Exploration of the Potential Bioactive Molecules of Tamarillo (Cyphomandra betacea): Antioxidant Properties and Prebiotic Index
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Samples Preparation
2.3. Preparation of Ethanolic Extracts
2.4. Bromatological Analysis
2.5. Antioxidant Properties
2.6. Spectroscopic Methods
2.7. Prebiotic Activity Score
3. Results and Discussions
3.1. Bromatological Analysis
3.2. Antioxidant Properties
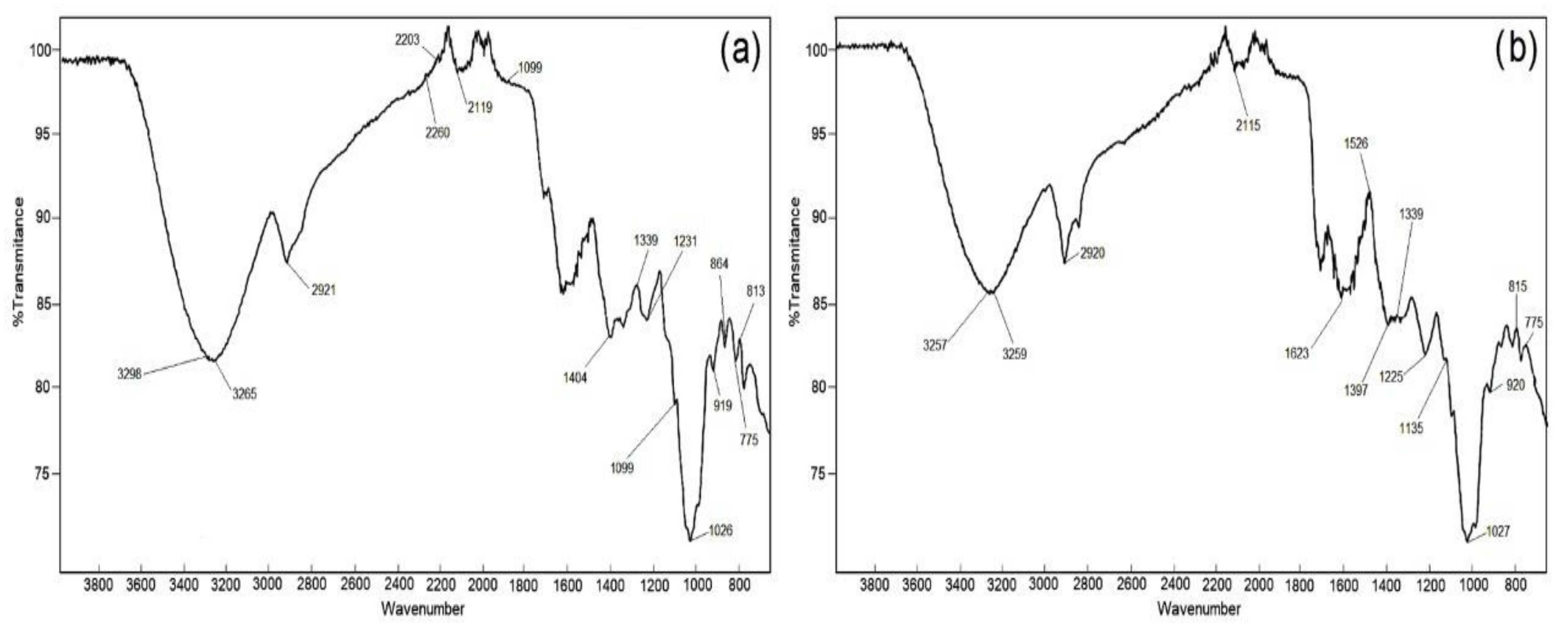
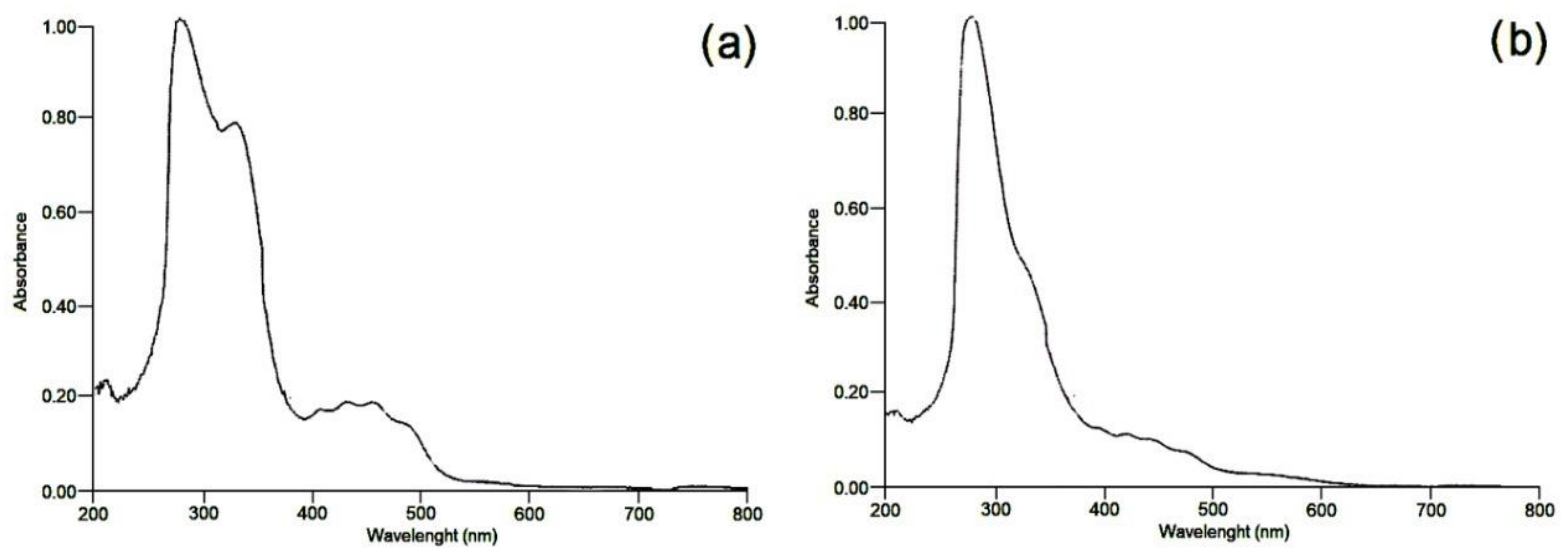
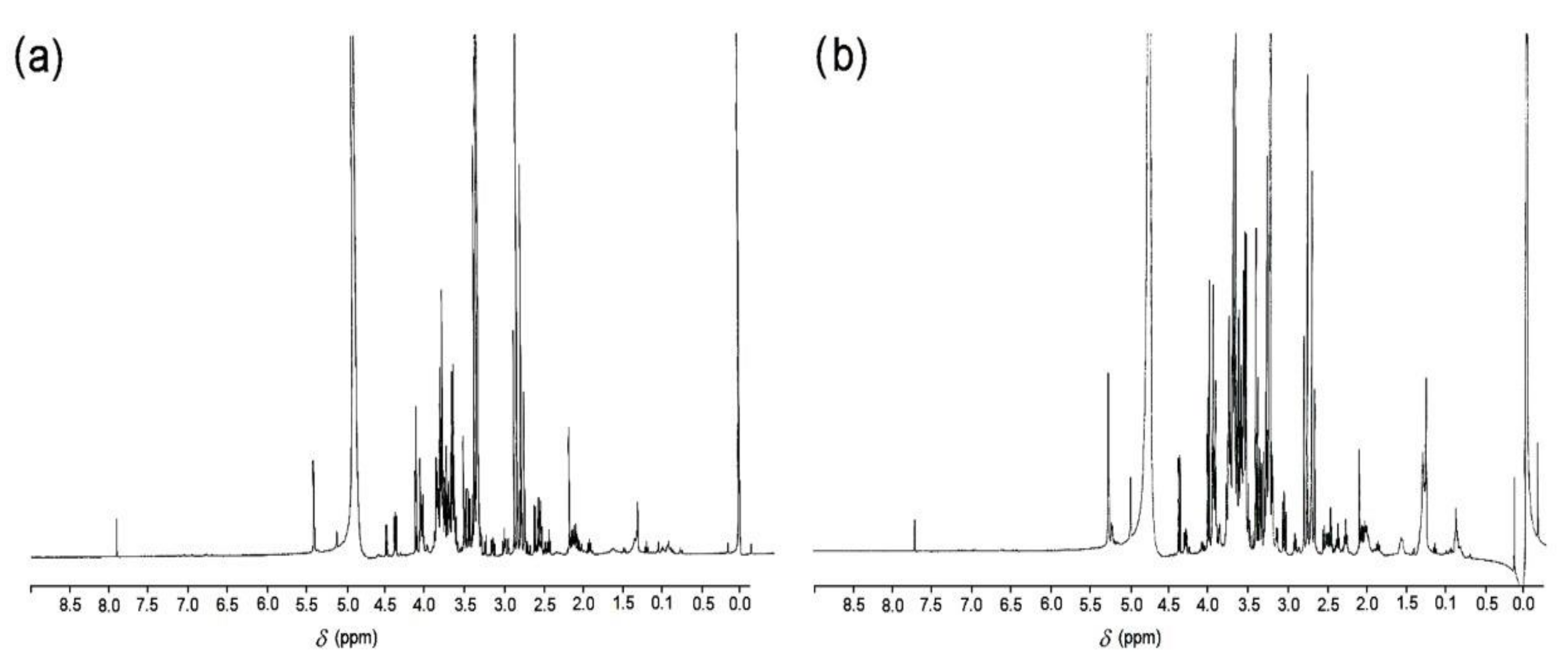
3.3. Spectroscopic Methods
3.4. Prebiotic Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- CONABIO. 2021. Sistema Nacional de Información sobre Biodiversidad (SNIB). Registros de Ejemplares, Versión 2021–03. México. Available online: http://www.snib.mx/ejemplares/docs/CONABIO-SNIB-Version202103.pdf (accessed on 3 November 2021).
- Duarte, O. Cocona (Solanum sessiliflorum Dunal). In Postharvest Biology and Technology of Tropical and Subtropical Fruits, 1st ed.; Yahia, E.M., Ed.; Woodhead Publishing: Sawston, UK, 2011; pp. 1–7. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, F. Tamarillo (Solanum betaceum): Chemical composition, biological properties, and product innovation. Trends Food Sci. Technol. 2020, 95, 45–58. [Google Scholar] [CrossRef]
- Prohens, J.; Nuez, F. The tamarillo (Cyphomandra betacea). Small Fruits Rev. 2001, 1, 43–68. [Google Scholar] [CrossRef]
- Vasco, C.; Avila, J.; Ruales, J.; Svanberg, U.; Kamal-Eldin, A. Physical and chemical characteristics of golden-yellow and purple-red varieties of tamarillo fruit (Solanum betaceum Cav.). Int. J. Food Sci. Nutr. 2009, 60, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Orqueda, M.E.; Rivas, M.; Zampini, I.C.; Alberto, M.R.; Torres, S.; Cuello, S.; Sayago, J.; Thomas-Valdes, S.; Jiménez-Aspee, F.; Schmeda-Hirschmann, G.; et al. Chemical and functional characterization of seed, pulp and skin powder from chilto (Solanum betaceum), an Argentine native fruit. Phenolic fractions affect key enzymes involved in metabolic syndrome and oxidative stress. Food Chem. 2017, 216, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Jauregui, A.M.; Ramos-Escudero, F.; Alvarado-Ortiz Ureta, C.; Castañeda Castañeda, B.; Lizaraso Caparó, F. Evaluación de compuestos con actividad biológica en cáscara de camu camu (Myrciaria dubia), guinda (Prunus serotina), tomate de árbol (Cyphomandra betacea) y carambola (Averrhoa carambola L.) cultivadas en Perú. Rev. Soc. Chem. Perú 2009, 75, 431–438. [Google Scholar]
- Gannasin, S.P.; Ramakrishnan, Y.; Adzahan, N.M.; Muhammad, K. Functional and preliminary characterisation of hydrocolloid from tamarillo (Solanum betaceum Cav.) puree. Molecules 2012, 17, 6869–6885. [Google Scholar] [CrossRef] [PubMed]
- Ranadheera, R.; Baines, S.K.; Adams, M.C. Importance of food in probiotic efficacy. Food Res. Int. 2010, 43, 1–7. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of Association of Official Analytical Chemists, 18th ed.; AOAC: Washington, DC, USA, 2010; Available online: http://URL (accessed on 2 July 2019).
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Singleton, W.T.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1956, 16, 144–158. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Kasote, D.M.; Katyare, S.S.; Hegdem, M.V.; Bae, H. Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int. J. Biol. Sci. 2015, 11, 982–991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huebner, J.; Wehling, R.L.; Hutkins, R.W. Functional activity of commercial prebiotics. Int. Dairy J. 2007, 17, 770–775. [Google Scholar] [CrossRef]
- Morillas-Ruiz, J.M.; Delgado-Alarcón, J.M. Análisis nutricional de alimentos vegetales con diferentes orígenes: Evaluación de capacidad antioxidante y compuestos fenólicos totales. Nutri. Clín. Diet. Hosp. 2012, 32, 8–20. Available online: http://www.usfx.bo/nueva/vicerrectorado/citas/SALUD_10/Nutricion_y_Dietetica/71.pdf (accessed on 2 November 2021).
- Brasil, I.; Siddiqui, M. Chapter 1: Postharvest Quality of Fruits and Vegetables: An Overview. In Preharvest Modulation of Postharvest Fruit and Vegetable Quality; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar] [CrossRef]
- Acosta-Quezada, P.G.; Raigón, M.D.; Riofrío-Cuenca, T.; García-Martínez, M.D.; Plazas, M.; Burneo, J.I.; Figueroa, J.G.; Vilanova, S.; Prohens, J. Diversity for chemical composition in a collection of different varietal types of tree tomato (Solanum betaceum Cav.), an Andean exotic fruit. Food Chem. 2015, 169, 327–335. [Google Scholar] [CrossRef]
- Lister, C.; Morrison, S.; Kerkhofs, N.; Wright, K. The nutritional composition and health benefits of New Zealand tamarillos. Crop. Food Res. Confid. Rep. 2005, 1281, 29. [Google Scholar]
- Mutalib, M.A.; Rahmat, A.; Ali, F.; Othman, F.; Ramasamy, R. Nutritional compositions and antiproliferative activities of different solvent fractions from ethanol extract of Cyphomandra betacea (Tamarillo) fruit. Malays. J. Med. Sci. 2017, 24, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Vergara-Valencia, N.; Granados-Pérez, E.; Agama-Acevedo, E.; Tovar, J.; Ruales, J.; Bello-Pérez, L.A. Fibre concentrate from mango fruit: Characterization, associated antioxidant capacity and application as a bakery product ingredient. LWT-Food Sci. Technol. 2007, 40, 722–729. [Google Scholar] [CrossRef]
- Mudgil, D.; Barak, S. Composition, properties and health benefits of indigestible carbohydrate polymers as dietary fiber: A review. Int. J. Biol. Macromol. 2013, 61, 1–6. [Google Scholar] [CrossRef]
- Yaich, H.; Garna, H.; Bchir, B.; Besbes, S.; Paquot, M.; Richel, A.; Blecker, C.; Attia, H. Chemical composition and functional properties of dietary fibre extracted by Englyst and Prosky methods from the alga Ulva lactuca collected in Tunisia. Algal Research. 2015, 9, 65–73. [Google Scholar] [CrossRef]
- Espin, S.; González-Manzano, S.; Taco, V.; Poveda, C.; Ayuda-Durán, B.; González-Paramas, A.M.; Santos-Buelga, C. Phenolic composition and antioxidant capacity of yellow and purple-red Ecuadorian cultivars of tree tomato (Solanum betaceum Cav.). Food Chem. 2016, 194, 1073–1780. [Google Scholar] [CrossRef] [PubMed]
- Peluso, I.; Palmery, M. Flavonoids at the pharma-nutrition interface: Is a therapeutic index in demand? Biomed. Pharmacother. 2015, 71, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, N.H.; Morales, A.L.; González-Miret, M.L.; Escudero-Gilete, M.L.; Heredia, F.J. Colour, pH stability and antioxidant activity of anthocyanin rutinosides isolated from tamarillo fruit (Solanum betaceum Cav.). Food Chem. 2009, 117, 88–93. [Google Scholar] [CrossRef]
- Ordóñez, R.M.; Cardozo, M.L.; Zampini, I.C.; Isla, M.I. Evaluation of antioxidant activity and genotoxicity of alcoholic and aqueous beverages and pomace derived from ripe fruits of Cyphomandra betacea Sendt. J. Agric. Food Chem. 2010, 58, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Rufino, M.d.S.M.; Alves, R.E.; de Brito, E.S.; Pérez-Jiménez, J.; Saura-Calixto, F.; Mancini-Filho, J. Bioactive compounds and antioxidant capacities of 18 non-traditional tropical fruits from Brazil. Food Chem. 2010, 121, 996–1002. [Google Scholar] [CrossRef] [Green Version]
- Saura-Calixto, F. Dietary fiber as a carrier of dietary antioxidants: An essential physiological function. J. Agric. Food Chem. 2011, 59, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Orqueda, M.E.; Torres, S.; Zampini, I.C.; Cattaneo, F.; Di Pardo, A.F.; Valle, E.M.; Jiménez-Aspee, F.; Schmeda-Hirschmann, G.; Isla, M.I. Integral use of Argentinean Solanum betaceum red fruits as functional food ingredient to prevent metabolic syndrome: Effect of in vitro simulated gastroduodenal digestion. Heliyon 2020, 6, e03387. [Google Scholar] [CrossRef] [Green Version]
- Rajendran, P.; Nandakumar, N.; Rengarajan, T.; Palaniswami, R.; Gnanadhas, E.N.; Lakshminarasaiah, U.; Gopas, J.; Nishigaki, I. Antioxidants and human diseases. Clin. Chim. Acta. 2014, 436, 332–347. [Google Scholar] [CrossRef]
- Brummer, Y.; Cui, S.W. Detection and determination of polysaccharides in foods. In Food Polysaccharides and Their Applications; Stephen, A.M., Phillips, G.O., Eds.; CRC Press: New York, NY, USA, 2006; pp. 704–705. [Google Scholar]
- Gannasin, S.P.; Mustafa, S.; Adzahan, N.M.; Muhammad, K. In vitro prebiotic activities of tamarillo (Solanum betaceum Cav.) hydrocolloids. J. Funct. Foods 2015, 19, 10–19. [Google Scholar] [CrossRef]
- Wei, L.; Tan, W.; Zhang, J.; Mi, Y.; Dong, F.; Li, Q.; Guo, Z. Synthesis, Characterization, and Antifungal Activity of Schiff Bases of Inulin Bearing Pyridine ring. Polymers 2019, 11, 371. [Google Scholar] [CrossRef] [Green Version]
- Kaijanen, L.; Paakkunainen, M.; Pietarinen, S.; Jernström, E.; Reinikainen, S.P. Ultraviolet detection of monosaccharides: Multiple wavelength strategy to evaluate results after capillary zone electrophoretic separation. Int. J. Electrochem. Sci. 2015, 10, 2950–2961. [Google Scholar]
- Osorio, C.; Hurtado, N.; Dawid, C.; Hofmann, T.; Heredia-Mira, F.J.; Morales, A.L. Chemical characterisation of anthocyanins in tamarillo (Solanum betaceum Cav.) and Andes berry (Rubus glaucus Benth.) fruits. Food Chem. 2012, 132, 1912–1921. [Google Scholar] [CrossRef]
- Barclay, T.; Ginic-Markovic, M.; Johnston, M.R.; Cooper, P.D.; Petrovsky, N. Analysis of the hydrolysis of inulin using real time 1H NMR spectroscopy. Carbohyd. Res. 2012, 352, 117–125. [Google Scholar] [CrossRef] [Green Version]
- Nascimento, G.E.; Iacomini, M.; Cordeiro, L.M.C. A comparative study of mucilage and pulp polysaccharides from tamarillo fruit (Solanum betaceum Cav.). Plant Physiol. Biochem 2016, 104, 278–283. [Google Scholar] [CrossRef]
- Nascimento, G.E.; Hamm, L.A.; Baggio, C.H.; Werner, M.F.P.; Iacomini, M.; Cordeiro, L.M.C. Structure of a galactoarabinoglucuronoxylan from tamarillo (Solanum betaceum), a tropical exotic fruit, and its biological activity. Food Chem. 2013, 141, 510–516. [Google Scholar] [CrossRef] [Green Version]
- Kou, M.C.; Yen, J.H.; Hong, J.T.; Wang, C.L.; Lin, C.W.; Wu, M.J. Cyphomandra betacea Sendt. phenolics protect LDL from oxidation and PC12 cells from oxidative stress. LWT Food Sci. Technol. 2009, 42, 458–463. [Google Scholar] [CrossRef]
- Diaz-Vela, J.; Totosaus, A.; Cruz-Guerrero, A.E.; Pérez-Chabela, M.L. In vitro evaluation of the fermentation of added-value agroindustrial by-products: Cactus pear (Opuntia ficus-indica L.) peel and pineapple (Ananas comosus) peel as functional ingredients. Int. J. Food Sci. Technol. 2013, 48, 1460–1467. [Google Scholar] [CrossRef]
- Minzanova, S.T.; Mironov, V.F.; Arkhipova, D.M.; Khabibullina, A.V.; Mironova, L.G.; Zakirova, Y.M.; Milyukov, V.A. Biological Activity and Pharmacological Application of Pectic Polysaccharides: A Review. Polymers 2018, 10, 1407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salazar-Lugo, R.; Barahona, A.; Ortiz, K.; Chávez, C.; Freire, P.; Méndez, J. Efecto del consumo de jugo de tomate de árbol (Cyphomandra betacea) sobre el perfil lipídico y las concentraciones de glucosa en adultos con hiperlipidemia, Ecuador. Arch. Latinoam. Nutr. 2016, 66, 121–128. [Google Scholar] [PubMed]

| Parameter 1 | Pulp | Epicarp |
| Moisture | 86.75 ± 0.35 | 68 ± 0.00 |
| Fat | 0.18 ± 0.01 | 1.71 ± 0.17 |
| Ash | 1.1 ± 0.02 | 2.1 ± 0.04 |
| Protein | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Crude fiber | 3.57 ± 0.09 | 30.12 ± 0.17 |
| Total sugars | 61.30 ± 0.21 | 20.51 ± 0.01 |
| Soluble dietary fiber | 0.12 ± 0.00 | 0.14 ± 0.01 |
| Insoluble dietary fiber | 3.92 ± 0.02 | 8.44 ± 0.03 |
| * Total dietary fiber | 4.07 ± 0.01 | 8.58 ± 0.00 |
| Phenolic compounds 2 | 206.23 ± 0.4 | ND |
| Antioxidant capacity 3 | 6.27 ± 0.01 | ND |
| Antioxidant activity 4 | 131.26 ± 0.00 | ND |
| Substrate/Flour | L. casei | L. plantarum | L. paracasei | p |
|---|---|---|---|---|
| DPFC | 0.08 ± 0.00 c | 1.49 ± 0.01 a | 0.33 ± 0.01 b | 0.0001 |
| DEFC | −0.35 ± 0.02 b | 1.30 ± 0.01 a | −0.02 ± 0.01 c | 0.0001 |
| P | 0.0001 | 0.5482 | 0.0002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reyes-García, V.; Totosaus, A.; Pérez-Chabela, L.; Juárez, Z.N.; Cardoso-Ugarte, G.A.; Pérez-Armendáriz, B. Exploration of the Potential Bioactive Molecules of Tamarillo (Cyphomandra betacea): Antioxidant Properties and Prebiotic Index. Appl. Sci. 2021, 11, 11322. https://doi.org/10.3390/app112311322
Reyes-García V, Totosaus A, Pérez-Chabela L, Juárez ZN, Cardoso-Ugarte GA, Pérez-Armendáriz B. Exploration of the Potential Bioactive Molecules of Tamarillo (Cyphomandra betacea): Antioxidant Properties and Prebiotic Index. Applied Sciences. 2021; 11(23):11322. https://doi.org/10.3390/app112311322
Chicago/Turabian StyleReyes-García, Verónica, Alfonso Totosaus, Lourdes Pérez-Chabela, Zaida Nelly Juárez, Gabriel Abraham Cardoso-Ugarte, and Beatriz Pérez-Armendáriz. 2021. "Exploration of the Potential Bioactive Molecules of Tamarillo (Cyphomandra betacea): Antioxidant Properties and Prebiotic Index" Applied Sciences 11, no. 23: 11322. https://doi.org/10.3390/app112311322
APA StyleReyes-García, V., Totosaus, A., Pérez-Chabela, L., Juárez, Z. N., Cardoso-Ugarte, G. A., & Pérez-Armendáriz, B. (2021). Exploration of the Potential Bioactive Molecules of Tamarillo (Cyphomandra betacea): Antioxidant Properties and Prebiotic Index. Applied Sciences, 11(23), 11322. https://doi.org/10.3390/app112311322







