Absorption of Peptide Drugs by Pulmonary Administration. J. Pharm. Sci. 2013, 102, 1281–1289. [Google Scholar] [CrossRef] [PubMed]
- Brück, A.; Abu-dahab, R.; Borchard, G.; Schäfer, U.F.; Lehr, C.M. Lectin-Functionalized Liposomes for Pulmonary Drug Delivery: Interaction with Human Alveolar Epithelial Cells. J. Drug Target. 2001, 9, 241–251. [Google Scholar] [CrossRef]
- Zhang, N.; Ping, Q.; Huang, G.; Xu, W.; Cheng, Y.; Han, X. Lectin-modified solid lipid nanoparticles as carriers for oral administration of insulin. Int. J. Pharm. 2006, 327, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Makhlof, A.; Fujimoto, S.; Tozuka, Y.; Takeuchi, H. In vitro and in vivo evaluation of WGA–carbopol modified liposomes as carriers for oral peptide delivery. Eur. J. Pharm. Biopharm. 2011, 77, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Ezpeleta, I.; Arangoa, M.A.; Irache, J.M.; Stainmesse, S.; Chabenat, C.; Popineau, Y.; Orecchioni, A.-M. Preparation of Ulex europaeus lectin-gliadin nanoparticle conjugates and their interaction with gastrointestinal mucus. Int. J. Pharm. 1999, 191, 25–32. [Google Scholar] [CrossRef]
- Montisci, M.-J.; Dembri, A.; Giovannuci, G.; Chacun, H.; Duchêne, D.; Ponchel, G. Gastrointestinal Transit and Mucoadhesion of Colloidal Suspensions of Lycopersicon Esculentum L. and Lotus Tetragonolobus Lectin-PLA Microsphere Conjugates in Rats. Pharm. Res. 2001, 18, 829–837. [Google Scholar] [CrossRef]
- Adebisi, A.O.; Conway, B.R. Lectin-conjugated microspheres for eradication of Helicobacter pylori infection and interaction with mucus. Int. J. Pharm. 2014, 470, 28–40. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.-A.; Shin, M.S.; Yang, J.-W. Preparation and characterization of hydrophobically modified alginate. Polym. Bull. 2002, 47, 429–435. [Google Scholar] [CrossRef]
- Banks, S.R.; Enck, K.; Wright, M.; Opara, E.C.; Welker, M.E. Chemical Modification of Alginate for Controlled Oral Drug Delivery. J. Agric. Food Chem. 2019, 67, 10481–10488. [Google Scholar] [CrossRef]



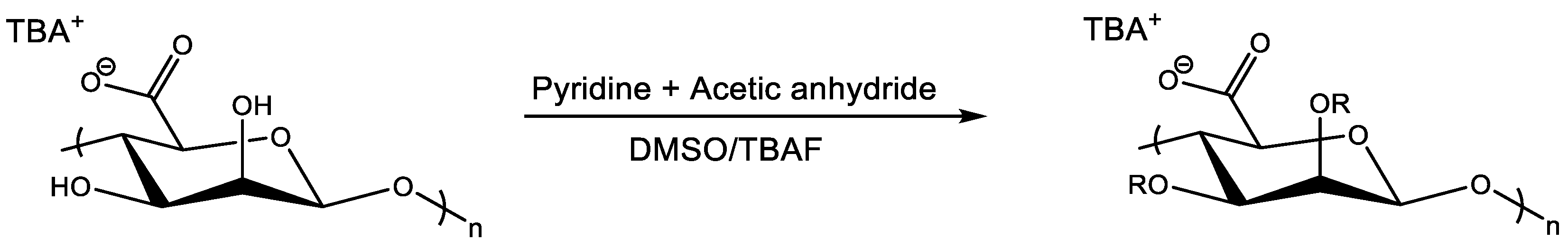

| Carrier | Lectin | Glycan Preference | Carrier-Lectin Conjugation Method | Targeted Cell/Organ | Ref. |
|---|---|---|---|---|---|
| PLGA nanoparticles | Wheat germ agglutinin (WGA) | Sialic acid and N-acetyl glucosamine (GlcNac) | Carbodiimide | Caco-2 cells Caco-2 and HT-29 cells (colon cancer) A549 cells (Type II alveolar epithelial cells) Wistar rats | [8,113,114,115] |
| PLGA nanoparticles containing isopropyl myristate (IPM) | Wheat germ agglutinin (WGA) | Sialic acid and N-acetyl glucosamine (GlcNac) | Carbodiimide | A549 cells, H1299 cells (non-small cell lung carcinoma), CCL-186 cells (lung fibroblast IMR-90) | [116] |
| Carboxymethylated kappa-carrageenan microparticles | Wheat germ agglutinin (WGA) | Sialic acid and N-acetyl glucosamine (GlcNac) | Glutaraldehyde linker | Caco-2 cells | [117] |
| Poly-l-glutamic acid (PGA) and α-poly-(l)-glutamic acid (PGA) | Wheat germ agglutinin (WGA) | Sialic acid and N-acetyl glucosamine (GlcNac) | Carbodiimide | 5637 cells (urothelial carcinoma) | [118,119] |
| Methoxy poly(ethylene glycol)-poly(ε-caprolactone) (MPEG-PCL) nanoparticles | Wheat germ agglutinin (WGA) | Sialic acid and N-acetyl glucosamine (GlcNac) | Carbodiimide | U87MG cells (glioblastoma) | [120] |
| Thiolated alginate nanoparticles | Wheat germ agglutinin (WGA) | Sialic acid and N-acetyl glucosamine (GlcNac) | Carbodiimide | HT-29 | [121] |
| Chitosan-Ca-alginate (CTS-Ca-ALG) microparticles | Wheat germ agglutinin (WGA) | Sialic acid and N-acetyl glucosamine (GlcNac) | Carbodiimide | Caco-2 cells | [122] |
| Liposomes | Wheat germ agglutinin (WGA) | Sialic acid and N-acetyl glucosamine (GlcNac) | Carbodiimide | A549 cells | [123] |
| Liposomes | Concanavalin A (Con A), Wheat germ agglutinin (WGA), and Soybean agglutinin (SBA) | α-D-mannose, α-D-glucose, and N-acetyl glucosamine (GlcNac) | Neutravidin-Biotin-complexes linker | A549 cells | [124] |
| Liposomes | Wheat germ agglutinin (WGA), Tomato lectin (TL), and Ulex europaeus agglutinin 1 (UEA1) | Sialic acid, N-acetyl glucosamine (GlcNac), and α-L-fucose | Carbodiimide | Mice | [125] |
| Liposomes nanocarriers | Wheat germ agglutinin-carbopol (WGA-CP) conjugate | N-acetyl glucosamine (GlcNac) | Carbodiimide | Caco-2 cells and intestinal membrane of rats | [126] |
| Gliadin nanoparticle (GNP) | Ulex europaeus agglutinin 1 (UEA1) | α-L-fucose | Carbodiimide | Bovine submaxillary gland mucin (BSM) | [127] |
| Poly(lactide) (PLA) microspheres | Lycopersicon esculentum agglutinin (LEA) | N-acetyl-d-glucosamine and α-L-fucose | PVA linker | Rat intestinal mucosa | [128] |
| Ethylcellulose (EC) and chitosan floating-mucoadhesive microp[articles | Concanavalin A (Con A) | α-D-mannose and α-D-glucose | Carbodiimide | Pig gastric mucosa | [129] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Putri, A.P.; Picchioni, F.; Harjanto, S.; Chalid, M. Alginate Modification and Lectin-Conjugation Approach to Synthesize the Mucoadhesive Matrix. Appl. Sci. 2021, 11, 11818. https://doi.org/10.3390/app112411818
Putri AP, Picchioni F, Harjanto S, Chalid M. Alginate Modification and Lectin-Conjugation Approach to Synthesize the Mucoadhesive Matrix. Applied Sciences. 2021; 11(24):11818. https://doi.org/10.3390/app112411818
Chicago/Turabian StylePutri, Arlina Prima, Francesco Picchioni, Sri Harjanto, and Mochamad Chalid. 2021. "Alginate Modification and Lectin-Conjugation Approach to Synthesize the Mucoadhesive Matrix" Applied Sciences 11, no. 24: 11818. https://doi.org/10.3390/app112411818
APA StylePutri, A. P., Picchioni, F., Harjanto, S., & Chalid, M. (2021). Alginate Modification and Lectin-Conjugation Approach to Synthesize the Mucoadhesive Matrix. Applied Sciences, 11(24), 11818. https://doi.org/10.3390/app112411818








